Abstract
The purpose of this position paper is to present a critical analysis of the challenges and limitations of the most widely used fluorescent probes for detecting and measuring reactive oxygen and nitrogen species. Where feasible, we have made recommendations for the use of alternate probes and appropriate analytical techniques that measure the specific products formed from the reactions between fluorescent probes and reactive oxygen and nitrogen species. We have proposed guidelines that will help present and future researchers with regard to the optimal use of selected fluorescent probes and interpretation of results.
Introduction
The generation of reactive oxygen and nitrogen species has been implicated in the onset and progression of several diseases (e.g., atherosclerosis, cancer, diabetes, neurodegeneration) [1]. At a molecular level, reactive oxygen and nitrogen species exhibit signaling and cell-function-modifying roles [2]. As pointed out in a recent review [3], reactive oxygen and nitrogen species are not single entities but represent a broad range of chemically distinct reactive species with diverse biological reactivities [3,4]. To clearly attribute a particular cell signaling event to a specific reactive oxygen or nitrogen species, it is essential to detect and characterize these species accurately. Several analytical approaches (EPR, chemiluminescence, fluorescence) have been used to detect reactive oxygen and nitrogen species. Many reviews on this subject have been published in this journal. In this position paper, we discuss the relative merits and weaknesses of the fluorescent probes (e.g., dichlorodihydrofluorescein, hydroethidine, and dihydrorhodamine) that are being used frequently for measuring hydrogen peroxide, superoxide, and peroxynitrite in biological systems.
Is dichlorodihydrofluorescein diacetate (DCFH-DA) a suitable probe for measuring intracellular H2O2 and other oxidants?
DCFH-DA is the most widely used probe for detecting intracellular H2O2 and oxidative stress. This probe is cell-permeable and is hydrolyzed intracellularly to the DCFH carboxylate anion, which is retained in the cell. Two-electron oxidation of DCFH results in the formation of a fluorescent product, dichlorofluorescein (DCF), which can be monitored by several fluorescence-based techniques (e.g., confocal microscopy, flow cytometry). This is a relatively easy and user-friendly assay that has become immensely popular. Investigators have routinely used DCFH-DA to measure intracellular generation of H2O2 and other oxidants or monitor redox signaling changes in cells in response to intra- or extracellular activation with oxidative stimulus [5–10].
However, as shown in Fig. 1 and outlined below, the intracellular redox chemistry of DCFH is complex and there are several limitations and artifacts associated with the DCF assay for intracellular H2O2 measurement [11–13]. It is essential to keep these limitations in mind for proper interpretation of data obtained with the DCFH-DA probe. Specifically:
DCFH does not directly react with H2O2 to form the fluorescent product, DCF. Therefore, DCF fluorescence cannot be used as a direct measure of H2O2.
Several one-electron-oxidizing species will oxidize DCFH to DCF (Fig. 1). These include hydroxyl radicals (•OH), compounds I and II formed from peroxidase or heme interaction with H2O2, •NO2 formed from the myeloperoxidase/H2O2/ system, hypochlorous acid (HOCl), and reactive species formed from peroxynitrite (ONOO−/ONOOH) decomposition. Peroxynitrite decomposition also forms •OH or carbonate anion radicals ( ) in the presence of bicarbonate.
The intermediate radical, DCF•−, formed from the one-electron oxidation of DCFH, rapidly reacts with O2 (k=108 M−1 s−1) to form superoxide ( ) (Fig. 1). The dismutation of yields additional H2O2[14], which can establish a redox-cycling mechanism leading to artifactual amplification of the fluorescence signal intensity.
Cytochrome c, a heme protein that is released from mitochondria to the cytosol during apoptosis, is capable of oxidizing DCFH directly or indirectly via a peroxidase-type mechanism, forming DCF [8, 11]. The increase in DCF fluorescence that occurs during apoptosis of cells loaded with DCFH-DA has frequently been associated with enhanced oxidant production.
Redox-active metals (e.g., Fe2+) promote DCFH oxidation in the presence of oxygen or H2O2 [15].
In addition, one cannot always assume that control and experimental samples exhibit the same efficiency in DCF radical generation and the same linear dependence of self-propagating redox-cycling reactions induced by the DCF radical (Fig. 1).
Fig. 1.
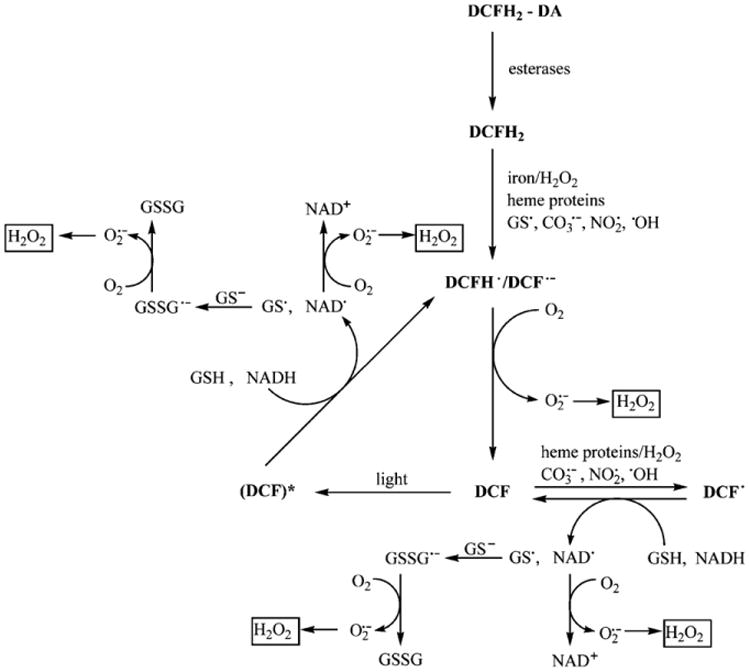
Oxidative reactions mediated by the dichlorodihydrofluorescein radical (DCF•−) in the presence of oxygen, heme proteins, and glutathione (reproduced from Ref. [35]).
Therefore, even if differences in DCF fluorescence are documented under control and experimental conditions, one has to consider the various caveats listed above for proper interpretation of the data. Failure to recognize these limitations often leads to erroneous interpretations and diverts the researchers' attention away from the more significant findings (e.g., increased peroxidase activity induced by the experimental conditions, or a new redox signaling/redox modification mechanism). Our recommendation to researchers in this field is that the DCFH-DA probe cannot be reliably used to measure intracellular H2O2 and other reactive oxygen species.
Now the important question: What type of useful information can one obtain using the fluorescent probe DCFH-DA? On a positive note, it is conceivable that useful redox-signaling mechanisms can be explored using this probe [6–8]. There are numerous examples in the literature that support the role of oxidant-induced iron signaling in DCFH oxidation, of which a few are discussed here. Previous studies implicated a role for redox-active iron in intracellular oxidation of DCFH to DCF [16], although the origin of the cellular iron was not known. In earlier studies, it was shown that H2O2- and lipid hydroperoxide-induced intracellular DCFH oxidation to DCF was mediated by transferrin receptor (TfR)-dependent uptake of iron [5–7]. Intracellular DCF fluorescence was inhibited by the monoclonal IgA-class anti-TfR antibody that blocked TfR endocytosis and the iron uptake. These studies also showed that peroxide-dependent DCF fluorescence was critically dependent on intracellular glutathione levels [5]. DCFH-DA was also used to monitor doxorubicin-induced iron signaling and the associated mitochondrial damage in endothelial cells [7]. DCFH-DA was used as an indicator for mitochondrial generation of oxidants and peroxynitrite in endothelial cells treated with oxidized low-density lipoprotein (oxLDL) [9]. A role for iron, endothelial nitric oxide synthase activity, and complex II activity in oxLDL-dependent DCF fluorescence was proposed [9,10,17]. DCFH-DA may be used as a redox indicator probe that responds to changes in intracellular iron signaling or peroxynitrite formation.
Are there other probes for measuring intracellular H2O2?
Very few small organic molecules stoichiometrically react with H2O2 to form a detectable intracellular fluorescent product. However, aromatic boronates (boronic acids and esters) react with H2O2 to form a single major product, the corresponding phenol (reaction yield is nearly 100% [18]). Recently, the boronate moiety was attached to a fluorophore (e.g., fluorescein) that, upon reaction with H2O2, forms a highly fluorescent molecule [19]. The boronate esters attached to a fluorophore are cell-permeable and interact directly with intracellular H2O2 to form fluorescent products in cells. However, the rate constant for the reaction between boronates and H2O2 is relatively low (0.1–1.0 M−1 s−1). It is unlikely to compete with H2O2-detoxifying enzymes such as the peroxiredoxins, which react 7 orders of magnitude faster with H2O2[3,4]. Nevertheless, even a small fraction of the reaction between the fluorescein boronate molecule and H2O2 would result in detectable intracellular fluorescence. This assay has been used to detect intracellular H2O2 formation [19,20]. Mitochondrial-targeted boronate (aromatic boronate conjugated to an alkyl triphenylphosphonium group) was recently used in vivo to quantitate H2O2 in fly mitochondria [21]. Mass spectrometry was used to quantitate the product [21]. However, aromatic boronates also react nearly stoichiometrically with ONOO− a million times faster than they do with H2O2[18,22], which should be taken into consideration (see Is dihydrorhodamine a specific probe for measuring intracellular peroxynitrite?). The use of inhibitors (L-NAME, catalase, PEG–catalase, SOD, or PEG–SOD) should help distinguish ONOO− from H2O2 in boronate-based fluorescence assays.
Another probe that is frequently used to measure H2O2 is Amplex red [23]. Amplex red is oxidized by horseradish peroxidase (HRP) and H2O2 to a fluorescent product, resorufin [23]. Although other one-electron oxidants are capable of oxidizing Amplex red to resorufin, the oxidation catalyzed by HRP in the presence of H2O2 is highly efficient and vastly increases the yield of resorufin. Therefore this assay is a viable method to continuously measure the extracellular formation of H2O2. A major complicating factor is light-mediated photochemical oxidation of resorufin in the presence of biological reductants (glutathione, NADH) that induce resorufin radical-mediated formation of and H2O2[24]. Because of the confounding radical reactions induced by resorufin, the use of Amplex red or cell-permeable Amplex red analog to measure intracellular H2O2 could pose problems. Under conditions where H2O2 is released from the cell or in isolated mitochondrial preparations, the Amplex red probe can be used to measure H2O2 in the presence of HRP.
Other approaches for monitoring intracellular H2O2 levels include the use of a redox-active biosensor, HyPer, whose design is based on OxyR, a natural bacterial H2O2 sensor [25]. Recent developments include HyPer targeted to various cellular compartments. This ratio-metric assay for H2O2 is potentially promising, although its range of applications in biology has not yet been critically examined.
Measurement of intracellular and mitochondrial superoxide using hydroethidine (HE) and Mito-SOX
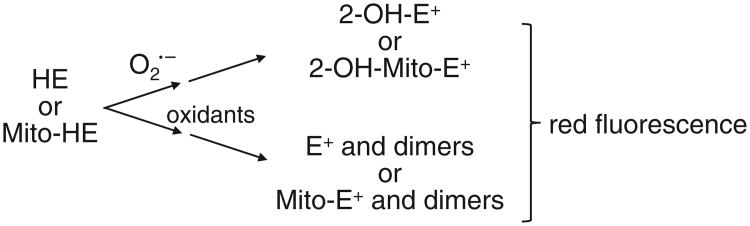
HE, or dihydroethidium, is another widely used probe for detecting intracellular [26]. The red fluorescence formed from the two-electron oxidation product, ethidium (E+), is usually equated to intracellular superoxide formation. Previous research suggests that E+ is not formed from the direct oxidation of HE by [27,28]. Instead, another product, 2-hydroxyethidium (2-OH-E+), with similar fluorescence characteristics, is formed from the HE/ reaction [29]. To the best of our knowledge, 2-OH-E+ is not formed during the reaction between HE and other oxidants (ONOO−, •OH, H2O2, compounds I and II), although they oxidize HE to E+ and other dimeric products. However, one cannot rule out the direct hydroxylation of HE to form 2-OH-E+ by cytochrome P-450 or by a similar enzymatic metabolism. Hydroethidine-derived radicals do not react with oxygen to form superoxide. Because of other oxidative reactions of HE, we suggest that 2-OH-E+ formation is only a qualitative and not a quantitative indicator of intracellular and/or extracellular [30] (Scheme 1). However, it will not be possible to use fluorescence-based intracellular techniques alone to assess formation because intracellular red fluorescence detection, using HE, does not automatically translate to measurement. One has to confirm the formation of 2-OH-E+ using other analytical techniques (HPLC-fluorescence or electrochemical detection or LC–MS). Another advantage of measuring E+ and other dimers of HE (HE–HE, HE–E+, E+–E+) is that they can be used as an indicator for one-electron oxidant formation in cells.
Scheme 1.
Oxidative reactions of HE and Mito-HE.
Mitochondrial-targeted HE or Mito-SOX, a triphenylphosphonium cation conjugated to HE via a linker carbon–carbon alkyl chain, has been used to measure mitochondrial [31]. The chemistry of Mito-SOX with is similar to that of HE and [32] and the same caveats apply as above. Being a positively charged molecule, Mito-SOX reacts slightly faster with compared to HE [31]. Mito-SOX reacts with and forms a red-fluorescent product, 2-hydroxymitoethidium (2-OH-Mito-E+), and not Mito-E+ (Scheme 1). 2-OH-Mito-E+ (specific reaction product of with Mito-SOX) and Mito-E+ (nonspecific oxidation product of Mito-SOX) have overlapping fluorescence spectra. Thus, the red fluorescence formed from Mito-SOX localized in mitochondria is not a reliable indicator of mitochondrial formation. Mito-SOX red fluorescence could arise from the product, from a composite of product and an oxidation product, or solely from an oxidation product of Mito-SOX induced by one-electron oxidants (cytochrome c, peroxidase, and H2O2). As shown for the HE probe, it is necessary to separate and identify 2-OH-Mito-E+ by HPLC. For details, see previous publications [29–31].
Is dihydrorhodamine a specific probe for measuring intracellular peroxynitrite?
Dihydrorhodamine (DHR) is the most frequently used probe for measuring ONOO−[33,34]. This assay is based on the oxidative conversion of DHR to its corresponding two-electron-oxidized fluorescent product, rhodamine. In many respects, the oxidative mechanisms of DHR and DCFH are very similar [15]. DHR oxidation to rhodamine is triggered by several oxidants (•OH, compounds I and II, and •NO2), similar to DCFH oxidation by oxidants as shown in Fig. 1. Thus, DHR oxidation to rhodamine is also nonspecific. The oxidative conversion of DHR to rhodamine is mediated by an intermediate DHR radical. The overall reaction profile of DHR to rhodamine in the presence of cogenerated •NO and is influenced by the free radical chemistry of DHR radical, showing a bell-shaped response [34]. The oxidation of DHR by cogenerated •NO and is mediated by oxidants (•NO2 and •OH) formed from the rapid and spontaneous decomposition of ONOO−, and DHR oxidation is not induced directly by ONOO−. The intermediate radical, DHR•, formed from the one-electron oxidation of DHR, also rapidly reacts with oxygen (k=7×108 M−1 s−1) [15,33], setting up a redox cycling mechanism leading to artifactual amplification of the fluorescence signal intensity (see Fig. 1). The limitations and caveats associated with DCF assay apply to DHR assay as well. The roles of •NO and or iron in intracellular DHR oxidation should be independently confirmed with appropriate inhibitors (e.g., L-NAME, PEG–SOD, desferrioxamine). Thus, DHR can be used only as a nonspecific indicator of intracellular ONOO− and HOCl formation [33].
Emerging probes for measurement of peroxynitrite
Recent research shows that aromatic boronates can be rapidly oxidized by ONOO− yielding the corresponding phenols as a major product (85% yield) [18,22]. Boronate-containing fluorophores (e.g., coumarin boronate) react in a similar fashion with ONOO− giving rise to the corresponding fluorescent products. Some of these boronate-based fluorophores are cell-permeable and can be effectively used to measure intracellular ONOO−. With increased availability of these probes, significant advances and new understanding in reactive oxygen and nitrogen research are likely. Synthesis of newer cell-permeable probes, coupled with enhanced understanding of their radical/nonradical chemistry, will provide additional research tools with which the complex roles of reactive oxygen and nitrogen species in cell signaling, cell growth, and cell differentiation can be unraveled.
We hope that this position paper clarifies some aspects of the appropriate use of fluorescent probes in the detection and characterization of reactive oxygen and nitrogen species in biology, and that it also highlights the many challenges and limitations of detection, characterization, and measurement of intracellular reactive oxygen and nitrogen species. The advantages, disadvantages, and recommendations of selected fluorescent probes listed in Table 1 should help alleviate some confusion with regard to the choice of suitable probes for detecting reactive oxygen and nitrogen species.
Table 1.
Guidelines for proper use of fluorescent probes.
| Fluorogenic probe | Advantages | Disadvantages | Recommendations |
|---|---|---|---|
HE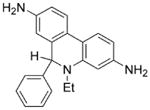
|
Reacts with
to form a diagnostic marker product, 2-OH-E+. Reacts with other oxidants (e.g., •OH, ONOO−) to form E+ and dimers. Intermediate HE-derived radical does not react with molecular oxygen to form and H2O2. |
2-OH-E+ and E+ have similar fluorescence spectral characteristics. Further extraction and HPLC analyses are needed for proper identification. |
Suitable for detection of intracellular
. Suitable for detection of intracellular oxidants (e.g., iron and H2O2, cytochrome c and H2O2). However, red fluorescence measurements using fluorescence or confocal microscopy will not be able to distinguish between E+ and 2-OH-E+. HPLC or other analytical measurements of products are crucial. |
Mito-SOX or Mito-HE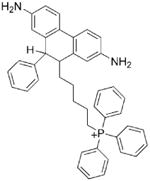
|
Localizes into mitochondria. Reacts with to form a diagnostic marker product, 2-OH-Mito-E+. Reacts with other oxidants (•OH, ONOO−) to form Mito-E+ and dimers. Intermediate Mito-HE-derived radical does not react with O2 to form and H2O2. |
2-OH-Mito-E+ and Mito-E+ have similar fluorescence spectral parameters. HPLC analyses of products are necessary for proper identification probe. Mitochondrial toxicity is a concern. |
Suitable for detection of mitochondrial
. Suitable for detection of intracellular oxidants (e.g., iron and H2O2, cytochrome c and H2O2). However, the “red fluorescence” measurements alone will not allow distinction between the hydroxylated and the oxidized Mito-HE. HPLC or other analytical measurements of products are crucial. |
DCFH-DA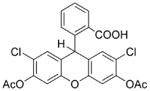
|
Cell-permeable Easy to use. Responds to changes in intracellular iron signaling or enhanced peroxidase activity (see Ref. [36]). |
Artifactual amplification of the fluorescence intensity via a redox-cycling mechanism involving an intermediate radical, DCF•−. | Not suitable for measuring intracellular H2O2 or other oxidants. May be used as a redox indicator probe for uncovering new redox signaling mechanisms keeping in mind the various caveats. |
DHR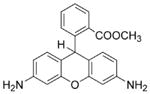
|
Cell-permeable Easy to use. Responds to cogenerated •NO and via a predictable radical chemistry. |
Artifactual amplification of the fluorescence intensity via a redox-cycling mechanism involving an intermediate radical, DHR•. | Not suitable for measuring intracellular ONOO− or other oxidants. May be used as a nonspecific indicator for intracellular ONOO− or oxidants derived from it. |
Coumarin boronate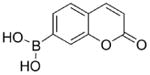
|
Reacts very rapidly and nearly stoichiometrically with ONOO− to form a fluorescent product. | Further metabolism of the product (7-hydroxycoumarin) and possible excretion out of cells may be a hindrance. | Suitable for measuring extracellular ONOO− formation. As this probe also reacts with H2O2 (albeit slowly), proper controls using appropriate inhibitors should be performed. |
Amplex red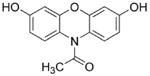
|
HRP/H2O2-dependent oxidation of Amplex red to resorufin is very efficient. The intermediate Amplex red-derived radical does not react with O2 for form additional and H2O2. The intermediate Amplex red-derived radical will react with to decrease resorufin formation (see Ref. [37]). Reducing agents (e.g., ascorbate) and peroxidase substrates (nitrite anion) will inhibit resorufin formation. |
Room light-mediated photochemical oxidation of resorufin in the presence of GSH, NAD(P)H, or ascorbate will greatly increase artifactual generation of H2O2. Amplex red-derived radical can rapidly react with and inhibit product formation. |
Suitable for measuring extracellular H2O2 under conditions limiting the secondary radical reactions induced by resorufin. |
References
- 1.Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. 3rd. Oxford Univ Press; New York: 1999. [Google Scholar]
- 2.D'Autreaux B, Toledano MB. ROS as signaling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2006;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 3.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 4.Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornally PJ, Partridge L, Gems D, Nyström T, Belousov V, Schumacker PT, Winterbourn CC. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–364. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tampo Y, Kotamraju S, Chitambar CR, Kalivendi SV, Keszler A, Joseph J, Kalyanaraman B. Oxidative stress-induced iron signaling is responsible for peroxide-dependent oxidation of dichlorodihydrofluorescein in endothelial cells: role of transferrin receptor-dependent iron uptake in apoptosis. Circ Res. 2003;92:56–63. doi: 10.1161/01.res.0000048195.15637.ac. [DOI] [PubMed] [Google Scholar]
- 6.Kotamraju S, Tampo Y, Keszler A, Chitambar CR, Joseph J, Haas AL, Kalyanaraman B. Nitric oxide inhibits H2O2-induced transferrin receptor-dependent apoptosis in endothelial cells: role of ubiquitin–proteasome pathway. Proc Natl Acad Sci U S A. 2003;100:10653–10658. doi: 10.1073/pnas.1933581100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotamraju S, Kalivendi SV, Konorev E, Chitambar CR, Joseph J, Kalyanaraman B. Oxidant-induced iron signaling in doxorubicin-mediated apoptosis. Methods Enzymol. 2004;378:362–382. doi: 10.1016/S0076-6879(04)78026-X. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson M, Kurz T, Brunk UT, Nilsson SE, Frennesson CI. What does the commonly used DCF test for oxidative stress really show? Biochem J. 2010;428:183–190. doi: 10.1042/BJ20100208. [DOI] [PubMed] [Google Scholar]
- 9.Zmijewski JW, Moellering DR, Le Goffe C, Landar A, Ramachandran A, Darley-Usmar VM. Oxidized LDL induces mitochondrially associated reactive oxygen/nitrogen species formation in endothelial cells. Am J Physiol Heart Circ Physiol. 2005;289:H852–H861. doi: 10.1152/ajpheart.00015.2005. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Gharavi NM, Honda H, Chang I, Kim B, Jen N, Li R, Zimman A, Berliner JA. A role for NADPH oxidase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free Radic Biol Med. 2009;47:145–151. doi: 10.1016/j.freeradbiomed.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkitt MJ, Wardman P. Cytochrome c is a potent catalyst of dichlorofluorescein oxidation: implications for the role of reactive oxygen species in apoptosis. Biochem Biophys Res Commun. 2001;282:329–333. doi: 10.1006/bbrc.2001.4578. [DOI] [PubMed] [Google Scholar]
- 12.Bonini MG, Rota C, Tomasi A, Mason RP. The oxidation of 2′,7′-dichlorofluorescein to reactive oxygen species: a self-fulfilling prophesy? Free Radic Biol Med. 2006;40:968–975. doi: 10.1016/j.freeradbiomed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 13.Hempel SL, Buettner GR, O'Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5 (and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine. Free Radic Biol Med. 1999;27:146–159. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 14.Folkes LK, Patel KB, Wardman P, Wrona M. Kinetics of reaction of nitrogen dioxide with dihydrorhodamine and the reaction of the dihydrorhodamine radical with oxygen: implications for quantifying peroxynitrite formation in cells. Arch Biochem Biophys. 2009;484:122–126. doi: 10.1016/j.abb.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Qian SY, Buettner GR. Iron and dioxygen chemistry is an important route to initiation of biological and free radical oxidations: an electron paramagnetic resonance spin trapping study. Free Radic Biol Med. 1999;26:1447–1456. doi: 10.1016/s0891-5849(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 16.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 17.Kooy NW, Royall JA, Ischiropoulos H. Oxidation of 2′,7′-dichlorofluorescin by peroxynitrite. Free Radic Res. 1997;27:245–254. doi: 10.3109/10715769709065763. [DOI] [PubMed] [Google Scholar]
- 18.Sikora A, Zielonka J, Lopez M, Joseph J, Kalyanaraman B. Direct oxidation of boronates by peroxynitrite: mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic Biol Med. 2009;47:1401–1407. doi: 10.1016/j.freeradbiomed.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller EW, Abers AE, Pralle A, Isacoff EY, Chang CJ. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J Am Chem Soc. 2005;127:16652–16659. doi: 10.1021/ja054474f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickenson BC, Huynh C, Chang CJ. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J Am Chem Soc. 2010;132:5906–5915. doi: 10.1021/ja1014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cochemé HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, Prime TA, Abakumova I, Patel JV, Fearnley IM, James AM, Porteous CM, Smith RA, Saeed S, Carré JE, Singer M, Gems D, Hartley RC, Partridge L, Murphy MP. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011;13:340–350. doi: 10.1016/j.cmet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide. J Biol Chem. 2010;285:14210–14216. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishin V, Gray JP, Heck DE, Laskin DL, Laskin JD. Application of the Amplex red/horseradish peroxidase assay to measure hydrogen peroxide generation by recombinant microsomal enzymes. Free Radic Biol Med. 2010;48:1485–1491. doi: 10.1016/j.freeradbiomed.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao B, Ranguelova K, Jian JJ, Zielonka J, Kalyanaraman B, Mason R. Studies on the photosensitized reduction of resorufin and implications for the detection of oxidative stress with Amplex red. Free Radic Biol Med. 2010;49(Suppl. 1):S105–S106. doi: 10.1016/j.freeradbiomed.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 26.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dikalov S, Grienling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 31.Robinson KM, Janes MS, Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nat Protoc. 2008;3:941–947. doi: 10.1038/nprot.2008.56. [DOI] [PubMed] [Google Scholar]
- 32.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, Avadhani NG, Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic Biol Med. 2008;44:835–846. doi: 10.1016/j.freeradbiomed.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wardman P. Methods to measure the reactivity of peroxynitrite-derived oxidants toward reduced fluoresceins and rhodamines. Methods Enzymol. 2008;441:261–282. doi: 10.1016/S0076-6879(08)01214-7. [DOI] [PubMed] [Google Scholar]
- 34.Jourd'heuil D, Jourd'heuil FL, Kutchukian PS, Musah RA, Wink DA, Grisham MB. Reaction of superoxide and nitric oxide with peroxynitrite. J Biol Chem. 2001;276:28799–28805. doi: 10.1074/jbc.M102341200. [DOI] [PubMed] [Google Scholar]
- 35.Zielonka J, Kalyanaraman B. “ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis”—a critical commentary. Free Radic Biol Med. 2008;45:1217–1219. doi: 10.1016/j.freeradbiomed.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130:1910–1917. [PubMed] [Google Scholar]
- 37.Kettle AJ, Carr AC, Winterbourn CC. Assays using horseradish peroxidase and phenolic substrates require superoxide dismutase for accurate determination of hydrogen peroxide production by neutrophils. Free Radic Biol Med. 1994;17:161–164. doi: 10.1016/0891-5849(94)90111-2. [DOI] [PubMed] [Google Scholar]