Abstract
For development of an effective T cell-based AIDS vaccine, it is critical to define the antigens that elicit the most potent responses. Recent studies have suggested that Gag-specific and possibly Vif/Nef-specific CD8+ T cells can be important in control of the AIDS virus. Here, we tested whether induction of these CD8+ T cells by prophylactic vaccination can result in control of simian immunodeficiency virus (SIV) replication in Burmese rhesus macaques sharing the major histocompatibility complex class I (MHC-I) haplotype 90-010-Ie associated with dominant Nef-specific CD8+ T-cell responses. In the first group vaccinated with Gag-expressing vectors (n = 5 animals), three animals that showed efficient Gag-specific CD8+ T-cell responses in the acute phase postchallenge controlled SIV replication. In the second group vaccinated with Vif- and Nef-expressing vectors (n = 6 animals), three animals that elicited Vif-specific CD8+ T-cell responses in the acute phase showed SIV control, whereas the remaining three with Nef-specific but not Vif-specific CD8+ T-cell responses failed to control SIV replication. Analysis of 18 animals, consisting of seven unvaccinated noncontrollers and the 11 vaccinees described above, revealed that the sum of Gag- and Vif-specific CD8+ T-cell frequencies in the acute phase was inversely correlated with plasma viral loads in the chronic phase. Our results suggest that replication of the AIDS virus can be controlled by vaccine-induced subdominant Gag/Vif epitope-specific CD8+ T cells, providing a rationale for the induction of Gag- and/or Vif-specific CD8+ T-cell responses by prophylactic AIDS vaccines.
INTRODUCTION
Human immunodeficiency virus (HIV) infection induces persistent viral replication, leading to AIDS onset in humans. Virus-specific CD8+ T-cell responses play a central role in the resolution of acute peak viremia (1–4) but mostly fail to contain viral replication in HIV infection. Prophylactic vaccination resulting in more effective CD8+ T-cell responses postexposure than those in natural HIV infections might contribute to HIV control. Current trials in macaque AIDS models have shown that vaccine induction of T-cell responses can result in control of postchallenge viral replication (5–10). It is now critical to define the antigens that elicit the most potent responses for development of an effective T-cell-based AIDS vaccine.
Recent studies have implicated Gag-specific CD8+ T cells in the control of HIV and simian immunodeficiency virus (SIV) replication (11–16). Several HLA or major histocompatibility complex class I (MHC-I) alleles have been shown to be associated with lower viral loads (17–25). Virus control associated with some of these protective MHC-I alleles is attributed to Gag epitope-specific CD8+ T-cell responses (26–29). For instance, CD8+ T-cell responses specific for the HLA-B*57-restricted Gag240–249 TW10 and HLA-B*27-restricted Gag263–272 KK10 epitopes exert strong suppressive pressure on HIV replication and frequently select for escape mutations with viral fitness costs, leading to lower viral loads (27, 30–33). Thus, certain individuals possessing MHC-I alleles associated with dominant Gag-specific CD8+ T-cell responses could have a greater chance to control HIV replication than those without these alleles. For those individuals that do not express these MHC-I alleles, the question arises as to whether prophylactic vaccination inducing Gag epitope-specific CD8+ T-cell responses might contribute to HIV control. Furthermore, recent studies have shown that CD8+ T-cell responses targeting SIV antigens other than Gag, such as Mamu-B*08- or Mamu-B*17-restricted Vif and Nef epitopes, exert strong suppressive pressure on SIV replication (10, 34, 35).
We previously developed a prophylactic AIDS vaccine consisting of a DNA prime and a boost with a Sendai virus (SeV) vector expressing SIVmac239 Gag (SeV-Gag) (36). Our trial showed vaccine-based control of an SIVmac239 challenge in a group of Burmese rhesus macaques sharing the MHC-I haplotype 90-120-Ia (5, 37). Unvaccinated animals possessing 90-120-Ia dominantly elicited CD8+ T-cell responses specific for the Gag206–216 (IINEEAADWDL) and the Gag241–249 (SSVDEQIQW) epitopes after SIV challenge (38, 39). DNA/SeV-Gag-vaccinated 90-120-Ia-positive macaques showed enhanced Gag206–216-specific and Gag241–249-specific CD8+ T-cell responses in the acute phase after SIV challenge, resulting in viremia control (37). This implies virus control by vaccine-based enhancement of Gag-specific CD8+ T-cell responses in animals possessing MHC-I alleles associated with dominant Gag CD8+ T-cell epitopes. However, we have not defined the efficacy of prophylactic vaccination inducing Gag-specific CD8+ T-cell responses against HIV/SIV infection in the hosts possessing MHC-I alleles not associated with dominant Gag CD8+ T-cell epitopes.
In the present study, we first examined efficacy of prophylactic vaccination inducing Gag-specific CD8+ T-cell responses against SIVmac239 challenge in a group of macaques that possess the 90-010-Ie MHC-I haplotype (referred to as E) associated with dominant Nef-specific CD8+ T-cell responses (39, 40). Furthermore, we examined the efficacy of prophylactic vaccination inducing Vif/Nef-specific CD8+ T-cell responses in these E+ macaques. Our results show SIV control in those vaccinees that mounted efficient Gag- or Vif-specific CD8+ T-cell responses in the acute phase postchallenge.
MATERIALS AND METHODS
Animal experiments.
Animal experiments were carried out in Tsukuba Primate Research Center, National Institute of Biomedical Innovation (NIBP), with the help of the Corporation for Production and Research of Laboratory Primates after approval by the Committee on the Ethics of Animal Experiments of NIBP (permission number DS21-28 and DS23-19) under the guideline for animal experiments at NIBP and National Institute of Infectious Diseases, which is in accordance with the Guidelines for Proper Conduct of Animal Experiments established by Science Council of Japan (http://www.scj.go.jp/ja/info/kohyo/pdf/kohyo-20-k16-2e.pdf). Blood collection, vaccination, and SIV challenge were performed under ketamine anesthesia.
We used Burmese rhesus macaques (Macaca mulatta) possessing the MHC-I haplotype 90-010-Ie (E) (39, 40). The determination of MHC-I haplotypes was based on the family study in combination with the reference strand-mediated conformation analysis of Mamu-A and Mamu-B genes and detection of major Mamu-A and Mamu-B alleles by cloning the reverse transcription (RT)-PCR products as described previously (39–41). Confirmed MHC-I alleles consisting of the MHC-I haplotype E are Mamu-A1*066:01, Mamu-B*005:02, and Mamu-B*015:04. Unvaccinated R01-011, R05-007, R08-003, R08-007, R09-011, and R06-038 and Gag-vaccinated R01-010 and R01-008 used in our previous experiments (39, 42) are included in the present study. At week 1, unvaccinated macaque R06-038 was intravenously infused with 300 mg of nonspecific immunoglobulin G purified from uninfected rhesus macaques as described before (43). All animals were intravenously challenged with 1,000 50% tissue culture infective doses (TCID50) of SIVmac239 (44).
Macaques R01-010, R05-010, R01-008, R08-002, and R08-006 received prophylactic DNA prime/SeV-Gag boost vaccination (referred to as Gag vaccination) (5). The DNA used for the vaccination, cytomegalovirus (CMV)-SHIVdEN, was constructed from env-deleted and nef-deleted simian-human immunodeficiency virus SHIVMD14YE (45) molecular clone DNA (SIVGP1) and has the genes encoding SIVmac239 Gag, Pol, Vif, and Vpx and HIV Tat and Rev. At the DNA vaccination, animals received 5 mg of CMV-SHIVdEN DNA intramuscularly. Six weeks after the DNA prime, animals received a single boost intranasally with 6 × 109 cell infectious units (CIU) of F-deleted replication-defective Sendai virus (SeV) expressing SIVmac239 Gag (SeV-Gag) (46).
Macaques R08-012, R10-012, R10-013, R10-010, R10-011, and R10-014 received prophylactic DNA prime/SeV-VifNef boost vaccination (referred to as Vif/Nef vaccination). The Vif-expressing DNA used for the vaccination, pcDNA-SIVvif-opt, was constructed by introducing an optimized SIVmac239 Vif cDNA (GenScript) into pcDNA3.1. The Nef-expressing DNA used for the vaccination, pcDNA-SIVnef-G2A, has an SIVmac239 Nef cDNA with a mutation resulting in glycine (G) to alanine (A) at the 2nd amino acid (aa) in Nef. Animals intramuscularly received 3 mg of Vif-expressing DNA at the first DNA vaccination and 3 mg of Vif-expressing DNA and 3 mg of Nef-expressing DNA at the second DNA vaccination. Six weeks after the first DNA prime, animals received a single boost intranasally with 1 × 109 CIU of F-deleted SeV expressing Vif-opt (SeV-Vif) and 1 × 109 CIU of F-deleted SeV expressing Nef-G2A (SeV-Nef) (47).
Analysis of antigen-specific CD8+ T-cell responses.
We measured virus-specific CD8+ T-cell frequencies by flow cytometric analysis of gamma interferon (IFN-γ) induction after specific stimulation as described previously (48, 49). Autologous herpesvirus papio-immortalized B-lymphoblastoid cell lines (B-LCLs) were pulsed with each peptide (at a final concentration of 1 μM) or peptide pools (at a final concentration of 1 to 2 μM for each peptide) using panels of overlapping peptides spanning the entire SIVmac239 Gag, Vif, and Nef amino acid sequences (Sigma-Aldrich Japan) for 1 h. Peripheral blood mononuclear cells (PBMCs) were cocultured with these pulsed B-LCLs in the presence of GolgiStop (monensin; BD) for 6 h. Intracellular IFN-γ staining was performed with a Cytofix/Cytoperm kit (BD) and fluorescein isothiocyanate-conjugated anti-human CD4 (BD), peridinin chlorophyll protein-conjugated anti-human CD8 (BD), allophycocyanin (APC)-Cy7-conjugated anti-human CD3 (BD), and phycoerythrin (PE)-conjugated anti-human IFN-γ monoclonal antibodies (BioLegend). In the flow cytometric analysis, PBMCs were gated in forward scatter-side scatter dot plots, and B-LCLs were excluded in this step. Specific T-cell frequencies were calculated by subtracting nonspecific IFN-γ T-cell frequencies (less than 100 per million PBMCs) from those after peptide-specific stimulation. Specific T-cell frequencies lower than 100 per million PBMCs were considered negative.
Sequencing analysis of plasma viral genomes.
Viral RNAs were extracted using the high pure viral RNA kit (Roche Diagnostics, Tokyo, Japan) from macaque plasma obtained around 1 year after challenge. Fragments of cDNAs encoding SIVmac239 Gag, Vif, and Nef were amplified by nested RT-PCR (25 cycles at the first RT-PCR using the PrimeScript one-step RT-PCR kit, version 2 [TaKaRa] and 30 cycles at the second PCR using KOD-Plus, version 2 [Toyobo]) from plasma RNAs and subjected to direct sequencing by using dye terminator chemistry and an automated DNA sequencer (Applied Biosystems, Tokyo, Japan) as described before (39). Predominant nonsynonymous mutations were determined.
Statistical analysis.
Statistical analysis was performed with Prism software version 4.03, with significance levels set at a P value of <0.050 (GraphPad Software, Inc.). Antigen-specific CD8+ T-cell frequencies were compared by the nonparametric Mann-Whitney U test. Correlation was analyzed by the Pearson test.
RESULTS
Plasma viral loads after SIVmac239 challenge.
We used a group of Burmese rhesus macaques possessing the MHC-I haplotype 90-010-Ie (E). In our previous study (39), unvaccinated E+ macaques consistently showed persistent viremia after SIVmac239 challenge. CD4+ T-cell percentage in PBMCs declined to less than 20% in a year. In the present study, we compared viral loads in vaccinated animals with those in these unvaccinated animals.
The first vaccine group of five E+ macaques received a DNA prime and an SeV-Gag boost vaccination, followed by an SIVmac239 challenge. Two of these Gag-vaccinated animals failed to control viral replication, but the remaining three showed SIV control (Fig. 1). In the latter controllers, plasma viremia became undetectable in a few months. Macaques R01-008 and R08-006 rapidly controlled SIV replication and maintained high CD4 levels (Fig. 1).
FIG 1.
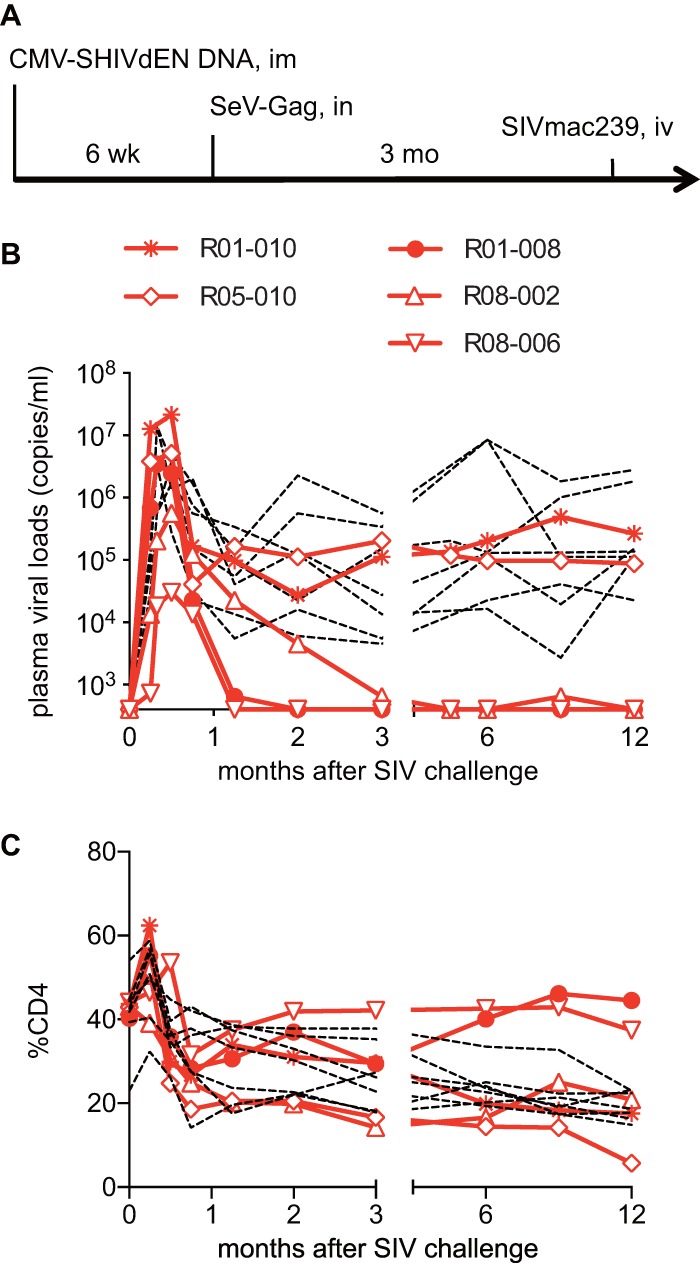
Viral loads and percentages of CD4 in Gag-vaccinated animals after SIVmac239 challenge. (A) Protocol of Gag vaccination and SIVmac239 challenge. (B) Plasma viral loads (SIV gag RNA copies/ml plasma) determined as described previously (5). The lower limit of detection is approximately 4 × 102 copies/ml. (C) Percentages of CD4+ T cells in PBMCs. In panels B and C, data on unvaccinated animals (n = 7) are shown by dotted lines for comparison. Data on six unvaccinated (39) and two Gag-vaccinated (R01-010 and R01-008) (42) animals used in our previous studies are included.
The second group of six E+ macaques received a DNA prime and an SeV-Vif/Nef boost vaccination, followed by an SIVmac239 challenge. The vaccine protocol first delivered Vif-expressing DNA, with the second vaccination consisting of Vif-expressing and Nef-expressing DNAs, and the third with Vif-expressing and Nef-expressing SeVs (SeV-Vif and SeV-Nef) with intervals of 3 weeks. After SIV challenge, three of these Vif/Nef-vaccinated animals failed to control viral replication and had high levels of set-point viral loads equivalent to those in unvaccinated macaques, but the remaining three showed SIV control with low levels of set-point viral loads (geometric mean of viral loads from 6 months to 1 year in each controller, <2.0 × 103 copies/ml) and maintained higher CD4 levels (Fig. 2). Indeed, these six SIV controllers, consisting of three Gag-vaccinated and three Vif/Nef-vaccinated animals, showed significantly higher percentages of CD4 at 1 year than those in the remaining noncontrollers (see Fig. S1 in the supplemental material).
FIG 2.
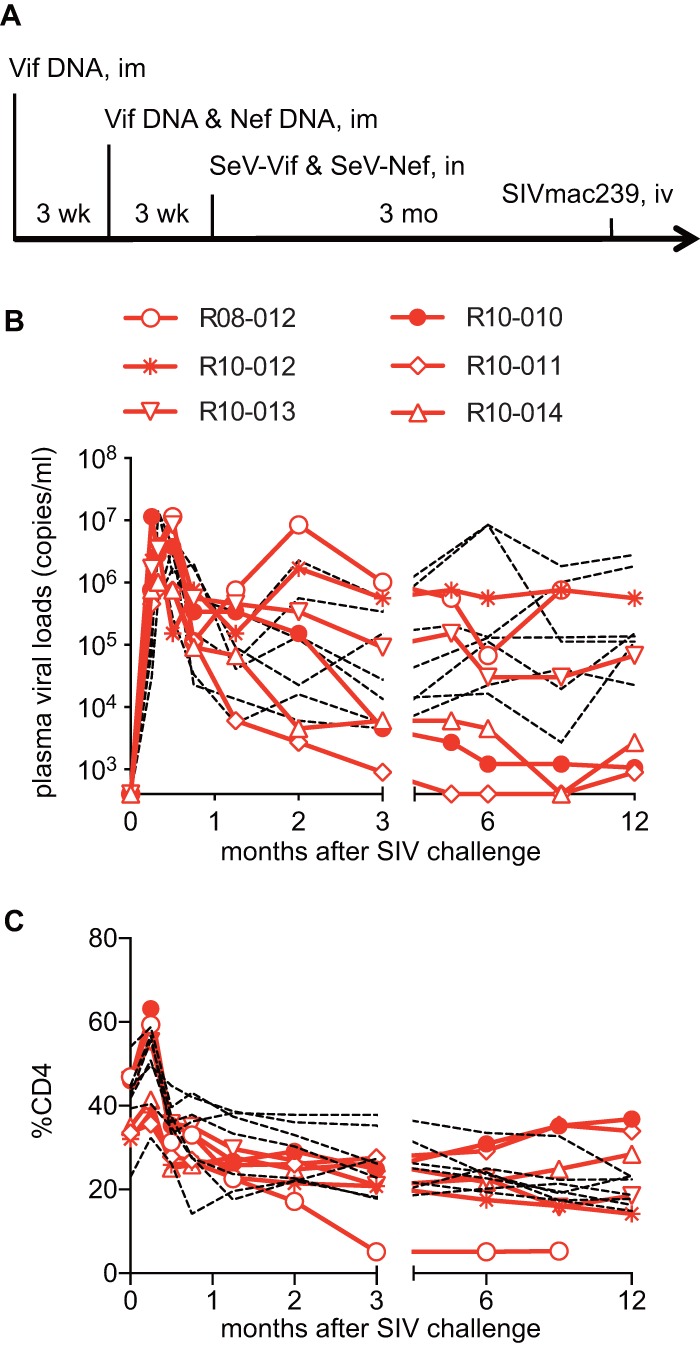
Viral loads and percentages of CD4 in Vif/Nef-vaccinated animals after SIVmac239 challenge. (A) Protocol of Vif/Nef vaccination and SIVmac239 challenge; (B) plasma viral loads; (C) percentages of CD4+ T cells in PBMCs. In panels B and C, data on unvaccinated animals are shown by dotted lines for comparison.
Gag-, Vif-, and Nef-specific CD8+ T-cell responses in unvaccinated and vaccinated animals.
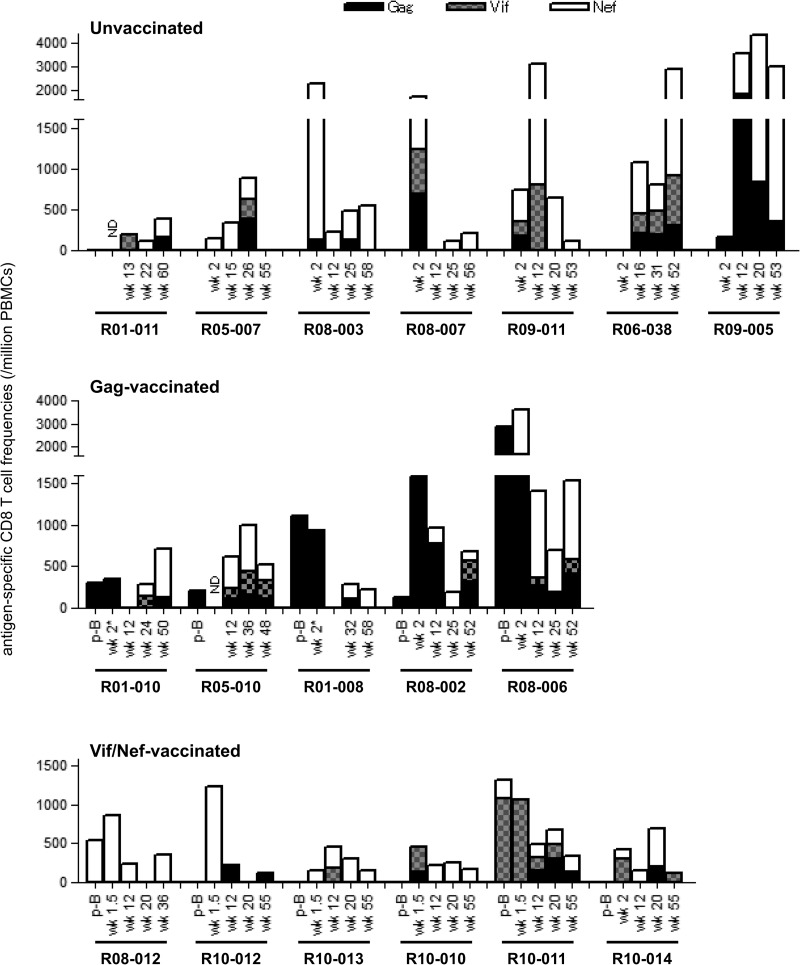
We examined Gag-, Vif-, and Nef-specific CD8+ T-cell responses in these animals. Unvaccinated macaques showed SIV-specific CD8+ T-cell responses equivalent to those observed in Indian rhesus macaques (8) (Fig. 3). All of these E+ unvaccinated macaques elicited immunodominant Nef-specific CD8+ T-cell responses, consistent with our previous study analyzing other E+ macaques (50). Gag-specific and Vif-specific CD8+ T-cell responses were detected but were not immunodominant in these animals.
FIG 3.
SIV Gag/Vif/Nef-specific CD8+ T-cell responses in macaques. We examined CD8+ T-cell responses specific for Gag, Vif, and Nef 1 week after SeV-Gag boost (p-B) and approximately 2 weeks, 3 months, 6 months, and 1 year after SIV challenge in unvaccinated (top), Gag-vaccinated (middle), and Vif/Nef-vaccinated (bottom) animals. We examined only Gag-specific CD8+ T-cell responses but not Vif- or Nef-specific ones at week 2 in macaques R01-010 and R01-008 (indicated by asterisks). ND, not determined.
In contrast, all Gag-vaccinated E+ macaques showed Gag-specific CD8+ T-cell responses after the SeV-Gag boost and in the early phase after SIV challenge (Fig. 3). In these animals, Nef-specific CD8+ T-cell responses mostly became immunodominant in the later phase. Importantly, all three animals that controlled SIV replication showed efficient Gag-specific CD8+ T-cell responses in the acute phase postchallenge, suggesting a significant contribution of these Gag-specific CD8+ T-cell responses to SIV control.
In the second group of Vif/Nef-vaccinated E+ animals, analysis of Gag-specific, Vif-specific, and Nef-specific CD8+ T-cell responses showed different patterns of responses between SIV controllers and noncontrollers (Fig. 3). In the acute phase after SIV challenge, the noncontrollers (R08-012, R10-012, and R10-013) elicited immunodominant Nef-specific CD8+ T-cell responses, whereas the controllers (R10-010, R10-011, and R10-014) showed immunodominant Vif-specific CD8+ T-cell responses. This suggests that the Vif-specific CD8+ T-cell responses contributed to primary SIV control. In the chronic phase, Nef-specific CD8+ T-cell responses were immunodominant except for one noncontroller, R10-012.
Thus, among 18 E+ animals, consisting of seven unvaccinated, five Gag-vaccinated, and six Vif/Nef-vaccinated animals, three Gag-vaccinated and three Vif/Nef-vaccinated animals controlled SIV replication. Comparison between these six SIV controllers and the remaining 12 noncontrollers showed no significant difference in the sum of Gag-, Vif-, and Nef-specific CD8+ T-cell frequencies in the acute phase (data not shown). The sum of Gag- and Vif-specific CD8+ T-cell frequencies in the acute phase, however, was significantly higher in the controllers than in the noncontrollers (P = 0.0031 by Mann-Whitney U test) (Fig. 4A). Indeed, the sum of Gag- and Vif-specific CD8+ T-cell frequencies in the acute phase was inversely correlated with postpeak plasma viral loads (P = 0.0268, R = −0.5205 with viral loads at 3 months [data not shown]; P = 0.0017, R = −0.6849 with viral loads at 1 year [Fig. 4B] by Pearson test). When we focused on seven unvaccinated and five Gag-vaccinated animals, three Gag-vaccinated controllers showed significantly higher Gag-specific CD8+ T-cell frequencies in the acute phase than the remaining nine noncontrollers (P = 0.0045 by Mann-Whitney U test) (Fig. 4C). Also, in the analysis of seven unvaccinated and six Vif/Nef-vaccinated animals, Vif-specific CD8+ T-cell frequencies in the acute phase were significantly higher in three Vif/Nef-vaccinated controllers than in the remaining 10 noncontrollers (P = 0.0140 by Mann-Whitney U test) (Fig. 4D). These results suggest that efficient Gag- or Vif-specific CD8+ T-cell responses in the acute phase can result in SIV control.
FIG 4.
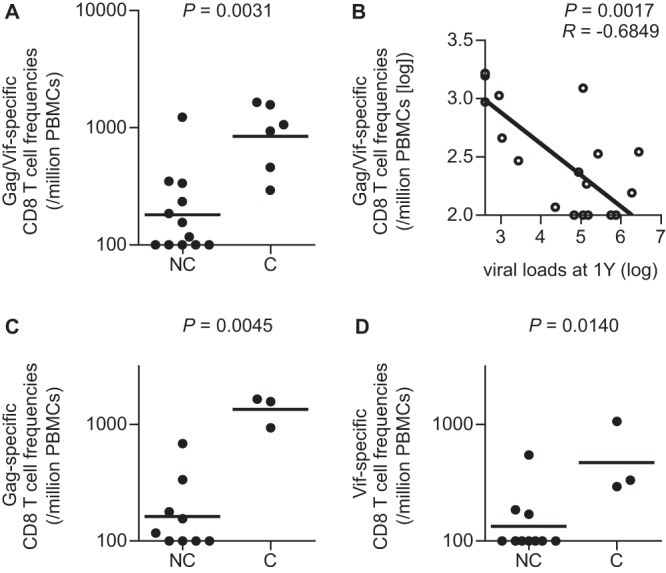
Comparison of Gag/Vif-specific CD8+ T-cell frequencies in the acute phase between SIV controllers (C) and noncontrollers (NC). Data on Gag- and Vif-specific CD8+ T-cell frequencies around week 2 postchallenge, which are shown in Fig. 3, were used. In macaques R01-011 and R05-010, samples at week 2 were unavailable, and data at week 12 were used. (A) Comparison of the sum of Gag- and Vif-specific CD8+ T-cell frequencies (Gag/Vif-specific CD8+ T-cell frequencies) between the controllers (three Gag-vaccinated and three Vif/Nef-vaccinated animals) and the noncontrollers in seven unvaccinated, five Gag-vaccinated, and six Vif/Nef-vaccinated animals (n = 18). The controllers showed significantly higher frequencies than the noncontrollers (P = 0.0031 by Mann-Whitney U test). (B) Correlation analysis of Gag/Vif-specific CD8+ T-cell frequencies in the acute phase with plasma viral loads at 1 year. The frequencies were inversely correlated with the viral loads (P = 0.0017, R = −0.6849 by Pearson test). (C) Comparison of Gag-specific CD8+ T-cell frequencies in seven unvaccinated and five Gag-vaccinated animals (n = 12). The three Gag-vaccinated controllers showed significantly higher frequencies than the noncontrollers (P = 0.0045 by Mann-Whitney U test). (D) Comparison of Vif-specific CD8+ T-cell frequencies in seven unvaccinated and six Vif/Nef-vaccinated animals (n = 13). The three Vif/Nef-vaccinated controllers showed significantly higher frequencies than the noncontrollers (P = 0.0140 by Mann-Whitney U test).
Viral gag, vif, and nef mutations in vaccinated animals.
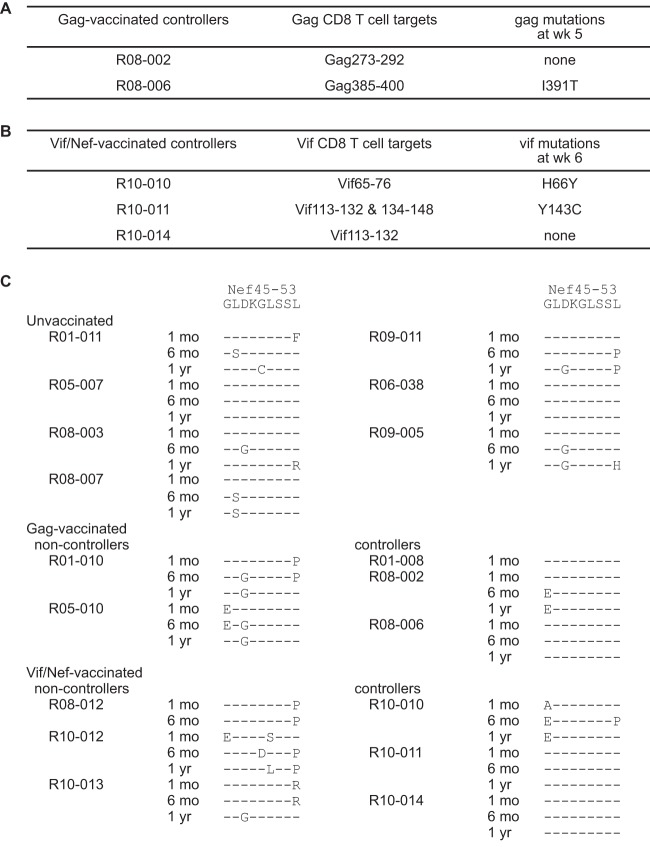
We then tried to define the CD8+ T-cell responses that might be contributing to the vaccine-based SIV control, although we were not able to map all of the CD8+ T-cell epitopes because of sample limitation. Among three Gag-vaccinated controllers, R01-008, R08-002, and R08-006, our previous study found Gag367–381-specific CD8+ T-cell responses at week 5 in macaque R01-008 (5). This animal showed rapid selection of a mutation leading to an isoleucine (I)-to-threonine (T) change at the 377th aa (I377T) in SIV Gag, which results in escape from Gag367–381-specific CD8+ T-cell recognition. This suggests that these Gag367–381-specific CD8+ T-cell responses may have played an important role in SIV control. Analysis in the present study found Gag385–400-specific CD8+ T-cell responses in the acute phase with rapid selection of a mutation leading to an I-to-T change at the 391st aa (I391T) in Gag in macaque R08-006 (Fig. 5A). We confirmed that this I391T substitution results in escape from Gag385–400-specific CD8+ T-cell recognition (data not shown), suggesting a contribution of these Gag385–400-specific CD8+ T-cell responses to the control of SIV. Macaque R08-002 mounted Gag273–292-specific CD8+ T-cell responses but showed no gag mutation in the early phase. None of the noncontrollers selected gag mutations at week 5 or 6.
FIG 5.
Predominant nonsynonymous mutations in CD8+ T-cell target-coding regions. (A) Gag target regions for CD8+ T-cell responses in the acute phase in Gag-vaccinated controllers. Macaque R01-008 induced Gag367–381-specific CD8+ T-cell responses and selected I377T mutation in 5 weeks as described before (5). (B) Vif target regions for CD8+ T-cell responses in the acute phase in Vif/Nef-vaccinated controllers. (C) Nonsynonymous mutations in Nef45–53 CD8+ T-cell epitope-coding regions of viral cDNAs at 1 month (1 mo), 6 months (6 mo), and 1 year (1 yr). Amino acid substitutions are shown.
Among three Vif/Nef-vaccinated controllers, R10-010, R10-011, and R10-014 (Fig. 5B), macaque R10-010 mounted Vif65–76-specific CD8+ T-cell responses in the acute phase that resulted in the rapid selection of a mutation leading to a histidine (H)-to-tyrosine (Y) change at the 66th aa (H66Y) in Vif. Macaque R10-011 mounted Vif113–132-specific and Vif134–148-specific CD8+ T-cell responses in the acute phase with rapid selection of a mutation leading to a Y-to-cysteine (C) change at the 143rd aa (Y143C) in Vif. We confirmed that this Y143C substitution results in escape from Vif134–148-specific CD8+ T-cell recognition (data not shown). None of the noncontrollers selected vif mutations at week 5 or 6. These suggest that Vif65–76-specific and Vif134–148-specific CD8+ T-cell responses contributed to SIV control in macaques R10-010 and R10-011, respectively. Macaque R10-014 mounted Vif113–132-specific CD8+ T-cell responses but showed no vif mutation in the early phase.
In E+ macaques, CD8+ T-cell responses specific for Nef38–66 and Nef101–138 regions were frequently observed (see Fig. S2 in the supplemental material). In all three Gag-vaccinated controllers, we confirmed both Nef38–66-specific and Nef101–138-specific CD8+ T-cell responses in the chronic phase, although we did not have available samples for analysis of these responses in the acute phase. In five Vif/Nef-vaccinated animals, we confirmed Nef38–66-specific CD8+ T-cell responses in the acute phase, followed by Nef101–138-specific CD8+ T-cell induction. Nef38–66-specific CD8+ T-cell responses became undetectable at week 12 in all the three noncontrollers but were maintained at detectable levels in controllers R10-010 and R10-011.
Further mapping defined the Nef45–53 CD8+ T-cell epitope. Mutations in the Nef45–53-coding region were selected after 1 year in five of seven unvaccinated E+ animals. Rapid selection of mutations at this Nef45–53-coding region in a month after SIV challenge was observed in both Gag-vaccinated noncontrollers and all three Vif/Nef-vaccinated noncontrollers (Fig. 5C). In contrast, out of six Gag-vaccinated or Vif/Nef-vaccinated controllers, only one animal (R10-010) rapidly selected a mutation in this region. We confirmed that the leucine (L)-to-proline (P) substitution at the 53rd aa (L53P) in Nef results in escape from Nef45–53-specific CD8+ T-cell recognition (data not shown). Thus, Nef45–53-specific CD8+ T-cell responses may have exerted strong suppressive pressure on SIV replication in the acute phase in Gag-vaccinated or Vif/Nef-vaccinated noncontrollers.
DISCUSSION
In this study, we examined efficacy of prophylactic DNA-prime/SeV-boost vaccines against SIVmac239 challenge in a group of Burmese rhesus macaques sharing the MHC-I haplotype E. Our previous study indicated that unvaccinated E+ animals show typical courses of SIV infection and AIDS progression (39). However, three of five Gag-vaccinated and three of six Vif/Nef-vaccinated E+ animals controlled SIV replication, indicating a possibility of virus control by prophylactic vaccination.
Unvaccinated E+ animals showed high-frequency Nef-specific CD8+ T-cell responses, particularly specific for the Nef38–66 and Nef101–138 regions, after SIVmac239 challenge. The Nef45–53 region is a candidate for a CD8+ T-cell target associated with MHC-I haplotype E, and the NefL53P mutation resulting in escape from Nef45–53-specific CD8+ T-cell recognition was often selected in E+ animals. These results imply suppressive pressure on SIV replication by Nef-specific CD8+ T-cell responses in macaques sharing this MHC-I haplotype.
Gag-vaccinated animals elicited detectable Gag-specific CD8+ T-cell responses after SeV-Gag boost. All three Gag-vaccinated controllers showed efficient Gag-specific CD8+ T-cell responses in the acute phase after SIV challenge. In particular, macaques R01-008 and R08-006 showed rapid SIV control without detectable plasma viremia after week 5. Gag367–381-specific CD8+ T-cell responses with rapid selection of a Gag367–381-specific CD8+ T-cell escape mutation, I377T, were observed in R01-008, whereas Gag385-400-specific responses were associated with an escape mutation, I391T, in R08-006. Our results suggest that the prophylactic Gag vaccination results in the efficient induction of these Gag-specific CD8+ T-cell responses in the acute phase, which then played an important role in the control of primary SIV replication. The MHC-I haplotypes other than E (see Table S1 in the supplemental material) may be associated with these effective Gag epitope-specific CD8+ T-cell responses. Nef-specific CD8+ T-cell responses became predominant after 3 or 6 months.
Vif/Nef-vaccinated animals induced Vif- or Nef-specific CD8+ T-cell responses in the acute phase after SIVmac239 challenge. Before challenge, detectable Vif-specific CD8+ T-cell responses were elicited after SeV-Vif/Nef boost only in macaque R10-011. It should be noted, however, that all three Vif/Nef-vaccinated controllers showed high-frequency Vif-specific CD8+ T-cell responses in the acute phase, while the three noncontrollers exhibited Nef-specific CD8+ T-cell responses. In particular, our results implicate Vif65-76-specific and Vif134-148-specific CD8+ T-cell responses in the control of primary viral replication in macaques R10-010 and R10-011, respectively. These CD8+ T-cell responses may be associated with the second MHC-I haplotypes (see Table S1 in the supplemental material). Even Vif/Nef-vaccinated controllers inducing Vif-specific CD8+ T-cell responses in the acute phase showed predominant Nef-specific CD8+ T-cell responses in the chronic phase.
Vif/Nef-vaccinated noncontrollers showed no Vif-specific CD8+ T-cell responses but mounted Nef-specific CD8+ T-cell responses in the acute phase. All three noncontrollers rapidly selected nef mutations in the Nef45-53-coding regions, and Nef45-53-specific CD8+ T-cell responses were undetectable after 3 months postchallenge. Interestingly, both Gag-vaccinated noncontrollers also showed rapid selection of nef mutations in the Nef45-53-coding regions. We speculate that, in these Gag-vaccinated or Vif/Nef-vaccinated noncontrollers, dominant Nef45-53-specific CD8+ T-cell responses may have exerted strong suppressive pressure on primary SIV replication without the help of other vaccine antigen-specific, effective CD8+ T-cell responses, leading to failure in virus control with rapid selection of escape mutations. Unvaccinated macaque R08-007 elicited Gag- and Vif-specific as well as Nef-specific CD8+ T-cell responses in the acute phase but failed to control SIV replication. The high magnitude of responses may reflect the highest peak viral loads (1.4 × 107 copies/ml) at day 10 in this animal among the unvaccinated. These naive-derived Gag- and Vif-specific CD8+ T-cell responses may have been less functional and insufficient for SIV control. In contrast, in vaccinated controllers, prophylactic vaccination resulted in effective Gag- or Vif-specific CD8+ T-cell responses postexposure, leading to primary SIV control, followed by Nef-specific CD8+ T-cell responses possibly contributing to maintenance of virus control. Induction of CD8+ T-cell responses specific for dominant Nef epitopes by prophylactic vaccination may not be good for SIV control in E+ animals. Several studies have indicated contribution of subdominant CD8+ T-cell responses to HIV or SIV suppression (51–53). Thus, induction of CD8+ T-cell responses specific for subdominant but not dominant epitopes by prophylactic vaccination may be a promising AIDS vaccine strategy resulting in effective, broader CD8+ T-cell responses postexposure.
In summary, this study demonstrates SIV control by prophylactic vaccination in hosts possessing MHC-I alleles associated with dominant non-Gag antigen-specific CD8+ T-cell responses. Our results suggest that prophylactic vaccination resulting in effective subdominant Gag/Vif epitope-specific CD8+ T-cell responses in the acute phase postexposure can lead to primary HIV control. This may imply a rationale of altering the hierarchy of postexposure CD8+ T-cell immunodominance toward HIV control.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology and grants-in-aid from the Ministry of Health, Labor, and Welfare in Japan.
We thank F. Ono, K. Oto, K. Komatsuzaki, A. Hiyaoka, M. Hamano, K. Hanari, S. Okabayashi, H. Akari, and Y. Yasutomi for their assistance in animal experiments.
Footnotes
Published ahead of print 23 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02634-13.
REFERENCES
- 1.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103–6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matano T, Shibata R, Siemon C, Connors M, Lane HC, Martin MA. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857–860. 10.1126/science.283.5403.857 [DOI] [PubMed] [Google Scholar]
- 5.Matano T, Kobayashi M, Igarashi H, Takeda A, Nakamura H, Kano M, Sugimoto C, Mori K, Iida A, Hirata T, Hasegawa M, Yuasa T, Miyazawa M, Takahashi Y, Yasunami M, Kimura A, O'Connor DH, Watkins DI, Nagai Y. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 199:1709–1718. 10.1084/jem.20040432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, Yang ZY, Chakrabarti B, Rao SS, Schmitz JE, Montefiori DC, Barker BR, Bookstein FL, Nabel GJ. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530–1533. 10.1126/science.1124226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, Gonzalez EJ, Yant LJ, Maness NJ, May GE, Soma T, Reynolds MR, Rakasz E, Rudersdorf R, McDermott AB, O'Connor DH, Friedrich TC, Allison DB, Patki A, Picker LJ, Burton DR, Lin J, Huang L, Patel D, Heindecker G, Fan J, Citron M, Horton M, Wang F, Liang X, Shiver JW, Casimiro DR, Watkins DI. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80:5875–5885. 10.1128/JVI.00171-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91. 10.1038/nature07469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–527. 10.1038/nature10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, Piaskowski SM, Duan L, Seese A, Gladden AD, Weisgrau KL, Furlott JR, Kim YI, Veloso de Santana MG, Rakasz E, Capuano S, III, Wilson NA, Bonaldo MC, Galler R, Allison DB, Piatak M, Jr, Haase AT, Lifson JD, Allen TM, Watkins DI. 2012. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature 491:129–133. 10.1038/nature11443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivière Y, McChesney MB, Porrot F, Tanneau-Salvadori F, Sansonetti P, Lopez O, Pialoux G, Feuillie V, Mollereau M, Chamaret S, Tekaia F, Montagnier L. 1995. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res. Hum. Retroviruses 11:903–907. 10.1089/aid.1995.11.903 [DOI] [PubMed] [Google Scholar]
- 12.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. 2002. Magnitude of functional CD8+ T-cell responses to the Gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 76:2298–2305. 10.1128/jvi.76.5.2298-2305.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novitsky V, Gilbert P, Peter T, McLane MF, Gaolekwe S, Rybak N, Thior I, Ndung'u T, Marlink R, Lee TH, Essex M. 2003. Association between virus-specific T-cell responses and plasma viral load in human immunodeficiency virus type 1 subtype C infection. J. Virol. 77:882–890. 10.1128/JVI.77.2.882-890.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masemola A, Mashishi T, Khoury G, Mohube P, Mokgotho P, Vardas E, Colvin M, Zijenah L, Katzenstein D, Musonda R, Allen S, Kumwenda N, Taha T, Gray G, McIntyre J, Karim SA, Sheppard HW, Gray CM, HIVNET 028 Study Team 2004. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. J. Virol. 78:3233–3243. 10.1128/JVI.78.7.3233-3243.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuñiga R, Lucchetti A, Galvan P, Sanchez S, Sanchez C, Hernandez A, Sanchez H, Frahm N, Linde CH, Hewitt HS, Hildebrand W, Altfeld M, Allen TM, Walker BD, Korber BT, Leitner T, Sanchez J, Brander C. 2006. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J. Virol. 80:3122–3125. 10.1128/JVI.80.6.3122-3125.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53. 10.1038/nm1520 [DOI] [PubMed] [Google Scholar]
- 17.Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, Hallahan CW, Selig SM, Schwartz D, Sullivan J, Connors M. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. U. S. A. 97:2709–2714. 10.1073/pnas.050567397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mühl T, Krawczak M, Ten Haaft P, Hunsmann G, Sauermann U. 2002. MHC class I alleles influence set-point viral load and survival time in simian immunodeficiency virus-infected rhesus monkeys. J. Immunol. 169:3438–3446 [DOI] [PubMed] [Google Scholar]
- 19.Tang J, Tang S, Lobashevsky E, Myracle AD, Fideli U, Aldrovandi G, Allen S, Musonda R, Kaslow RA, Zambia-UAB HIV Research Project 2002. Favorable and unfavorable HLA class I alleles and haplotypes in Zambians predominantly infected with clade C human immunodeficiency virus type 1. J. Virol. 76:8276–8284. 10.1128/JVI.76.16.8276-8284.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altfeld M, Addo MM, Rosenberg ES, Hecht FM, Lee PK, Vogel M, Yu XG, Draenert R, Johnston MN, Strick D, Allen TM, Feeney ME, Kahn JO, Sekaly RP, Levy JA, Rockstroh JK, Goulder PJ, Walker BD. 2003. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 17:2581–2591. 10.1097/00002030-200312050-00005 [DOI] [PubMed] [Google Scholar]
- 21.Kiepiela P, Leslie AJ, Honeyborne I, Ramduth D, Thobakgale C, Chetty S, Rathnavalu P, Moore C, Pfafferott KJ, Hilton L, Zimbwa P, Moore S, Allen T, Brander C, Addo MM, Altfeld M, James I, Mallal S, Bunce M, Barber LD, Szinger J, Day C, Klenerman P, Mullins J, Korber B, Coovadia HM, Walker BD, Goulder PJ. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432:769–775. 10.1038/nature03113 [DOI] [PubMed] [Google Scholar]
- 22.Bontrop RE, Watkins DI. 2005. MHC polymorphism: AIDS susceptibility in nonhuman primates. Trends Immunol. 26:227–233. 10.1016/j.it.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 23.Yant LJ, Friedrich TC, Johnson RC, May GE, Maness NJ, Enz AM, Lifson JD, O'Connor DH, Carrington M, Watkins DI. 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 80:5074–5077. 10.1128/JVI.80.10.5074-5077.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T, Bean AT, Beal DR, Wilson NA, Rehrauer WM, Lifson JD, Carrington M, Watkins DI. 2007. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 81:8827–8832. 10.1128/JVI.00895-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi N, Nomura T, Takahara Y, Yamamoto H, Shiino T, Takeda A, Inoue M, Iida A, Hara H, Shu T, Hasegawa M, Sakawaki H, Miura T, Igarashi T, Koyanagi Y, Naruse TK, Kimura A, Matano T. 2013. A novel protective MHC-I haplotype not associated with dominant Gag-specific CD8+ T-cell responses in SIVmac239 infection of Burmese rhesus macaques. PLoS One 8:e54300. 10.1371/journal.pone.0054300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedrich TC, Dodds EJ, Yant LJ, Vojnov L, Rudersdorf R, Cullen C, Evans DT, Desrosiers RC, Mothé BR, Sidney J, Sette A, Kunstman K, Wolinsky S, Piatak M, Lifson J, Hughes AL, Wilson N, O'Connor DH, Watkins DI. 2004. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat. Med. 10:275–281. 10.1038/nm998 [DOI] [PubMed] [Google Scholar]
- 27.Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, Tang Y, Holmes EC, Allen T, Prado JG, Altfeld M, Brander C, Dixon C, Ramduth D, Jeena P, Thomas SA, St John A, Roach TA, Kupfer B, Luzzi G, Edwards A, Taylor G, Lyall H, Tudor-Williams G, Novelli V, Martinez-Picado J, Kiepiela P, Walker BD, Goulder PJ. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10:282–289. 10.1038/nm992 [DOI] [PubMed] [Google Scholar]
- 28.Feeney ME, Tang Y, Roosevelt KA, Leslie AJ, McIntosh K, Karthas N, Walker BD, Goulder PJ. 2004. Immune escape precedes breakthrough human immunodeficiency virus type 1 viremia and broadening of the cytotoxic T-lymphocyte response in an HLA-B27-positive long-term-nonprogressing child. J. Virol. 78:8927–8930. 10.1128/JVI.78.16.8927-8930.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goulder PJR, Watkins DI. 2008. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat. Rev. Immunol. 8:619–630. 10.1038/nri2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goulder PJR, Watkins DI. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4:630–640. 10.1038/nri1417 [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Picado J, Prado JG, Fry EE, Pfafferott K, Leslie A, Chetty S, Thobakgale C, Honeyborne I, Crawford H, Matthews P, Pillay T, Rousseau C, Mullins JI, Brander C, Walker BD, Stuart DI, Kiepiela P, Goulder P. 2006. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J. Virol. 80:3617–3623. 10.1128/JVI.80.7.3617-3623.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneidewind A, Brockman MA, Yang R, Adam RI, Li B, Le Gall S, Rinaldo CR, Craggs SL, Allgaier RL, Power KA, Kuntzen T, Tung CS, LaBute MX, Mueller SM, Harrer T, McMichael AJ, Goulder PJ, Aiken C, Brander C, Kelleher AD, Allen TM. 2007. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J. Virol. 81:12382–12393. 10.1128/JVI.01543-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura T, Brockman MA, Schneidewind A, Lobritz M, Pereyra F, Rathod A, Block BL, Brumme ZL, Brumme CJ, Baker B, Rothchild AC, Li B, Trocha A, Cutrell E, Frahm N, Brander C, Toth I, Arts EJ, Allen TM, Walker BD. 2009. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte recognition. J. Virol. 83:2743–2755. 10.1128/JVI.02265-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loffredo JT, Bean AT, Beal DR, León EJ, May GE, Piaskowski SM, Furlott JR, Reed J, Musani SK, Rakasz EG, Friedrich TC, Wilson NA, Allison DB, Watkins DI. 2008. Patterns of CD8+ immunodominance may influence the ability of Mamu-B*08-positive macaques to naturally control simian immunodeficiency virus SIVmac239 replication. J. Virol. 82:1723–1738. 10.1128/JVI.02084-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valentine LE, Loffredo JT, Bean AT, León EJ, MacNair CE, Beal DR, Piaskowski SM, Klimentidis YC, Lank SM, Wiseman RW, Weinfurter JT, May GE, Rakasz EG, Wilson NA, Friedrich TC, O'Connor DH, Allison DB, Watkins DI. 2009. Infection with “escaped” virus variants impairs control of simian immunodeficiency virus SIVmac239 replication in Mamu-B*08-positive macaques. J. Virol. 83:11514–11527. 10.1128/JVI.01298-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matano T, Kano M, Nakamura H, Takeda A, Nagai Y. 2001. Rapid appearance of secondary immune responses and protection from acute CD4 depletion after a highly pathogenic immunodeficiency virus challenge in macaques vaccinated with a DNA prime/Sendai virus vector boost regimen. J. Virol. 75:11891–11896. 10.1128/JVI.75.23.11891-11896.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawada M, Tsukamoto T, Yamamoto H, Iwamoto N, Kurihara K, Takeda A, Moriya C, Takeuchi H, Akari H, Matano T. 2008. Gag-specific cytotoxic T-lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J. Virol. 82:10199–10206. 10.1128/JVI.01103-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawada M, Igarashi H, Takeda A, Tsukamoto T, Yamamoto H, Dohki S, Takiguchi M, Matano T. 2006. Involvement of multiple epitope-specific cytotoxic T-lymphocyte responses in vaccine-based control of simian immunodeficiency virus replication in rhesus macaques. J. Virol. 80:1949–1958. 10.1128/JVI.80.4.1949-1958.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nomura T, Yamamoto H, Shiino T, Takahashi N, Nakane T, Iwamoto N, Ishii H, Tsukamoto T, Kawada M, Matsuoka S, Takeda A, Terahara K, Tsunetsugu-Yokota Y, Iwata-Yoshikawa N, Hasegawa H, Sata T, Naruse TK, Kimura A, Matano T. 2012. Association of major histocompatibility complex class I haplotypes with disease progression after simian immunodeficiency virus challenge in Burmese rhesus macaques. J. Virol. 86:6481–6490. 10.1128/JVI.07077-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naruse TK, Chen Z, Yanagida R, Yamashita T, Saito Y, Mori K, Akari H, Yasutomi Y, Miyazawa M, Matano T, Kimura A. 2010. Diversity of MHC class I genes in Burmese-origin rhesus macaques. Immunogenetics 62:601–611. 10.1007/s00251-010-0462-z [DOI] [PubMed] [Google Scholar]
- 41.Argüello JR, Little AM, Pay AL, Gallardo D, Rojas I, Marsh SG, Goldman JM, Madrigal JA. 1998. Mutation detection and typing of polymorphic loci through double-strand conformation analysis. Nat. Genet. 18:192–194. 10.1038/ng0298-192 [DOI] [PubMed] [Google Scholar]
- 42.Kawada M, Tsukamoto T, Yamamoto H, Takeda A, Igarashi H, Watkins DI, Matano T. 2007. Long-term control of simian immunodeficiency virus replication with central memory CD4+ T-cell preservation after nonsterile protection by a cytotoxic T lymphocyte-based vaccine. J. Virol. 81:5202–5211. 10.1128/JVI.02881-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto H, Kawada M, Takeda A, Igarashi H, Matano T. 2007. Postinfection immunodeficiency virus control by neutralizing antibodies. PLoS One 2:e540. 10.1371/journal.pone.0000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kestler HW, III, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651–662. 10.1016/0092-8674(91)90097-I [DOI] [PubMed] [Google Scholar]
- 45.Shibata R, Maldarelli F, Siemon C, Matano T, Parta M, Miller G, Fredrickson T, Martin MA. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176:362–373. 10.1086/514053 [DOI] [PubMed] [Google Scholar]
- 46.Takeda A, Igarashi H, Nakamura H, Kano M, Iida A, Hirata T, Hasegawa M, Nagai Y, Matano T. 2003. Protective efficacy of an AIDS vaccine, a single DNA priming followed by a single booster with a recombinant replication-defective Sendai virus vector, in a macaque AIDS model. J. Virol. 77:9710–9715. 10.1128/JVI.77.17.9710-9715.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schindler M, Münch J, Brenner M, Stahl-Hennig C, Skowronski J, Kirchhoff F. 2004. Comprehensive analysis of nef functions selected in simian immunodeficiency virus-infected macaques. J. Virol. 78:10588–10597. 10.1128/JVI.78.19.10588-10597.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donahoe SM, Moretto WJ, Samuel RV, Metzner KJ, Marx PA, Hanke T, Connor RI, Nixon DF. 2000. Direct measurement of CD8+ T cell responses in macaques infected with simian immunodeficiency virus. Virology 272:347–356. 10.1006/viro.2000.0404 [DOI] [PubMed] [Google Scholar]
- 49.Iwamoto N, Tsukamoto T, Kawada M, Takeda A, Yamamoto H, Takeuchi H, Matano T. 2010. Broadening of CD8+ cell responses in vaccine-based simian immunodeficiency virus controllers. AIDS 24:2777–2787. 10.1097/QAD.0b013e3283402206 [DOI] [PubMed] [Google Scholar]
- 50.Nakamura M, Takahara Y, Ishii H, Sakawaki H, Horiike M, Miura T, Igarashi T, Naruse TK, Kimura A, Matano T, Matsuoka S. 2011. Major histocompatibility complex class I-restricted cytotoxic T lymphocyte responses during primary simian immunodeficiency virus infection in Burmese rhesus macaques. Microbiol. Immunol. 55:768–773. 10.1111/j.1348-0421.2011.00384.x [DOI] [PubMed] [Google Scholar]
- 51.Frahm N, Kiepiela P, Adams S, Linde CH, Hewitt HS, Sango K, Feeney ME, Addo MM, Lichterfeld M, Lahaie MP, Pae E, Wurcel AG, Roach T, St. John MA, Altfeld M, Marincola FM, Moore C, Mallal S, Carrington M, Heckerman D, Allen TM, Mullins JI, Korber BT, Goulder PJ, Walker BD, Brander C. 2006. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat. Immunol. 7:173–178. 10.1038/ni1281 [DOI] [PubMed] [Google Scholar]
- 52.Friedrich TC, Valentine LE, Yant LJ, Rakasz EG, Piaskowski SM, Furlott JR, Weisgrau KL, Burwitz B, May GE, León EJ, Soma T, Napoe G, Capuano SV, III, Wilson NA, Watkins DI. 2007. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J. Virol. 81:3465–3476. 10.1128/JVI.02392-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brennan CA, Ibarrondo FJ, Sugar CA, Hausner MA, Shih R, Ng HL, Detels R, Margolick JB, Rinaldo CR, Phair J, Jacobson LP, Yang OO, Jamieson BD. 2012. Early HLA-B*57-restricted CD8+ T lymphocyte responses predict HIV-1 disease progression. J. Virol. 86:10505–10516. 10.1128/JVI.00102-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.