Abstract
Cerium oxide nanoparticles (nanoceria) are direct antioxidants; they inhibit pathological neovascularization following a single intravitreal injection into new born very low density lipoprotein receptor knockout (vldlr−/−) mice. However, the long-term therapeutic effects and mechanisms of nanoceria action on regression of the existing pathologic neovascularization in the eyes are unknown. We intravitreally injected P28 vldlr−/− mice and extended the endpoint for analysis until P70. The data demonstrate that nanoceria sustained their therapeutic function up to 6 weeks. Multiple parameters for nanoceria effects were examined including: regression of existing abnormal blood vessels, reduction of vascular leakage, down-regulation of the expression of vascular endothelial growth factor (VEGF), acrolein, glial fibrillary acidic protein (GFAP) and caspase 3 as well as up-regulation of the expression of rod- and cone-opsin genes. Regulation of ASK1-P38/JNK-NF-κB signaling pathway by nanoceria was investigated. Our data demonstrated that a single intravitreal injection of nanoceria in P28 vldlr−/− mice produced sustained regression of existing oxidative stress-induced neovascularizations, prevented blood vessel leakage and inhibited apoptosis via down-regulation of the ASK1-P38/JNK-NF-κB signaling pathway.
Keywords: Nanoceria, vldlr−/− mouse, neovascularization, retinal degeneration, signal transduction
1. Introduction
Age-related macular degeneration (AMD) is a heterogeneous human eye disorder that damages the central region of the retina (macula) and is the leading cause of irreversible blindness in adults over 50 years old in developed countries [1, 2]. AMD is categorized into two forms, wet and dry. The very low density lipoprotein receptor knockout (vldlr−/−) mouse is a model for human retinal angiomatous proliferation (RAP) [3, 4], a distinct form of neovascular (“wet”) AMD. It is characterized by progressive neovascularizations which usually appear by postnatal day (P) 12–13. The excessive new blood vessels arise from the inner retina extending through the photoreceptors, invading the subretinal space [3–5] and connecting with the choroidal neovessels. This causes retinal pigment epithelium (RPE) disruption, Bruch’s membrane exposure, and eventually results in photoreceptor degeneration with significant fibrosis [4]. Published data [6–9] documented that degenerative diseases, including neovascular ocular diseases, are directly linked with oxidative stress, inflammation and other cellular stresses. These stress conditions, associated with the progression of AMD, induce the expression of vascular endothelial growth factor (VEGF) which results in neovascularization [10, 11]. Increased VEGF is a major causative factor for vasculature formation in the eye [11–13] and has been shown to be correlated with increased reactive oxygen species (ROS) and inappropriate angiogenesis [11, 12, 14, 15]. Currently there are no effective treatments to completely cure these ocular disorders. Anti-VEGF or humanized anti-VEGF monoclonal antibodies are the principle therapeutic agents for treatment of choroidal neovascularization (CNV) (see review [16]). However, these therapeutic antibodies require repeated intravitreal injections which can result in endophthalmitis or retinal detachment [17].
We have shown that inorganic cerium oxide nanoparticles (nanoceria) have numerous unique characteristics, such as biocompatibility, small size, cellular and nuclear membrane permeability and are excellent agents for biological applications [18–20]. Nanoceria exhibit catalytic activities which mimic those of superoxide dismutase and catalase [21]. Nanoceria were synthesized and characterized as described previously [22]. They regeneratively scavenge free-radicals by switching their valence states between +3 (fully reduced) and +4 (fully oxidized) as a result of increased oxygen vacancies in the crystal structure which are caused by the loss of oxygen and/or its electrons [23, 24]. We previously reported that nanoceria, acting as direct antioxidants and being preferentially taken up by the retina and retained for more than one year [25], are capable of preventing light-induced photoreceptor damage in wild type (wt) albino rats [18] and inherited retinal degeneration in tubby mice [26, 27]. We also showed that nanoceria down-regulate VEGF expression and inhibit the formation of new leaky blood vessels in vldlr−/− retinas when administered in the new born pups at P7 [15] and inhibit expression of inflammatory genes [28]. In this study, we further examined the long-term therapeutic effects of nanoceria on regression of the existing neovascularizations in vldlr−/− adult mice after intravitreal delivery at P28. In addition, the mechanism by which nanoceria accomplish their effects was investigated.
2. Materials and Methods
2.1. Animals
vldlr−/− mice on C57BL/6J background were purchased from Jackson laboratory. Animals were cared for and handled according to the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in vision and ophthalmic research. The protocols for this study were approved by the Institutional Animal Care and Use Committees (IACUC) of the University of Oklahoma Health Sciences Center and the Dean McGee Eye Institute.
2.2. Intravitreal injection
vldlr−/− mice at P28 were anesthetized by intraperitoneal injection of ketamine (85 mg/kg) and xylazine (14 mg/kg) (Pharmaceutical Systems Inc.). The eyes were dilated with one drop of 10% AK-dilate phenylephrine hydrochloride solution (Akorn Inc.) and a puncture through the sclera was made with a 30 gauge needle. A 34 gauge needle attached to a 10 µl syringe of a Nanofil® injection system (World Precision Instruments) was inserted into the puncture and 1 µl of either saline or 1 mM nanoceria (172 ng) in saline was delivered into the vitreous under an operating microscope (Carl Zeiss Surgical Inc.) as previous report [27]. The needle was kept in place for 30s to allow complete delivery. After recovery from anesthesia, the mice were returned to their original cages and maintained under the standard conditions until the scheduled time points (P49 and P70) for assays.
2.3. Fundoscopy and fluorescein angiography
The eyes of fully anesthetized mice were dilated and one drop of 2.5% Goniotaire hypromellose solution (Altaire pharmaceuticals, Inc.) was placed on the cornea. The mice were positioned on the bed of the Micron III fundoscopy system (Phoenix Research Labs), the positions of the mice and Micron III objective were adjusted until the objective contacted the cornea. After the fundus was clearly seen and images were taken, 15 µl of 5% AK-FLUOR(Alcon) was intraperitonealy injected. The photographs were captured using StreamPix software with UV filters at three times after injection at 0.5min, 1min and 4min.
2.4.Vascular filling assay
Fluorescein vascular filling assay was performed according to the protocol reported previously [15] with minor modification. Briefly, fully anesthetized mice were intracardially injected with 40 µl of 10 mg/ml high molecular weight fluorescein isothiocyanate (FITC) - dextran (Sigma-Aldrich, FD-2000S). Mice were killed; the eyes were harvested and fixed in 4% paraformaldehyde (PFA) for 1h. After rinsing in PBS buffer, the cornea and lens were removed, and the remaining parts of the eye were dissected into retina and SCR (sclera-choroid-RPE). They were flat mounted (SCR with sclera facing down and RPE facing up) on slides with 4–6 radial cuts. The slides were mounted with mounting media (Fluoromount-G, Southern Biotech) and coverslipped. Observations and imaging were conducted using an Olympus stereo MVX10 microscope with a UV filter under 5× and 6.3×.
2.5.RT-PCR and PCR array
3–4 pairs of retinas (for PCR array) or 3–6 eyecups without the lens and cornea (for quantitative real time RT-PCR (qRT-PCR) and semi-quantitative RT-PCR) were collected. Total RNA was isolated using TRIzol reagent (Invitrogen); 2 µg of total RNA was used for cDNA synthesis with first strand superscript III transcriptase and an Oligo dT primer (Invitrogen), and PCR arrays using “mouse oxidative stress and antioxidant defense” array plates were performed as previously reported [27]. The changes of gene expression in uninjected and nanoceria (CeO2) injected retinas are shown as fold changes compared to wt (vldlr−/−/wt and CeO2/wt) and to uninjected retinas (CeO2/vldlr−/−) using PCR array software (SABiosciences). Quantitative real time RT-PCR (qRT-PCR) performance and calculation of relative expression level of target genes against the house-keeping gene (GAPDH) [29] as well as the primers for VEGF [11], rhodopsin, M-opsin, and S-opsin are the same as previously reported [27]. NF-E2-related factor-2 (Nrf2) expression was analyzed by semi-quantitative RT-PCR using GAPDH as a control. The primer sequences of Nrf2 and densitometric band analysis are the same as previously reported [27].
2.6. Immunocytochemistry
Eye collection, fixation, embedding, cryosectioning and immunocytochemistry were performed as previously reported [27]. Briefly, eyes were enucleated and fixed in 4% PFA at room temperature for 1h. After the cornea and lens were removed, the eyecups were cryoprotected with 15% and 30% sucrose for 1h each. Individual eyecups were embedded in OCT media (Sakura Finetek USA, Inc) and frozen over liquid nitrogen. Cryosections of 10 µm in thickness were made with a cryostat (Leica, Japan) and collected on pre-cleaned Superfrost-Plus microscope slides (Fisher Scientific). The slides were incubated with rabbit anti-Acrolein (1:250, Abcam) and then incubated in AlexaFluor 488 conjugated anti-rabbit IgG. The slides were mounted with Vectashield mounting media for fluorescence with DAPI (Vector laboratories, Inc.) and coverslipped. Observation and image capture were conducted using a Nikon Eclipse 800 fluorescence microscope under 20×. The average of fluorescent intensity of images from 4–8 eyes per group was determined by regional intensity analysis.
2.7. Histology
The procedure for eye collection, fixation, sectioning, and slide staining is the same as previously reported [27]. Briefly, eyes were marked with green dye on the superior cornea, then enucleated, and fixed in Perfix at 4°C for at least 24 h. The eyes were embedded in paraffin after dehydration and were sectioned as 5 µm in thickness and stained with Hematoxylin and eosin (H&E). Representative photomicrographs were obtained from the superior-hemisphere at a distance of 0.96 mm from the optic nerve head (ONH) using a 40× and 10× lens. For morphometric analysis, three images were taken at both superior and inferior hemispheres with the first position at 0.48 mm from the ONH using a 40× lens. The number of nuclei in the outer nuclear layer (ONL) of images from 4–6 eyes per group was counted.
2.8. Western blot
Individual (6–8) eyecups (without lens and cornea) per group at P70 were homogenized on ice, centrifuged and the soluble phase was used for experiments [27]. Protein was quantified using the BCA assay, and 50 µg of soluble proteins were loaded on a 10% SDS-page gel and electrophoresed. The proteins were transferred to a nitrocellulose membrane and the membrane was blocked with 5% dry milk in TBST buffer. Specific proteins were detected with primary antibodies diluted in TBST buffer: mouse anti-Glial Fibrillary Acidic Protein (1:1000, Millipore); rabbit anti-phospho-ASK1 (Ser967), anti-phospho-SAPK/JNK (Thr183/Tyr185) and anti-phospho-p38 MAP Kinase (Thr180/Tyr182) (1:1,000, respectively, Cell signaling technology); sheep anti-VEGF and rabbit anti-NF-κB p65 (C-20) (1:1,000, respectively, Santa Cruz biotechnology). The same membrane was stripped and blotted with anti-β-actin antibody (HRP conjugate) (1:1,000, Cell signaling technology) as loading control. The membrane was developed with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific), the band detection and densitometric analyses of bands are the same as previously reported [27].
2.9. Statistical analysis
Results are expressed as mean ± SD or mean ± SEM and indicated in each figure. One – way ANOVA and/or two tailed student t-test were used to test the difference. P value of less than 0.05 (P<0.05) was considered to be significant.
3. Results
3.1. Neovascularization and photoreceptor death in vldlr−/− retina
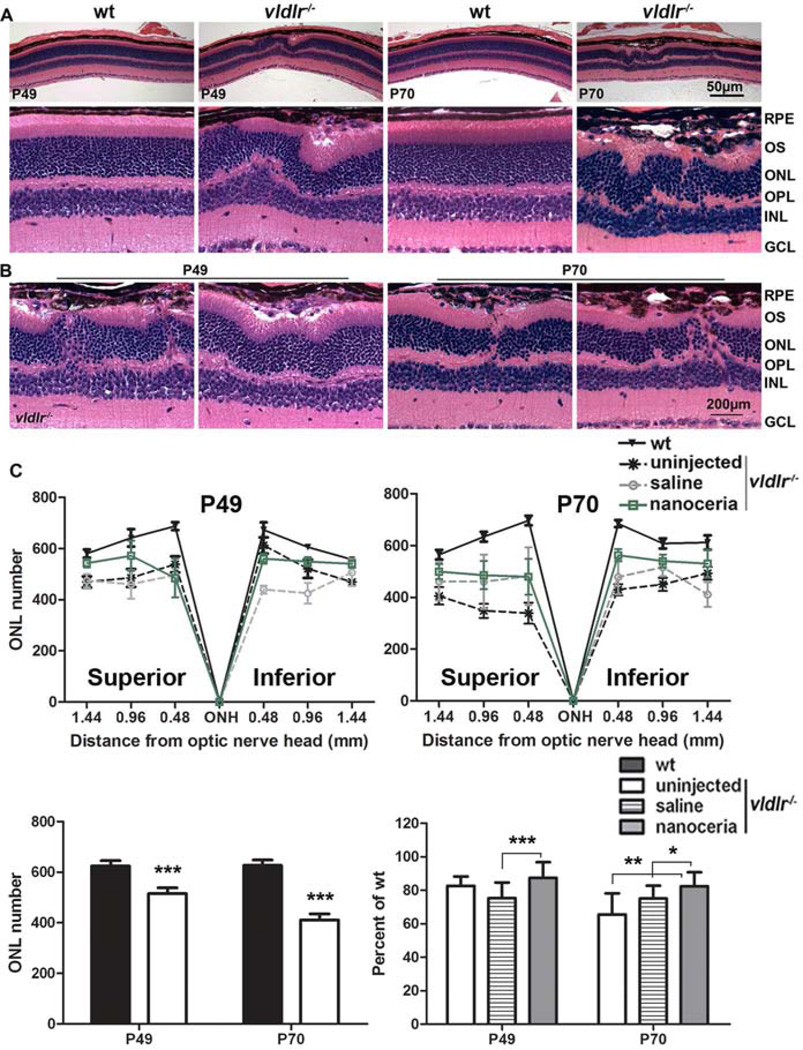
To determine the relationship between photoreceptor death and the progression of neovascularization, eyes from vldlr−/− and wt mice at P49 and P70 were used for histological analysis. The loss of photoreceptors and the reduction of the ONL thickness predominantly occurred in the 2/3 of the retina from the ONH with more severe loss in the superior hemisphere (Fig. 1C). This agrees with the observation previously reported by Dorrel and colleagues [11]. In the vascular lesion areas (Figs. 1A, 1B), hyper-pigmented RPE appeared as several cell layers. Irregular pattern and rosette-like structures of ONL and inner nuclear layer (INL), and/or a thinner ONL overlying the thickened RPE region were constantly observed (Figs. 1A, 1B). Abnormal intraretinal vessels originated from the inner retina were clearly seen to penetrate through the ONL to invade the subretinal space (Fig. 1B). The connection of blood vessels from the retina and choroid (retinal-choroidal anastomosis) resulted in the destruction of the outer segment (OS), ONL, outer plexiform layer (OPL) and INL (Fig. 1B). The number of nuclei in the ONL in vldlr−/− mice is reduced to 83% and 66% of wt at P49 and P70, respectively (Fig. 1C). Nanoceria treatment at P28 prevented photoreceptor death in vldlr−/− retinas with the nuclear number in the ONL increased to 87% and 83% of wt respectively (Fig. 1C). The protection of the retina against degeneration by nanoceria is more apparent at P70.
Figure 1.
Neovascularizations destroy the retinal architecture and are correlated with cell death in vldlr−/− retina. (A, B) Histological photomicrographs at P49 and P70 show that the ONL is irregular beneath the hypertrophied RPE in vldlr−/− retina (A, bottom) and illicit blood vessels in different areas of vldlr−/− retina penetrate through the ONL damaging the ONL structure (B). N=4–6 eyes per group. RPE: retinal pigment epithelium, OS+IS: outer segment plus inner segment, ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer. Scale bar, 50 µm and 200 µm. (C) Morphometric analysis demonstrates that the number of nuclei in the ONL of uninjected vldlr−/− is greatly reduced, especially in the 2/3 of the retina from the ONH to the ora serrata (top), however nanoceria treatment significantly increased the nuclear number in the ONL. The average of all the measurements in the same group are shown (bottom). N=24–36 measurements per group. *P<0.05, **P<0.005, ***P<0.001.
3.2. Inhibition of existing neovascularizations and fluid leakage
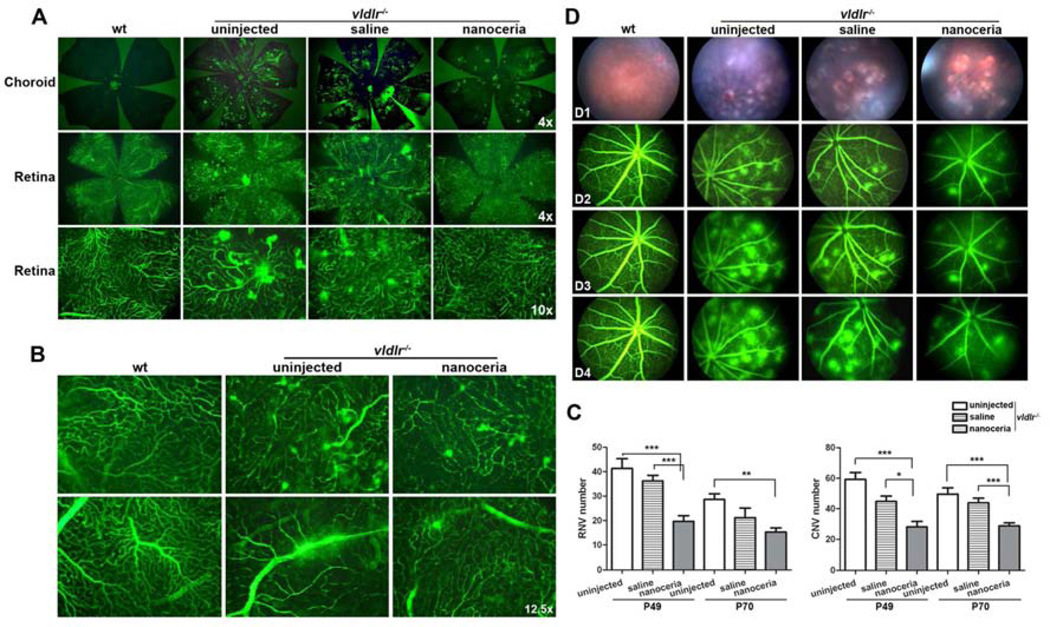
Retinal and choroidal blood vessels are the two separate blood supply networks for the eye [30]. The patterns and abnormalities of these blood vessels are easily seen using a fluorescent microscope and a vascular filling assay [15]. In P49 wt, the retinal blood vessels are shown as a uniform and well organized network. The choroidal vasculature is not visible in the wt because there are no neovascularizations extruding from the retina connecting with the choroidal blood network. But the vldlr−/− retina exhibits extruded “bulb-shaped” pathological blood vessels representing retinal neovascular “blebs” (RNV), whereas the vldlr−/− choroid has massive and disordered aggregates of blood vessels representing choroidal neovascular “tufts” (CNV) which project through the RPE layer (Fig. 2A). Under a higher magnification, the extravasation of fluorescein dextran from the retinal “neo” vessels and the original large vessels was frequently seen in vldlr−/− retina (Fig. 2B). Nanoceria treatment markedly reduced the number of RNV and CNV (Fig. 2A) and blocked the fluid leakage (Fig. 2B). Similar results were obtained from P70 retinas and choroids (Supplemental Fig. 1). The number of RNV and CNV is easily counted under a higher magnification. The number of RNV and CNV in uninjected vldlr−/− mice is 42.57 ± 9.71 and 59.27 ± 17.1 respectively at P49, and is decreased to 28.67 ± 7.98 and 49.58 ± 14.23 respectively at P70 due to the fusion of the neovascularizations. Saline treatment had little effect on these numbers. However, nanoceria treatment markedly reduced the number of RNV and CNV to 19.67 ± 9.89 and 28.22 ± 14.96 at P49 (P<0.0001 for RNV and CNV), and 15.27 ± 8.09 and 28.68 ± 9.51 at P70, respectively (PRNV=0.0009 and PCNV<0.0001), which represent a reduction of about 50% for both RNV and CNV at both time points, compared to uninjected controls (Fig. 2C).
Figure 2.
Nanoceria regress existing neovascularizations and prevent vascular leakage in vldlr−/− mice. (A) Uninjected vldlr−/− eyes at P49 exhibit numerous choroidal neovascular “tufts” (top) and intraretinal neovascular “blebs” (bottom two panels) compared to wt which have no fluorescein labeling in the choroid and have uniform organized retinal blood vessels. Nanoceria treatment markedly reduced the number of “tufts” and “blebs”. (B) High magnification of the retina reveals the leakage of both the neovasculature (top) and the original larger vessels (bottom) in untreated vldlr−/− retina compared to wt. However, nanoceria injection not only reduced the “blebs” number, but also prevented the larger vessel leakage. Representative images from 10–22 eyes per group are shown. (C) The quantitation of the RNV and CNV number (N=10–22 eyes per group) is presented and shown as mean ± SD. *P<0.05, **P<0.001, ***P<0.0001. (D) Fundoscopy at P49 revealed that untreated vldlr−/− mice exhibit numerous fundus patches of neovascularization (D1) which were highlighted by fluorescent angiography (D2, D3 and D4). The blood vessel leakage in the uninjected group was progressive and severe over extended times. Saline treatment did not affect the leakage. However nanoceria injection remarkably reduced neovascularization and neovascular leakage.
Fundoscopic examination of untreated and saline injected vldlr−/− mice at P49 showed numerous flecks representing retinal neovascularizations which are scattered all over the fundus (Fig. 2 D1). Fluorescein angiographies (Fig. 2 D2, D3, D4) show these neovascularizations as areas of focal leakage (intense hyperfluorescent spots) in the retina. Because of the vascular leakage in vldlr−/− mice, fluorescein diffused from the blood vessels very rapidly and progressively over time whereas wt mice do not exhibit any leakage. Nanoceria treatment reduced the number of fluorescent spots and prevented the blood vessel leakage compared to uninjected or saline treated vldlr−/− mice (Fig. 2D). Similar data were obtained from P70 eyes (Supplemental Fig. 2).
3.3. Oxidative stress and oxidative stress-associated gene expression
Acrolein is a commonly used oxidative stress marker for detecting lipid peroxidation [6]. Immunocytochemistry at P49 (Fig. 3A) showed that acrolein was localized in almost all the layers in vldlr−/− retina and its level is clearly greater than that of age-matched wt controls. Quantitation of the intensity of acrolein demonstrates that uninjected vldlr−/− eyes exhibit 1.4 fold higher intensity than wt (wt: 342.67 ± 51.17, uninjected: 470.3 ± 90.9, P=0.0092). Saline injection did not affect the fluorescent intensity (saline: 484.2 ± 133.5, P=0.8291). Nanoceria treatment significantly decreased the intensity to wt level compared to uninjected and saline injected eyes (nanoceria: 354.6 ± 50.6, P=0.0078 and P=0.0504, respectively) (Fig. 3B). To determine nanoceria effects on oxidative stress associated gene expression, PCR arrays were performed using P49 retinas. The data (Table 1) show that nanoceria differentially regulated the expression of genes having functions associated with molecular/cellular protection. For example, the expression of Gpx4, Prdx6-rs1, Tmod1 and Txnrd2 is down-regulated in uninjected vldlr−/− retinas and they are up-regulated by nanoceria treatment. However, another group of genes such as Aass, Apc, Cat, Ercc6, Nxn, Prdx3, Serpinb1b, Sod1 and Sod2, having similar functions, are down-regulated after nanoceria injection. It is well known that Nrf2 is a central gene in modulation of its downstream target genes of defensive networks [31]. Semi-quantitative RT-PCR at P49 and P70 demonstrates that nanoceria treatment significantly increased Nrf2 mRNA level 2 fold higher than untreated vldlr−/− mice (Figs. 3C, 3D).
Figure 3.
Increased oxidative stress is alleviated by nanoceria via Nrf2 up-regulation. (A) Immunocytochemistry at P49 shows that the level of acrolein (green) was greater in the entire retina in uninjected vldlr−/− mice. However, anoceria treatment greatly reduced acrolein level. Nuclei were counterstained with DAPI. Scale bar, 100 µm. RPE: retinal pigment epithelium, OS+IS: outer segment plus inner segment, ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer. (B) Average intensity (mean ± SD) analysis of sections from 4–8 eyes per group demonstrates that untreated and saline treated retinas exhibit higher fluorescent intensities compared to wt, and nanoceria treatment significantly decreased the fluorescent intensity. (C, D) Nrf2 expression level in untreated vldlr−/− eyes was increased by nanoceria treatment. Representative semi-quantitative RT-PCR data (C) and densitometric analysis of the bands (D) are shown (mean ± SD). N=3–6 eyes per group, *P<0.05, **P<0.01.
Table 1.
Mouse oxidative stress and antioxidant defense genes with statistically significant changes in expression in vldlr−/− mice (≥1.5 fold) before and after nanoceria treatment.
| Symbol | Gene name | vldlr−/−/wt | CeO2/wt | CeO2/vldlr−/− | |||
|---|---|---|---|---|---|---|---|
| antioxidants | fold | P value | fold | P value | fold | P value | |
| Cyba | Cytochrome b-245, alpha polypeptide | −1.88 | 0.0033** | −1.21 | 0.4534 | 1.55 | 0.1391 |
| Gpx4 | Glutathione peroxidase 4 | −2.55 | 0.0637 | −1.47 | 0.0334* | 1.74 | 0.3968 |
| Prdx6-rs1 | Peroxiredoxin 6, related sequence 1 | −1.51 | 0.8250 | 1.04 | 0.8221 | 1.56 | 0.7266 |
| Sod3 | Superoxide dismutase 3, extracellular | −3.04 | 0.1486 | −2.11 | 0.1244 | 1.44 | 0.8652 |
| Tmod1 | Tropomodulin 1 | −1.72 | 0.1518 | −1.33 | 0.3394 | 1.30 | 0.5464 |
| Txnrd2 | Thioredoxin reductase 2 | −1.57 | 0.4587 | −1.06 | 0.8665 | 1.48 | 0.5558 |
| Aass | Aminoadipate-semialdehyde synthase | −1.34 | 0.4148 | −2.12 | 0.0018** | −1.58 | 0.2353 |
| Apc | Adenomatosis polyposis coli | −1.38 | 0.2484 | −2.05 | 0.0028** | −1.48 | 0.2212 |
| Cat | Catalase | −1.97 | 0.0292* | −2.13 | 0.0175* | −1.08 | 0.6089 |
| Ercc6 | Excision repair cross-complementing rodent repair deficiency, complementation group 6 | −1.33 | 0.4227 | −1.95 | 0.0143* | −1.47 | 0.2030 |
| Gpx3 | Glutathione peroxidase 3 | −3.93 | 0.1469 | −4.56 | 0.1332 | −1.16 | 0.5127 |
| Mpp4 | Membrane protein, palmitoylated 4 (MAGUK p55 subfamily member 4) | −1.84 | 0.1437 | −2.28 | 0.0701 | −1.24 | 0.6545 |
| Nxn | Nucleoredoxin | −1.08 | 0.8587 | −1.72 | 0.3267 | −1.59 | 0.4085 |
| Prdx3 | Peroxiredoxin 3 | −1.04 | 0.9271 | −1.51 | 0.0882 | −1.46 | 0.2881 |
| Serpinb1b | Serine (or cysteine) peptidase inhibitor, clade B, member 1b | 1.24 | 0.2492 | −1.27 | 0.3336 | −1.58 | 0.1400 |
| Sod1 | Superoxide dismutase 1, soluble | −1.10 | 0.7133 | −1.67 | 0.1749 | −1.52 | 0.2494 |
| Sod2 | Superoxide dismutase 2, mitochondrial | −1.18 | 0.5598 | −1.97 | 0.1465 | −1.67 | 0.2951 |
N=3 – 4,
P<0.05,
P<0.01
3.4. VEGF level and Müller cells in vldlr−/− retina
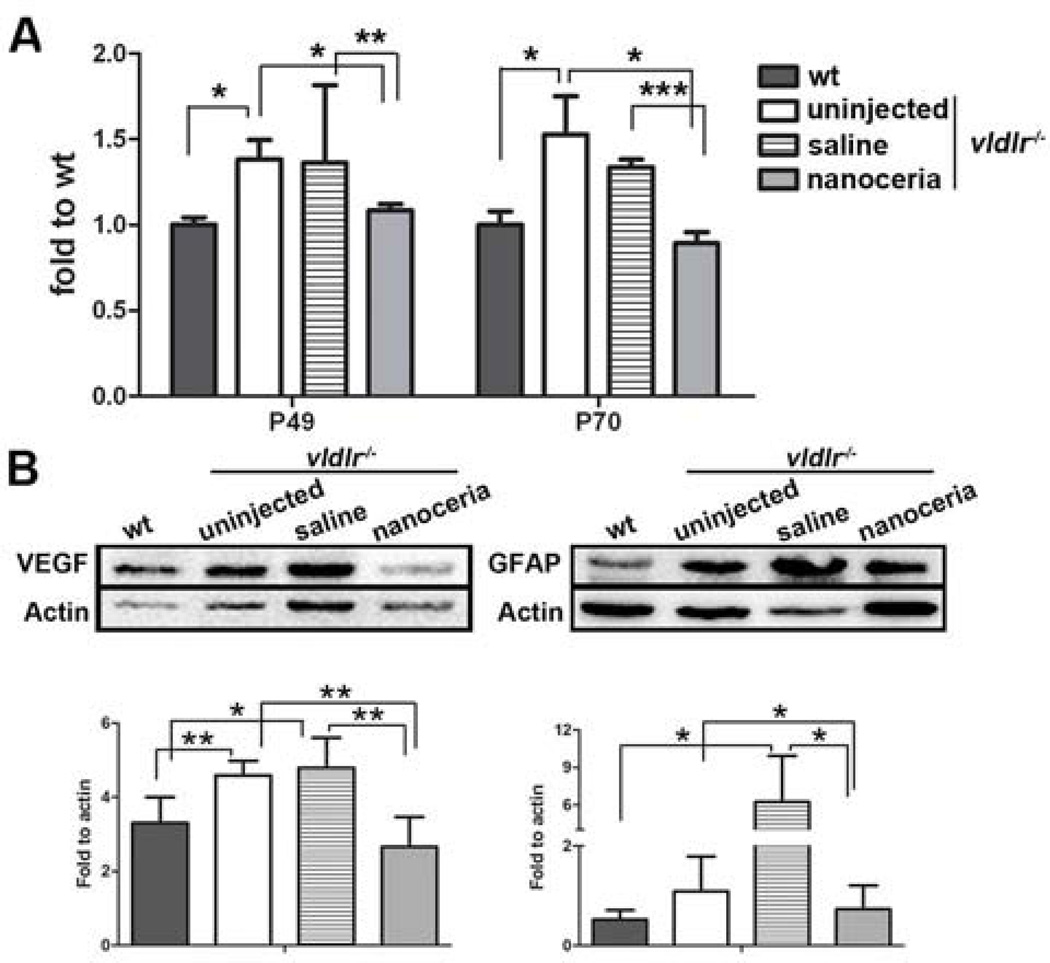
We next examined the effect of nanoceria on the regulation of VEGF expression. qRT-PCR (Fig. 4A) showed that the VEGF mRNA level in uninjected vldlr−/− eyes was increased about 1.5 fold compared to wt (P<0.05 at P49 and P70) wherease saline injection only slightly changed VEGF expression in comparison to uninjected vldlr−/− (P=0.9483 at P49 and P=0.3820 at P70). However, nanoceria treatment significantly decreased the VEGF levels compared to uninjected and saline injected vldlr−/− mice (nanoceria: 1.08, uninjected: 1.38, saline: 1.36, P<0.05 and P<0.005 respectively at P49 and 0.89, 1.53, 1.34, P<0.05 and P<0.0005 respectively at P70), and are equivalent to the wt level. Western blots using P70 eyecups, in agreement with our previous report [15], demonstrate that nanoceria decreased VEGF expression compared to untreated and saline treated samples (Fig. 4B left).
Figure 4.
Levels of VEGF and GFAP were reduced by nanoceria treatment. (A) qRT-PCR at P49 and P70 demonstrated that nanoceria reduced the elevated VEGF mRNA levels in vldlr−/− eyes. Data shown are mean ± SD. N=3–6 eyes per group. *P<0.05, **P<0.005, ***P<0.0005. (B) Western blots of VEGF and GFAP at P70 showed both factors increased their expression in uninjected and saline treated vldlr−/− mice and nanoceria treatment markedly decreased their levels. Representative band from 6–8 eyecups per group is shown (top). Densitometric band analysis (mean ± SD, bottom) indicated that the difference is significant. *P<0.05, **P<0.01.
Müller cells are one of the main sources of VEGF [32] and GFAP is a commonly used marker of Müller glial activation [33]. We next examined the expression of GFAP by western blot at P70. Consistent with VEGF expression, GFAP expression in uninjected eyes is higher than the wt, and saline treatment induced an even higher GFAP level, possibly injection of saline caused activation of glia cells. However, nanoceria injection greatly reduced the amount of GFAP (Fig. 4B right) suggesting that nanoceria, by decreasing oxidative stress, inhibit Müller glial activation and thereby prevent VEGF synthesis and/or secretion.
3.5. Expression of photoreceptor-specific genes and apoptosis effector gene in vldlr−/− retina
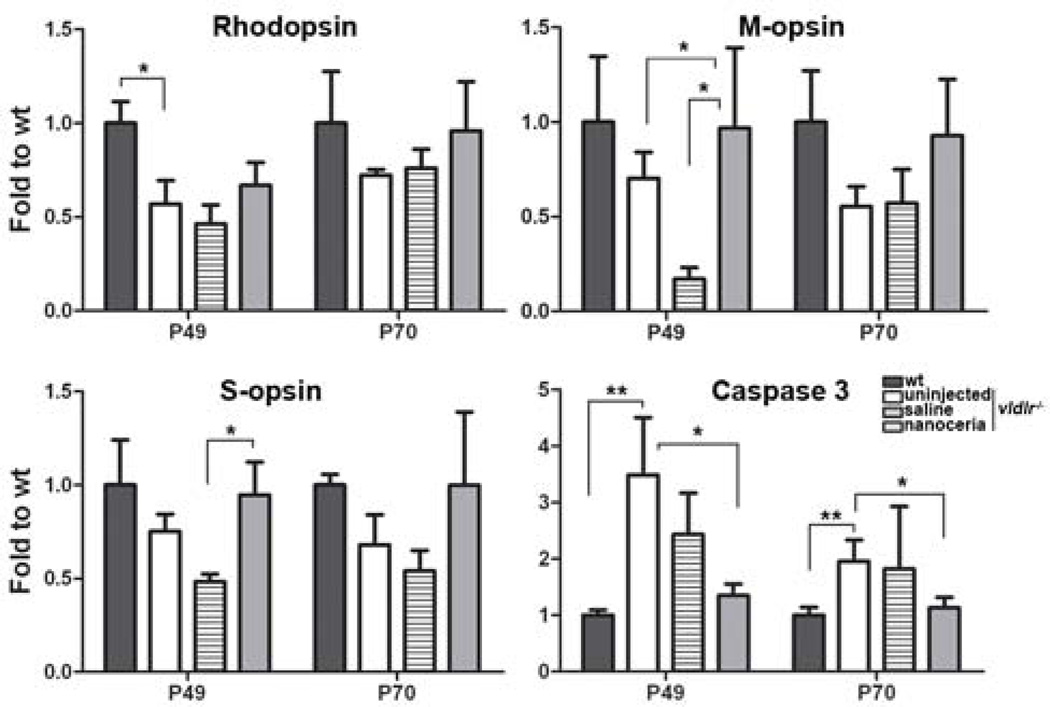
One of the abnormalities associated with neovascularizations in vldlr−/− mice is retinal degeneration. qRT-PCR demonstrated that the levels of rhodopsin, M-opsin and S-opsin were decreased by 1.8, 1.4, 1.3 fold at P49, and 1.4, 1.8, 1.5 fold at P70, respectively, compared to wt. And their levels were increased by nanoceria treatment to 1.2, 2.2, 1.3 fold at P49 and 1.3, 1.4, 1.5 fold at P70 respectively, compared to uninjected vldlr−/− mice (Fig. 5).
Figure 5.
Nanoceria up-regulate photoreceptor-specific gene and down-regulate caspase 3 expression. mRNA levels of rhodopsin, M-opsin and S-opsin in untreated and saline injected eyes were reduced compared to wt. Nanoceria treatment increased the expression of these genes. Caspase 3 expression was significantly elevated in uninjected and saline injected vldlr−/− eyes and was greatly reduced by nanoceria injection. Data shown are mean ± SD. N=3–6 eyecups per group. *P<0.05, **P<0.005
Caspase 3 is a major cell death effector in the apoptosis signaling pathway. qRT-PCR demonstrated that uninjected vldlr−/− mice have more than 4 fold and 2 fold higher levels of caspase 3 than wt at P49 and P70 respectively (Fig. 5) which are in accord with the histological observation that the number of ONL nuclei has declined in vldlr−/− mice (Fig. 1C). However, nanoceria treatment markedly decreased the levels of caspase 3 to wt level (Fig. 5) which is in agreement with the result showing that the number of ONL nuclei was higher following nanoceria treatment (Fig. 1C).
3.6. Regulation of the ASK1-P38/JNK-NF-κB signaling pathway
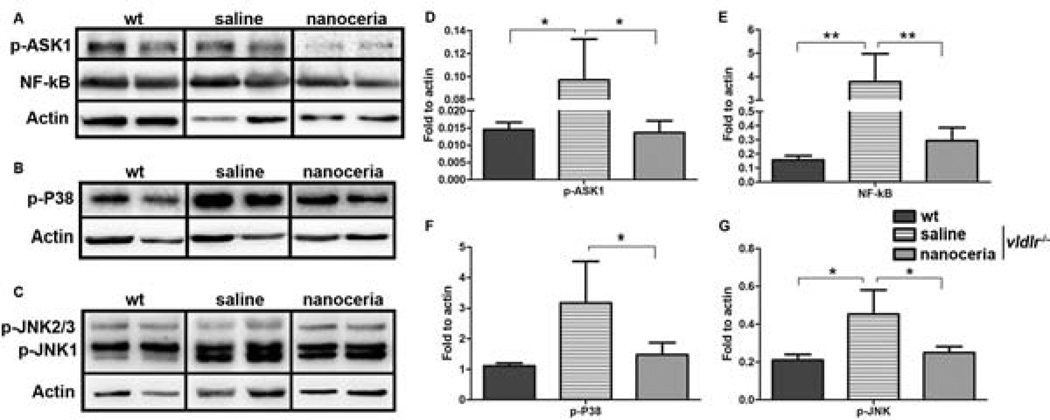
It was reported that oxidative stress increases the activity of ASK1 (apoptosis signal-regulating kinase 1), and ASK1 promotes early angiogenesis by inducing inflammation, monocyte chemoattractant protein-1 (MCP-1) and VEGF expression [34]. Phosphorylation of P38-MAPKs and c-Jun N-terminal kinases (JNKs) was induced in human umbilical vein endothelial cells (HUVECs) when VEGF was stimulated [35]. Nuclear factor kappa B (NF-κB) had been shown to be correlated with VEGF and angiogenesis in HUVECs, human lung cancer cells (NCI-H292) and mouse cornea [36, 37]. ASK1 is upstream of P38 and JNK, and also a positive regulator of NF-κB [38]. It is logical to hypothesize that if ROS were destroyed by nanoceria, the downstream effects should be decreased. To test this mechanism, western blots were carried out at P70 to analyze the levels of phosphorylated ASK1 (p-ASK1), p-P38 and p-JNK as well as NF-κB. Fig. 6 shows all these components are elevated in uninjected and saline injected vldlr−/− mice compared to wt, however their expression levels are remarkably reduced by nanoceria treatment.
Figure 6.
Nanoceria regulate the expression of ASK1-P38/JNK-NF-κB signaling pathway. (A–C) Two representative bands are shown by western blots from 6–8 eyecups per groups; (D–G) are densitometry of the bands. Activated ASK1 (A, D), P38 (B, F), JNK (C, G) and NF-κB (A, E) in saline treated eyes are higher than the wt, nanoceria injection decreased their levels. Densitometric analysis of the bands from 6–8 samples (mean ± SD) demonstrated that the difference is significant. *P<0.05, **P<0.005
4. Discussion
It is well accepted that oxidative stress promotes angiogenesis and ocular neovascularization [8, 11]. We previously have shown that the amount of ROS was increased in vldlr−/− retinas and demonstrated that oxidative stress is directly involved in the pathogenesis of the “neo” blood vessel development [15]. In this paper, we further confirmed our previous report by demonstrating that acrolein, a well-known oxidative stress marker, is higher in vldlr−/− retinas. In addition, the number of retinal neovascular ‘blebs” and choroidal neovascular “tufts” in vldlr−/− mice is significantly increased compared to age-matched wt, and these pathologic neovascularizations resulted in blood vessel leakage. Nanoceria treatment, by destroying ROS, greatly reduced the level of acrolein and decreased the number of neovascularizations in vldlr−/− retina and choroid resulting in inhibition of vascular leakage. These effects are sustained for up to 6 weeks following a single intravitreal injection of vldlr−/− mice at P28.
An elevated level of VEGF in the eyes is one of the distinct characteristics of vldlr−/−mice [11, 12, 14, 15]. VEGF had been reported to be mainly expressed in retinal Müller cells, RPE, ganglion cells, photoreceptors [15] and other cellular types [39, 40]. Although VEGF is an important factor required for normal retinal and choroidal vascular development, we and others demonstrated that increased VEGF level is tightly correlated with the abnormal retinal and choroidal neovascularizations [12, 15, 32]. The data we present in this study, demonstrating the increased VEGF levels in vldlr−/− eyes, are in agreement with previous reports. We also showed that GFAP, a marker for activated glial cells in the retina and is associated with the formation of intraretinal neovascularization in vldlr−/− mice [11, 13], was much more in vldlr−/− eyes. Therapeutic treatment with nanoceria markedly reduced the amounts of GFAP and VEGF, a further indication of the beneficial effects of nanoceria in the treatment of pathological angiogenesis.
We showed in this paper that there are 17% and 34% fewer photoreceptors in vldlr−/− retinas than in agematched wt litter mates at P49 and P70, respectively. Furthermore, we found that the expression of both rod and cone opsins was down-regulated and caspase 3 was up-regulated in vldlr−/− mice. Nanoceria increased rod- and cone-opsin expression levels, significantly reduced the expression of caspase 3, and prevented photoreceptor death. These effects are sustained for up to 6 weeks following one single intravitreal injection. Although we do not think nanoceria directly target these genes to alter their expressions, nanoceria might indirectly modulate the expression of these genes by regulating the production of oxidative stress associated genes through ROS scavenging. Indeed, a group of genes associated with oxidative stress and antioxidant defense, such as Gpx4, Prdx6-rs1, Sod3, Txnrd2, Aass, Apc and Ercc6 were down-regulated in vldlr−/− retinas. However, nanoceria treatment up-regulated the expression of some genes (Gpx4, Prdx6-rs1, Txnrd2) but further down regulated the expression of other genes (Aass, Apc, Ercc6, Prdx3, Sod1, Sod2, Nxn, Serpinb1b) having a similar function. A possible reason for the differential regulation might be that the structure and subcellular localization of these proteins or other regulators determines or modulates their temporal and spatial activity. Gpx4 had been reported to catalyze hydroperoxides and had been used as a therapeutic target for protection against oxidative stress-induced retinal degeneration and RPE damage by overexpression of an inducible Gpx4 transgene in photoreceptors [41]. Recently, Wnt (Wingless/Intergration, Wg/Int) activity was demonstrated to be increased in vldlr−/− mice [12]. Nxn knockout mice exhibit rapid degradation of Dishevelled (Dvl), an upstream activator of Wnt signaling pathway, suggesting that Nxn acts as a negative regulator of Dvl and interacts with Dvl to prolong inactivation and maintenance of Wnt/β-catenin signaling suppression [42, 43]. Nanoceria themselves are direct antioxidants and have the enzymatic activities of superoxide dismutase and catalase [21] suggesting that nanoceria supplementation would reduce the need for endogenous Sod1 and Sod2 expression. Nanoceria, because of their very small size, are not restricted to the compartments within the cells and they can even enter the nucleus [44] without restriction by normal barriers. Nrf2 is a central gene for up-regulation of multiple antioxidant defense associated genes including Sod3, Ercc6, Prdx6, Txnrd2, Nqo1, etc [31]. Here, our data demonstrate that Nrf2 expression is increased by nanoceria and genes associated with antioxidants or protective functions were differentially modulated. These data, in agreement with the work of Dorrel and colleges [11], suggest a direct link between oxidative stress, neovascularizations and photoreceptor apoptosis. Blockage of oxidative stress by nanoceria, inhibits neovascularizations and photoreceptor death in vldlr−/− mice.
Mitogen-activated protein kinase (MAPK) pathways are important intracellular signaling systems for the stress response. ASK1, a member of MAPK kinase kinase (MAP3K) family, is activated by many factors and oxidative stress is the most important activator to increase ASK1 activity [45–47]. ASK1 is identified as a kinase upstream of the stress response MAP kinases, p38 and JNKs [48]. It is essential to promote tumor necrosis factor α (TNF α) and oxidative stress-induced apoptosis [49] as well as VEGF-induced angiogenesis [34]. In addition, activated ASK1 also serves as an activator for transcription factor NF-κB [38], a central and key indicator of inflammatory responses and is involved in many cellular activities including apoptosis, stress response and angiogenesis [50]. Blockage of NF-κB inhibits angiogenesis in HUVECs, human NCI-H292 cells and mouse cornea [36, 37]. It had been shown that the expression of inflammatory factors, such as intercellular adhesion molecule-1 (ICAM-1), interleukin 18 (IL-18), and TNF α, was significantly greater in vldlr−/− mice at 3 weeks [13] and 2 months of age [10] suggesting that chronic inflammation and leukostasis are involved in the pathology in the retina and RPE, and contribute to VEGF expression and pathological neovascularizations [10, 13]. Furthermore, NF-κB [10] or MAPK/Akt/NF-κB signaling cascades [13] in vldlr−/− eyes were activated by inflammatory response factors. ASK1 knockout mice exhibit reduced protein levels of VEGF and MCP-1 and attenuate collateral vessels and angiogenesis [34]. Phosphorylation of JNK plays an important role in hypoxia-induced retinal VEGF production and pathological angiogenesis [51] whereas activation of p38 and JNKs was correlated with VEGF stimulation in HUVECs [35]. Inhibiting phosphorylation of p38 by crocetin suppresses VEGF-induced angiogenesis [52]. Our current study shows increased levels of VEGF and GFAP (confirming our previous study at 4 weeks of age [13]), and also demonstrates the up-regulation of ASK1, P38, JNK and NF-κB at P70 in vldlr−/− mice. These effects were reversed by nanoceria treatment.
Oxidative stress, a common node between cause and effect for many diseases, induces multiple kinases and transcription factors in a variety of signaling pathways which in turn promote angiogenesis and apoptosis [46]. We had shown that nanoceria can up-regulate phase II enzymes (a cellular defense network including thioredoxin (Trx), Trx reductase (Txnrd) and NADPH) by increasing Nrf2 expression [26]. Trx has a catalytic antioxidant property because its redox-active center (Cys-Gly-Pro-Cys) can reduce oxidized cysteine groups on proteins by scavenging ROS [53]. Trx is identified as an ASK1-interaction protein as it directly binds to the N-terminus of ASK1 and suppresses ASK1 activity [54]. Under oxidative stress conditions, oxidized Trx is dissociated from ASK1 [54] which switches ASK1 state from inactive to active and generates a phosphorylation-dependent signal. Similarly, nanoceria, by forming oxygen defects on their surface, can catalytically and regeneratively destroy ROS by switching their valance states between +3 (fully reduced) and +4 (fully oxidized). Therefore it is most likely that nanoceria maintain ASK1 in an inactive state by reducing Trx and enhancing its binding of ASK1 thereby preventing its activation of downstream components. Our histological observations of the retinas at P49 and P70 strongly suggest that photoreceptor loss is tightly linked with the development of pathological retinal and choroidal neovascularizations in vldlr−/− mice. So we think that oxidative stress promotes angiogenesis and apoptosis by induction of increased VEGF expression and via up-regulation of the ASK1-P38/JNK-NF-κB signaling pathway. Nanoceria, by changing their redox state, down-regulate phosphorylation of ASK1, P38/JNK, inhibit the activation of NF-κB and reduce VEGF, which prevents angiogenesis and apoptosis.
Conclusion
Our data demonstrated that a single intravitreal injection of catalytic nanoceria in young adult vldlr−/− mice, can modulate oxidative stress-associated gene expression, up regulate the expression of photoreceptor-specific genes, down regulate caspase 3 expression, regress existing neovascularization and prevent pathologic vascular leakage. These effects are achieved by down regulation of the ASK1-P38/JNK-NF-κB signaling pathway. On a more global basis, our data strongly suggest that nanoceria would be effective therapeutic agents for the treatment of human eye diseases involving oxidative stress and ROS, including AMD, Diabetic Retinopathy (DR), Retinopathy of Prematurity (ROP), macular edema and inherited retinal degeneration.
Supplementary Material
Supplemental Figure 1. Nanoceria regress developed neovascularizations and prevent blood vessel leakage. Uninjected vldlr−/− eyes at P70 exhibit numerous choroidal neovascular “tufts” and intraretinal neovascular “blebs” compared to wt which have no fluorescein labeling in the choroid and have well organized retinal blood vessels. Nanoceria treatment markedly reduced the number of “tufts” and “blebs”.
Supplemental Figure 2. Nanoceria prevent “neo” blood vessel leakage. Fundoscopy at P70 revealed that the fundus of untreated vldlr−/− mice exhibit numerous neovascularizations (A) which were showed as fluorescent spots under fluorescent angiography (B, C and D). The uninjected mice exhibit severe blood vessel leakage. Saline injection did not prevent the leakage. However nanoceria treatment greatly reduced neovascularizations and illicit blood vessel leakage.
Acknowledgements
We thank the personnel in the NEI/DMEI Cellular Imaging Core Facility and the Live Animal Imaging Facility for their assistance. We also thank Dr. Soumen Das (University of Central Florida), Lijuan Chen, Jiali Dong and Steven A. Sezate (University of Oklahoma Health Sciences Center) for technical assistance. This work was supported by NIH NEI grant COBRE-P20 RR017703, P30-EY 12190, R21EY018306, R01EY18724, R01EY0221; National Science Foundation: CBET-0708172.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
Xue Cai: None.
Potential confliction of interests: An US patent (7347987) was issued to the University of Central Florida and University of Oklahoma Health Sciences Center with JFM and SS listed as inventors. JFM is a cofounder of Nantiox, a startup company licensed to use nanoceria.
References
- 1.Bird AC. Age-related macular disease. Br J Ophthalmol. 1996;80:2–3. doi: 10.1136/bjo.80.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest. 2010;120:3033–3041. doi: 10.1172/JCI42437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heckenlively JR, Hawes NL, Friedlander M, Nusinowitz S, Hurd R, Davisson M, et al. Mouse model of subretinal neovascularization with choroidal anastomosis. Retina. 2003;23:518–522. doi: 10.1097/00006982-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Hu W, Jiang A, Liang J, Meng H, Chang B, Gao H, et al. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model's retinal angiomatous proliferation. Invest Ophthalmol Vis Sci. 2008;49:407–415. doi: 10.1167/iovs.07-0870. [DOI] [PubMed] [Google Scholar]
- 5.Frykman PK, Brown MS, Yamamoto T, Goldstein JL, Herz J. Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1995;92:8453–8457. doi: 10.1073/pnas.92.18.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen JK, Dong A, Hackett SF, Bell WR, Green WR, Campochiaro PA. Oxidative damage in age-related macular degeneration. Histol Histopathol. 2007;22:1301–1308. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- 7.Cai X, McGinnis JF. Oxidative stress: the achilles' heel of neurodegenerative diseases of the retina. Front Biosci. 2012;17:1976–1995. doi: 10.2741/4033. [DOI] [PubMed] [Google Scholar]
- 8.Dong A, Xie B, Shen J, Yoshida T, Yokoi K, Hackett SF, et al. Oxidative stress promotes ocular neovascularization. J Cell Physiol. 2009;219:544–552. doi: 10.1002/jcp.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salminen A, Kauppinen A, Hyttinen JM, Toropainen E, Kaarniranta K. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010;16:535–542. doi: 10.2119/molmed.2010.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Hu Y, Moiseyev G, Zhou KK, Chen D, Ma JX. Photoreceptor degeneration and retinal inflammation induced by very low-density lipoprotein receptor deficiency. Microvasc Res. 2009;78:119–127. doi: 10.1016/j.mvr.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorrell MI, Aguilar E, Jacobson R, Yanes O, Gariano R, Heckenlively J, et al. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J Clin Invest. 2009;119:611–623. doi: 10.1172/JCI35977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Hu Y, Lu K, Flannery JG, Ma JX. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J Biol Chem. 2007;282:34420–34428. doi: 10.1074/jbc.M611289200. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Huang Z, Kingsley R, Zhou X, Li F, Parke DW, 2nd, et al. Biochemical alterations in the retinas of very low-density lipoprotein receptor knockout mice: an animal model of retinal angiomatous proliferation. Arch Ophthalmol. 2007;125:795–803. doi: 10.1001/archopht.125.6.795. [DOI] [PubMed] [Google Scholar]
- 14.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Wong LL, Karakoti AS, Seal S, McGinnis JF. Nanoceria inhibit the development and promote the regression of pathologic retinal neovascularization in the Vldlr knockout mouse. PLoS One. 2011;6:e16733. doi: 10.1371/journal.pone.0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovach JL, Schwartz SG, Flynn HW, Jr, Scott IU. Anti-VEGF treatment strategies for wet AMD. J Ophthalmol. 2012;2012:786870. doi: 10.1155/2012/786870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarthy U, Evans J, Rosenfeld PJ. Age related macular degeneration. BMJ. 2010;340:c981. doi: 10.1136/bmj.c981. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Patil S, Seal S, McGinnis JF. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 19.Perez JM, Asati A, Nath S, Kaittanis C. Synthesis of biocompatible dextran-coated nanoceria with pH-dependent antioxidant properties. Small. 2008;4:552–556. doi: 10.1002/smll.200700824. [DOI] [PubMed] [Google Scholar]
- 20.Vincent A, Inerbaev TM, Babu S, Karakoti AS, Self WT, Masunov AE, et al. Tuning hydrated nanoceria surfaces: experimental/theoretical investigations of ion exchange and implications in organic and inorganic interactions. Langmuir. 2010;26:7188–7198. doi: 10.1021/la904285g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heckert EG, Karakoti AS, Seal S, Self WT. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials. 2008;29:2705–2709. doi: 10.1016/j.biomaterials.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S, Singh S, Dowding JM, Oommen S, Kumar A, Sayle TX, et al. The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomaterials. 2012;33:7746–7755. doi: 10.1016/j.biomaterials.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsunekawa S, Sahara R, Kawazoe Y, Ishikawa K. Lattice relaxation of manosize CeO2-X nanocrystalline particles. Appl Surf Sci. 1999;152:53–56. [Google Scholar]
- 24.Karakoti AS, Singh S, Kumar A, Malinska M, Kuchibhatla SV, Wozniak K, et al. PEGylated nanoceria as radical scavenger with tunable redox chemistry. J Am Chem Soc. 2009;131:14144–14145. doi: 10.1021/ja9051087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong LL, Hirst SM, Pye QN, Reilly CM, Seal S, McGinnis JF. Catalytic nanoceria are preferentially retained in the rat retina and are not cytotoxic after intravitreal injection. PLoS One. 2013;8:e58431. doi: 10.1371/journal.pone.0058431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong L, Cai X, Zhou X, Wong LL, Karakoti AS, Seal S, et al. Nanoceria extend photoreceptor cell lifespan in tubby mice by modulation of apoptosis/survival signaling pathways. Neurobiol Dis. 2011;42:514–523. doi: 10.1016/j.nbd.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai X, Sezate SA, Seal S, McGinnis JF. Sustained protection against photoreceptor degeneration in tubby mice by intravitreal injection of nanoceria. Biomaterials. 2012;33:8771–8781. doi: 10.1016/j.biomaterials.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyosseva SV, Chen L, Seal S, McGinnis JF. Nanoceria inhibit expression of genes associated with inflammation and angiogenesis in the retina of Vldlr null mice. Exp Eye Res. 2013;116C:63–74. doi: 10.1016/j.exer.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connolly SE, Hores TA, Smith LE, D'Amore PA. Characterization of vascular development in the mouse retina. Microvasc Res. 1988;36:275–290. doi: 10.1016/0026-2862(88)90028-3. [DOI] [PubMed] [Google Scholar]
- 31.Taylor RC, Acquaah-Mensah G, Singhal M, Malhotra D, Biswal S. Network inference algorithms elucidate Nrf2 regulation of mouse lung oxidative stress. PLoS Comput Biol. 2008;4:e1000166. doi: 10.1371/journal.pcbi.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai Y, Ma JX, Guo J, Wang J, Zhu M, Chen Y, et al. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009;219:446–454. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 33.Aartsen WM, van Cleef KW, Pellissier LP, Hoek RM, Vos RM, Blits B, et al. GFAP-driven GFP expression in activated mouse Muller glial cells aligning retinal blood vessels following intravitreal injection of AAV2/6 vectors. PLoS One. 2010;5:e12387. doi: 10.1371/journal.pone.0012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izumi Y, Kim-Mitsuyama S, Yoshiyama M, Omura T, Shiota M, Matsuzawa A, et al. Important role of apoptosis signal-regulating kinase 1 in ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2005;25:1877–1883. doi: 10.1161/01.ATV.0000174801.76234.bd. [DOI] [PubMed] [Google Scholar]
- 35.Wu G, Luo J, Rana JS, Laham R, Sellke FW, Li J. Involvement of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in vascular endothelial cells. Cardiovasc Res. 2006;69:512–519. doi: 10.1016/j.cardiores.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Watari K, Nakamura M, Fukunaga Y, Furuno A, Shibata T, Kawahara A, et al. The antitumor effect of a novel angiogenesis inhibitor (an octahydronaphthalene derivative) targeting both VEGF receptor and NF-kappaB pathway. Int J Cancer. 2012;131:310–321. doi: 10.1002/ijc.26356. [DOI] [PubMed] [Google Scholar]
- 37.Furuno A, Watari K, Nakamura M, Fukunaga Y, Jung JH, Ono M. A natural anti-inflammatory enone fatty acid inhibits angiogenesis by attenuating nuclear factor-kappaB signaling in vascular endothelial cells. Int J Oncol. 2011;38:493–501. doi: 10.3892/ijo.2010.856. [DOI] [PubMed] [Google Scholar]
- 38.Hirotani S, Otsu K, Nishida K, Higuchi Y, Morita T, Nakayama H, et al. Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation. 2002;105:509–515. doi: 10.1161/hc0402.102863. [DOI] [PubMed] [Google Scholar]
- 39.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev. 1997;13:37–50. doi: 10.1002/(sici)1099-0895(199703)13:1<37::aid-dmr174>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 41.Lu L, Oveson BC, Jo YJ, Lauer TW, Usui S, Komeima K, et al. Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxid Redox Signal. 2009;11:715–724. doi: 10.1089/ars.2008.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funato Y, Miki H. Redox regulation of Wnt signalling via nucleoredoxin. Free Radic Res. 2010;44:379–388. doi: 10.3109/10715761003610745. [DOI] [PubMed] [Google Scholar]
- 43.Funato Y, Terabayashi T, Sakamoto R, Okuzaki D, Ichise H, Nojima H, et al. Nucleoredoxin sustains Wnt/beta-catenin signaling by retaining a pool of inactive dishevelled protein. Curr Biol. 2010;20:1945–1952. doi: 10.1016/j.cub.2010.09.065. [DOI] [PubMed] [Google Scholar]
- 44.Singh S, Kumar A, Karakoti A, Seal S, Self WT. Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol Biosyst. 2010;6:1813–1820. doi: 10.1039/c0mb00014k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Homma K, Katagiri K, Nishitoh H, Ichijo H. Targeting ASK1 in ER stress-related neurodegenerative diseases. Expert Opin Ther Targets. 2009;13:653–664. doi: 10.1517/14728220902980249. [DOI] [PubMed] [Google Scholar]
- 46.Nagai H, Noguchi T, Takeda K, Ichijo H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J Biochem Mol Biol. 2007;40:1–6. doi: 10.5483/bmbrep.2007.40.1.001. [DOI] [PubMed] [Google Scholar]
- 47.Hattori K, Naguro I, Runchel C, Ichijo H. The roles of ASK family proteins in stress responses and diseases. Cell Commun Signal. 2009;7:9. doi: 10.1186/1478-811X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 49.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lan W, Petznick A, Heryati S, Rifada M, Tong L. Nuclear Factor-kappaB: central regulator in ocular surface inflammation and diseases. Ocul Surf. 2012;10:137–148. doi: 10.1016/j.jtos.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Guma M, Rius J, Duong-Polk KX, Haddad GG, Lindsey JD, Karin M. Genetic and pharmacological inhibition of JNK ameliorates hypoxia-induced retinopathy through interference with VEGF expression. Proc Natl Acad Sci U S A. 2009;106:8760–8765. doi: 10.1073/pnas.0902659106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Umigai N, Tanaka J, Tsuruma K, Shimazawa M, Hara H. Crocetin, a carotenoid derivative, inhibits VEGF-induced angiogenesis via suppression of p38 phosphorylation. Curr Neurovasc Res. 2012;9:102–109. doi: 10.2174/156720212800410830. [DOI] [PubMed] [Google Scholar]
- 53.Collet JF, Messens J. Structure, function, and mechanism of thioredoxin proteins. Antioxid Redox Signal. 2010;13:1205–1216. doi: 10.1089/ars.2010.3114. [DOI] [PubMed] [Google Scholar]
- 54.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Nanoceria regress developed neovascularizations and prevent blood vessel leakage. Uninjected vldlr−/− eyes at P70 exhibit numerous choroidal neovascular “tufts” and intraretinal neovascular “blebs” compared to wt which have no fluorescein labeling in the choroid and have well organized retinal blood vessels. Nanoceria treatment markedly reduced the number of “tufts” and “blebs”.
Supplemental Figure 2. Nanoceria prevent “neo” blood vessel leakage. Fundoscopy at P70 revealed that the fundus of untreated vldlr−/− mice exhibit numerous neovascularizations (A) which were showed as fluorescent spots under fluorescent angiography (B, C and D). The uninjected mice exhibit severe blood vessel leakage. Saline injection did not prevent the leakage. However nanoceria treatment greatly reduced neovascularizations and illicit blood vessel leakage.