Abstract
The nuclear transcription factor peroxisome proliferator-activated receptor-γ (PPARγ) is a key regulator of the inflammatory response to an array of biologic insults. We have previously demonstrated that PPARγ ligands reduce myocardial ischemia-reperfusion injury in rodents. In the current study, we directly determined the role of cardiomyocyte PPARγ in ischemia-reperfusion injury, employing a model of conditional cardiomyocyte-specific deletion of PPARγ in vivo. In mice, α-myosin heavy chain-restricted Cre-mediated PPARγ deficiency was induced by tamoxifen treatment (30 mg/kg intraperitoneally) for 4 days (PPARγ−/− mice); whereas controls included mice treated with the oil diluent vehicle (PPARγ+/+ mice). Western blot and histochemical analyses confirmed that expression of PPARγ protein was abolished in cardiomyocytes of mice treated with tamoxifen, but not with vehicle. After tamoxifen or vehicle treatment, animals were subjected to 30 min ligation of the left anterior descending coronary artery followed by 2 hrs reperfusion. In PPARγ−/− mice, myocardial ischemia and reperfusion induced extensive myocardial damage, which was associated with elevated tissue activity of myeloperoxidase, indicating infiltration of neutrophils, and elevated plasma levels of troponin-I when compared to PPARγ+/+ mice. PPARγ−/− mice also demonstrated ventricular dilatation and systolic dysfunction upon echocardiographic analysis. Plasma levels of the pro-inflammatory cytokines interleukin-1β and interleukin-6 were higher in PPARγ−/− mice when compared to PPARγ+/+ mice. These pathological events in PPARγ−/− mice were associated with enhanced nuclear factor-κB DNA binding in the infarcted hearts. Thus, our data suggests that cardiomyocyte PPARγ is a crucial protective receptor and may prevent reperfusion injury by modulating mechanisms of inflammation.
Keywords: Cre-loxP system, PPARγ, NF-κB, cardiomyocytes, neutrophils, IL-6, IL-1β
INTRODUCTION
Coronary artery disease and myocardial infarction remain a leading cause of morbidity and mortality in the United States (1). Modern therapies such as thrombolytic medications and coronary angioplasty reverse myocardial ischemia and restore coronary blood flow. However, concurrent with the restoration of blood flow and re-oxygenation, ischemic myocardial tissue may paradoxically suffer reperfusion injury. Myocardial ischemia and reperfusion injury results in further myocyte death and worsening myocardial dysfunction beyond that expected from the ischemic insult alone. Although the mechanisms underlying the phenomenon of ischemia and reperfusion injury have not been precisely defined, toxicity by reactive oxygen free radicals and oxidants, leukocyte-endothelial cell adhesion and a marked inflammatory reaction have been implicated in the process of injury (2, 3).
Peroxisome proliferator-activated receptor-γ (PPARγ) is a member of the superfamily of ligand-activated nuclear receptors. Upon activation, PPARγ binds to the retinoid X receptor, and this heterodimer subsequently serves as a transcription factor for the promoter regions of various target genes (4). PPARγ is expressed most prominently in adipose tissue, but is also seen in endothelium, vascular smooth muscle, monocytes, macrophages and cardiac myocytes (5). Functionally, PPARγ has a prominent role in several biologic processes, including lipid and glucose homeostasis, modulation of cellular proliferation, and regulation of several inflammatory disorders (4-6).
With the use of specific ligands for the receptor, we have previously indicated a role for PPARγ in regulating myocardial damage by governing the inflammatory process, which incites reperfusion injury at the nuclear level (7). In particular, in a rodent model of myocardial ischemia and reperfusion injury, treatment with the endogenous PPARγ ligand 15-deoxy-Δ12,14-prostaglandin J2 and the thiazolidinedione ciglitazone reduced myocardial damage, neutrophil infiltration and systemic cytokine production via enhanced PPARγ DNA binding and reduced activation of nuclear factor-κB (NF-κB) (7), an important transcription factor for pro-inflammatory genes (8). Other experimental studies have supported that treatment with thiazolidinedione PPARγ ligands reduce myocardial infarct size (9) and improve cardiac function following an acute myocardial ischemic insult (10-12).
Given the potential benefit for PPARγ ligands to attenuate myocardial ischemia and reperfusion injury, further investigation is merited to determine the exact biologic site of action of PPARγ within the heart. As PPARγ is expressed within cardiac myocytes as well as coronary endothelium and vascular smooth muscle (4-6), we sought to investigate the role of the cardiomyocyte PPARγ on myocardial ischemia and reperfusion injury by employing a conditional cardiomyocyte-specific PPARγ knockout animal model. Here, we provide evidence that cardiomyocyte PPARγ plays a prominent role in protecting the myocardium against the ischemia and reperfusion injury. This endogenous cardio-protective effect of PPARγ appears a result of nuclear regulation of NF-κB activation.
MATERIALS AND METHODS
Generation of cardiomyocyte-specific PPARγ knockout mice
The investigation conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee. Mice expressing a tamoxifen-inducible Cre recombinase fused to mutant estrogen-receptor ligand-binding domains (MerCreMer) under the control of the α-myosin heavy chain promoter (B6.Cg-Tg(Myh6-cre/Esr1)1Jmk/J) (13) and PPARγloxP mice (B6.129-Ppargtm2Rev/J), both on C57BL6 genetic background, were obtained from Jackson Laboratories (Bar Harbor, Maine). MerCreMer mice were crossed with PPARγloxP mice thus generating cardiac-specific inducible PPARγCre/loxP animals. At 8-12 weeks of age, male PPARγCre/loxP animals were given tamoxifen (30 mg/kg daily) intraperitoneally (ip) for four days in order to generate cardiomyocyte PPARγ knockout (PPARγ−/−) mice. Tamoxifen was dissolved in peanut oil at a concentration of 2.5 mg/ml. PPARγCre/loxP mice treated with oil alone served as controls (PPARγ+/+ mice).
Myocardial ischemia and reperfusion
Myocardial ischemia and reperfusion was conducted as previously described (7). Twenty-four hrs after the last treatment with tamoxifen or oil PPARγ+/+ and PPARγ−/− mice were anesthetized with thiopentone sodium (4 mg/ml, 10 μl/g body weight ip). A tracheostomy was performed to provide mechanical ventilation. The chest was opened via a left thoracotomy incision in order to expose the left ventricle. The left anterior descending (LAD) coronary artery was occluded for 30 minutes by ligation with a 6.0 silk suture passed underneath the LAD and subsequently anchored over a 3-mm air balloon, which was placed on top of the vessel. Reperfusion was allowed for 2 hrs following deflation of the balloon. Animals were euthanized at the end of the reperfusion period by a lethal dose of thiopentone sodium. Plasma samples and the left ventricles were collected for subsequent histological and biochemical studies. A separate group of mice underwent the above procedure without LAD ligation, thus serving as the sham control group.
Echocardiographic assessment of left ventricle structure and function
Cardiac function was assessed by echocardiography as previously described using a VisualSonics 2100 system equipped with a 30 MHz transducer (14). Left ventricle (LV) internal dimensions, including end-diastolic and end-systolic dimensions (LVIDd and LVIDs, respectively), interventricular septal thickness in diastole and systole (IVSd and IVSs, respectively) and LV posterior wall thickness in diastole and systole (LVPWd and LVPWs, respectively) were measured directly. Echocardiographic measurements were obtained before LAD ligation (baseline measurements, n=11-16 mice for each group) and at the end of the reperfusion period (n=3 mice for each group).
Histopathological analysis and immunohistochemical staining for PPARγ
Tissues were fixed in 4% paraformaldehyde and embedded in paraffin. For histological evaluation, sections were stained with hematoxylin and eosin. For immunohistochemistry of PPARγ expression, binding sites of PPARγ primary antibody were visualized with an avidin-biotin peroxidase complex immunoperoxidase technique using diaminobenzidine as recommended by the protocol provided by the manufacturer (Vector Laboratories, Burlingame, CA).
Plasma cardiac troponin activity
Plasma levels of troponin-I were evaluated as an index of cardiac cellular damage using a high sensitivity mouse cardiac troponin-I ELISA kit (Life Diagnostics, Inc., West Chester, PA).
Myeloperoxidase activity
Myeloperoxidase (MPO) activity was determined as a marker of neutrophil migration into myocardial tissue following ischemia-reperfusion. Cardiac tissues were homogenized in a solution containing 0.5% hexa-decyl-trimethyl-ammonium bromide in 10 mM potassium phosphate buffer (pH 7) and centrifuged for 30 min at 4,000 g at 4° C. An aliquot of the supernatant was allowed to react with a solution of tetra-methyl-benzidine (1.6 mM) and 0.1 mM hydrogen peroxide. The rate of change in absorbance was measured by spectrophotometry at 650 nm. A unit of MPO activity was defined as the quantity of enzyme that degraded 1 μmol of hydrogen peroxide per min at 37° C per 100 mg weight of tissue.
Plasma levels of cytokines
Plasma levels of interleukin (IL)-1β, IL-6, IL-10, keratinocyte-derived chemokine (KC), macrophage inflammatory protein-1α (MIP-1α) and tumor necrosis factor-α (TNF α) were evaluated by a commercially available Milliplex mouse cytokine magnetic bead panel kit (Millipore Corporation, Billerica, MA) using the protocols recommended by the manufacturer.
Subcellular fractionation and nuclear protein extraction
Heart samples were homogenized in a buffer containing 0.32 M sucrose, 10 mM tris-HCl pH 7.4, 1 mM EGTA, 2 mM EDTA, 5 mM sodium azide, 10 mM β-mercaptoethanol, 20 μM leupeptin, 0.15 μM pepstatin A, 0.2 mM phenylmethanesulfonyl fluoride, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 0.4 nM microcystin. The homogenates were centrifuged (1,000 g, 10 minutes). The pellets were solubilized in Triton buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl, pH 7.4, 1 mM EGTA, 1 mM EDTA, 0.2 mM sodium orthovanadate, 20 μM leupeptin A, 0.2 mM phenylmethanesulfonyl fluoride). The lysates were centrifuged (15,000 g, 30 minutes, 4° C), and the supernatant (nuclear extract) was collected for evaluation of content of PPARγ and DNA binding of NF-κB.
Western blot analysis
Nuclear expression of PPARγ was determined by immunoblot analysis. Nuclear extracts were boiled in loading buffer (125 mM Tris-HCl pH 6.8, 4% sodium dodecyl sulfate, 20% glycerol, and 10% 2-mercaptoethanol) and 35 μg of protein were loaded per lane on a 10% Tris-glycine gradient gel. Proteins were separated electrophoretically and transferred to nitrocellulose membranes. For immunoblotting, membranes were blocked with 5% nonfat dried milk in Tris-buffered saline for 1 h and then incubated with primary antibodies against PPARγ for 1 h. The membranes were washed in Tris-buffered saline with 0.1% Tween 20 and incubated with secondary peroxidase-conjugated antibody. Immunoreaction was visualized by chemiluminescence. Densitometric analysis was performed using ImageQuant (Molecular Dynamics).
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was performed using an oligonucleotide probe corresponding to NF-κB consensus sequence (5’-AGT TGA GGG GAC TTT CCC AGG C-3’) as previously described (9). The oligonucleotide probe was labeled with γ-(32P)ATP using T4 polynucleotide kinase and purified in Bio-Spin chromatography columns (BioRad, Hercules, CA). Twenty-five micrograms of nuclear protein was incubated with EMSA buffer (12 mM N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid pH 7.9, 4 mM Tris-HCl pH 7.9, 25 mM potassium chloride, 5 mM magnesium chloride, 1 mM EDTA, 1 mM dithiothreitol, 50 ng/ml poly (d[I-C]), 12% glycerol vol/vol, and 0.2 mM phenylmethanesulfonyl fluoride) and radiolabeled oligonucleotide. The specificity of the binding reactions was determined by co-incubating duplicate nuclear extract samples with unlabeled oligonucleotide (competitor assay). Protein-nucleic acid complexes were then resolved using a non-denaturing polyacrylamide gel and run in 0.5X Tris-HCl (45 mM), boric acid (45 mM), and EDTA (1mM) for 1 h at constant current (30 mA). Gels were then transferred to Whatman 3M paper, dried under a vacuum at 80° C for 1 h, and exposed to photographic film at −70° C with an intensifying screen. Densitometric analysis was performed using ImageQuant (Molecular Dynamics).
Materials
The primary antibody directed to PPARγ was obtained from Thermo Scientific (Rockford, IL). The oligonucleotide for NF-κB was obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). All other chemicals were obtained from Sigma/Aldrich (St. Louis, MO).
Statistical analysis
All values in the figures and text are expressed as mean ± SEM of n observations (n = 3-16 animals for each group). The results were examined by one-way analysis of variance followed by the Bonferroni’s correction post hoc t test. Data were analyzed using Systat software (Sigma Plot 12.3). A P value of < 0.05 was considered significant.
RESULTS
Conditional deletion of cardiomyocyte PPARγ by tamoxifen treatment
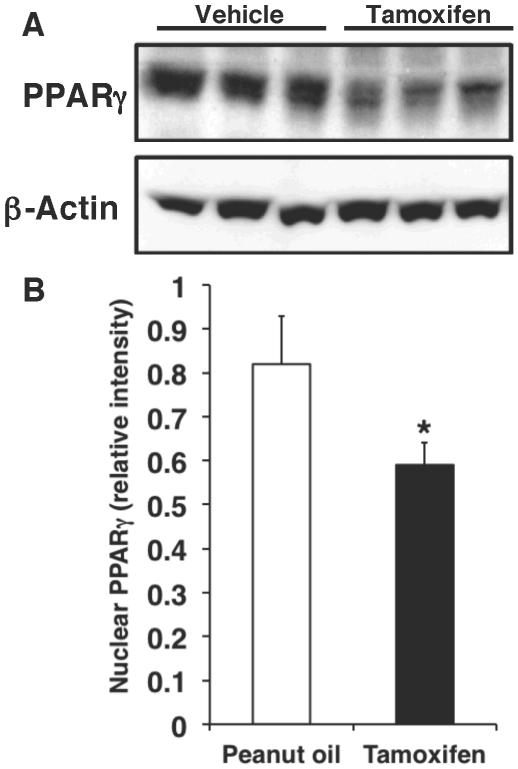
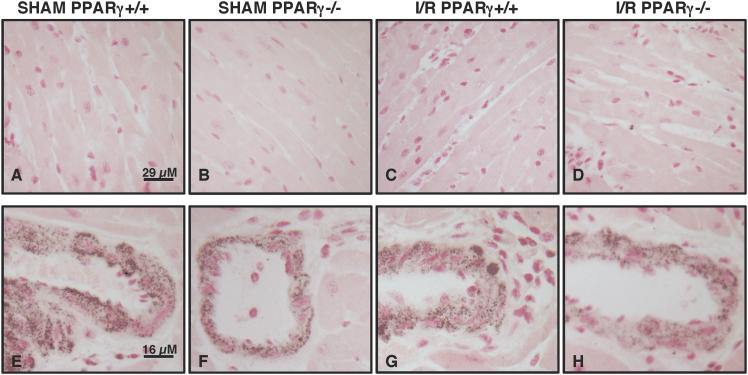
PPARγ conditional knockout mice were generated using the Cre-loxP system. PPARγCre/loxP animals were given tamoxifen (30 mg/kg, ip) or vehicle for four days in order to delete cardiomyocyte PPARγ. Western blot analysis revealed that PPARγ expression was markedly decreased in the heart samples of mice treated with tamoxifen (PPARγ−/− mice) when compared to control animals receiving vehicle alone (PPAR+/+ mice) (Figure 1). To confirm cell location of PPARγ deletion, we further performed immunohistochemical staining specific for PPARγ. At immunohistochemical evaluation cardiac sections of PPARγ+/+ mice treated with oil only for four days showed a dark staining in the nuclear compartment of cardiac cells and in vessels under sham conditions and after ischemia and reperfusion (Figure 2). Staining for PPARγ was absent in the myocytes of PPARγ−/− animals who received four days of tamoxifen therapy. Of note, PPARγ expression was maintained in the vasculature of these animals, thus confirming the cardiomyocyte specificity of the gene deletion (Figure 2).
FIG. 1. Nuclear PPARγ expression in cardiac samples.
(A) Representative Western blot of PPARγ in nuclear extracts form hearts of PPARγCre/loxP mice receiving tamoxifen (30 mg/kg daily, ip) or vehicle (peanut oil) for four days. Expression of β-actin was used as loading control protein. (B) Quantitative analysis of PPARγ expression determined by densitometry. Relative intensity was normalized to β-actin expression. *Represents P < 0.05 versus vehicle treatment.
FIG. 2. Immunohistochemistry of PPARγ in cardiac sections of PPARγ+/+ and PPARγ−/− mice subjected to sham procedure or myocardial ischemia and reperfusion (I/R).
Immunohistochemistry for PPARγ showed positive staining in PPARγ+/+ mice subjected to sham procedure or myocardial I/R both in cardiomyocytes (A and C) and endothelial cells (E and G). There was no staining for PPARγ in cardiomyocytes of PPARγ−/− mice subjected to sham procedure or myocardial I/R (B and D). Positive staining for PPARγ was observed in endothelial cells of PPARγ−/− mice subjected to sham procedure or myocardial I/R (F and H). Figure is representative of at least four different experiments.
Conditional deletion of cardiomyocyte PPARγ enhances cardiac dysfunction and tissue injury following myocardial ischemia and reperfusion
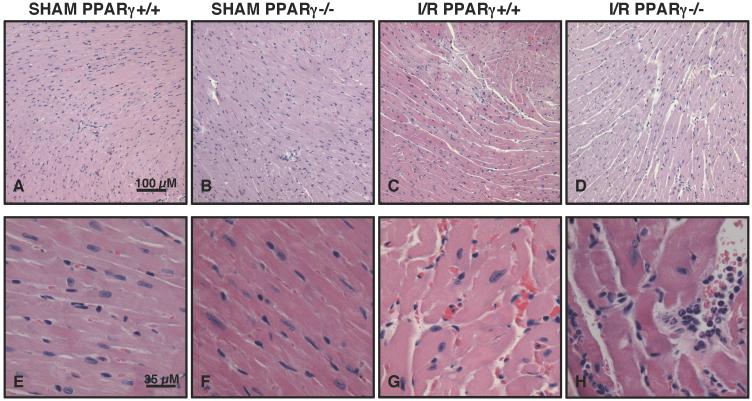
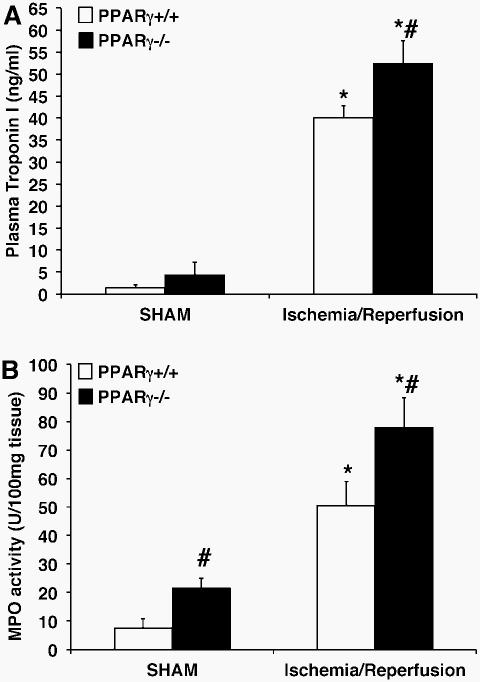
PPARγ−/− animals undergoing ischemia of the LAD coronary artery followed by reperfusion showed significant myocardial tissue injury, which was characterized by areas of interstitial edema, development of contracture bands, hemorrhage, margination of neutrophils along vessel walls and tissue infiltration of inflammatory cells, when compared to milder pathological signs of hemorrhage and edema of PPARγ+/+ mice (Figure 3). The severe histologic injury in knockout animals was paralleled by a rise in plasma cardiac-specific troponin-I (Figure 4A). Two hrs following reperfusion, plasma levels of troponin-I in PPARγ−/− animals (52.5 ± 5.1 ng/ml) were significantly elevated in comparison to those of PPARγ+/+ mice (40.1 ± 2.8 ng/ml; P < 0.05). By echocardiography analysis, mice of both genotypes exhibited a significant decrease in systolic function as evidenced by lower fractional shortening and ejection fraction after ischemia and reperfusion. However, PPARγ−/− mice had a more severe change when compared to baseline values. Furthermore, PPARγ−/− mice, but not wild-type mice, exhibited a significant increase in the left ventricle internal dimension both at diastole and systole when compared to baseline measurements, suggesting the occurrence of left ventricle dilatation most probably due to impairment of cardiac contractility (Table 1). Interestingly, the PPARγ−/− mice demonstrated decreased systolic tickening of the interventricular septum after ischemia and reperfusion. However, thickening of the LV posterior wall was not affected in these animals, thus suggesting that PPARγ deficiency renders the area at risk particularly vulnerable to reperfusion injury (Table 1).
FIG. 3. Histology of cardiac sections from PPARγ+/+ and PPARγ−/− mice subjected to sham procedure or myocardial ischemia and reperfusion (I/R).
Representative sections from sham PPARγ+/+ (A and E) and PPARγ−/− (B and F) mice revealed normal myocardial structure. After myocardial I/R areas of hemorrhage and extracellular edema were observed in cardiac sections of PPARγ+/+ mice (C and G). PPARγ−/− mice undergoing I/R exhibited a more severe damage characterized by disruption of myofibrils, larger vacuole of edema formation, neutrophil margination and infiltration (D and H). Figure is representative of at least four different experiments.
FIG. 4. Plasma levels of Troponin I (A) and cardiac myeloperoxidase activity (B) of PPARγ+/+ and PPARγ−/− mice subjected to sham procedure or myocardial ischemia and reperfusion.
Each data point represents the mean ± SEM of 4-8 animals for each group. *P < 0.05 versus sham mice of the same genotype; # P < 0.05 versus PPARγ+/+ mice.
Conditional deletion of cardiomyocyte PPARγ enhances neutrophil infiltration following ischemia and reperfusion
The activity of MPO, an enzyme specific to granulocytes, was used to quantify the degree of neutrophil infiltration into the myocardium following ischemia and reperfusion injury. MPO activity in the myocardial tissue of PPARγ−/− animals (77.95 ± 10.50 U/100 mg tissue) was significantly elevated compared to the PPARγ+/+ counterparts (50.60 ± 8.44 U/100 mg tissue, P < 0.05; Figure 4B).
Conditional deletion of cardiomyocyte PPARγ enhances levels of plasma pro-inflammatory cytokines
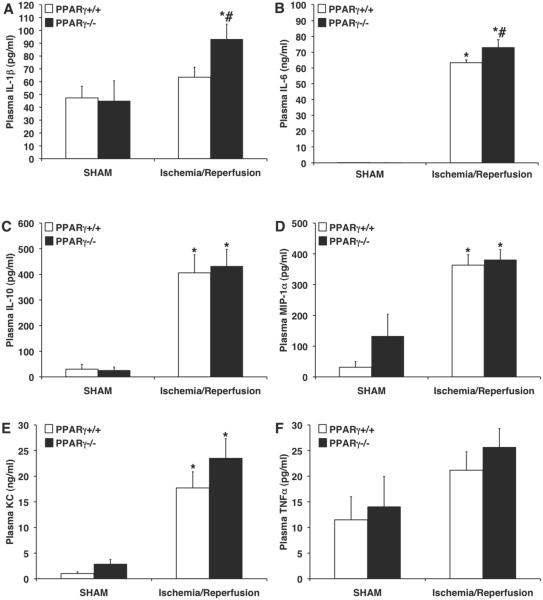
Plasma IL-1β levels of PPARγ−/− mice were significantly elevated at the end of reperfusion (93.1 ± 11.8 pg/ml) in comparison to PPARγ+/+ wild-type animals (63.5 ± 7.8 pg/ml, P < 0.05; Figure 5A). Similarly, PPARγ−/− mice had significantly higher plasma levels of IL-6 (72.9 ± 4.9 ng/ml) in comparison to PPARγ+/+ counterparts (63.3 ± 1.7 ng/ml, P < 0.05; Figure 5B). Interestingly, plasma levels of IL-10, MIP-1α, KC and TNF-α were not different between the two genotypes (Figure 5).
FIG. 5. Plasma levels of IL1-β (A), IL-6 (B), IL-10 (C), MIP-1α (D), KC (E) and TNF-α (F) of PPARγ+/+ and PPARγ−/− mice subjected to sham procedure or myocardial ischemia and reperfusion.
Each data point represents the mean ± SEM of 4-8 animals for each group. *P < 0.05 versus sham mice of the same genotype; # P < 0.05 versus PPARγ+/+ mice.
Conditional deletion of cardiomyocyte PPARγ enhances DNA binding of NF-κB
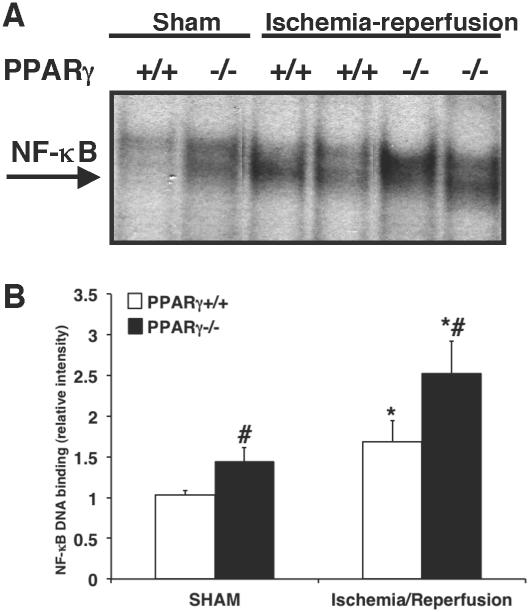
To investigate the mechanisms underlying the enhanced inflammatory response in the PPARγ−/− hearts we evaluated NF-κB activity. Cardiac DNA binding of NF-κB was significantly increased in PPARγ−/− animals undergoing sham procedure in comparison to PPARγ+/+ sham mice. After ischemia and reperfusion both genotypes experienced an elevation in NF-κB activity. However, the degree of NF-κB DNA binding was significantly higher in PPARγ−/− animals in comparison to PPARγ+/+ mice (Figure 6).
FIG. 6. DNA binding activity of NF-κB in cardiac samples of PPARγ+/+ and PPARγ−/− mice subjected to sham procedure or myocardial ischemia and reperfusion.
(A) Representative autoradiographs of electrophoretic mobility shift assay for NF-κB DNA binding in nuclear extracts form hearts of PPARγ+/+ and PPARγ−/− mice subjected to sham procedure or myocardial ischemia and reperfusion. (B) Quantitative analysis of NF-κB DNA binding determined by densitometry. Each data point represents the mean ± SEM of 4-7 animals for each group. *P < 0.05 versus sham mice of the same genotype; # P < 0.05 versus PPARγ+/+ mice.
DISCUSSION
The cardioprotective role of PPARγ ligands in myocardial ischemia-reperfusion injury has been well established (7, 9-12). While PPARγ is biologically active in many sites within the heart, the precise role of cardiomyocyte PPARγ during myocardial ischemia and reperfusion has not yet been elucidated. In the present study we demonstrated that cardiomyocyte-restricted PPARγ knockout enhanced myocardial ischemia and reperfusion injury. The underlying cellular mechanisms of this excessive vulnerability to injury related to increased neutrophil infiltration and increased production of IL-6 and IL-1β, most probably mediated by enhanced NF-κB activation.
Gene inactivation approaches have been used to investigate the functional role of PPARγ in the heart. These studies have demonstrated that PPARγ is clearly necessary for cellular development and metabolism. Studies using global homozygous PPARγ null mice have shown embryonic lethality with documented severe myocardial thinning (15). Conventional Cre-lox cardiac-specific PPARγ models have also demonstrated that mice with chronic cardiac PPARγ deficiency since early stages of embryonic development may survive to adulthood, but they develop age-dependent pathological changes, including cardiac hypertrophy and oxidative damage (16, 17). Thus, to avoid these potential pathological developmental confounds and other phenotypic differences, in our study we used a loss-of-function strategy of short-term deletion of PPARγ. Conditional knockout mice were generated using the Cre/LoxP system to allow for regional control of the deletion of the PPARγ gene through the Cre recombinase driven by the α-myosin heavy chain promoter. This Cre recombinase was fused to mutant estrogen-receptor ligand-binding domains (MerCreMer), which drives expression in cardiomyocytes even in adulthood after a short treatment with tamoxifen (13). Our data demonstrated that even an acute deficiency of cardiomyocyte-PPARγ rendered the heart more susceptible to reperfusion stress and caused severe damage, as demonstrated by histology assessment and high levels of plasma troponin I, the biomarker of infarct extension in humans and experimental animals (18-20). Importantly, the severe myocardial damage in PPARγ−/− mice translated into severe LV dysfunction and LV dilatation when compared to PPARγ+/+ mice as measured by echocardiography. It should be noted that other non-myocyte cell types, such as macrophages, endothelial cells, neutrophils, monocytes, and platelets contribute to the pathological processes of myocardial damage during ischemia and reperfusion (3). Our laboratory and others have previously demonstrated that PPARγ is also an important anti-inflammatory modulator in those cells (21-25). Our current study, therefore, suggest that PPARγ in myocytes constitutes an important requisite for regulating both the inflammatory response as well as the cardiac performance during early reperfusion injury. However, given the importance of PPARγ in myocardial metabolic homeostasis (4-6), it remains to be determined whether cardiomyocyte PPARγ deficiency may also impact the progressive process of cardiac remodeling and recovery.
Ischemia and reperfusion results in significant cardiac dysfunction and injury owing in part to the release of potent pro-inflammatory cytokines (2). The myocardium is critical for the production of several inflammatory cytokines during reperfusion injury as a mechanism to start tissue repair, but it also becomes a target of their cytotoxic effects (26). For example, in patients with myocardial infarction IL-6 appears to be synthesized in the myocardium during the ischemic period and its secretion is further triggered by the reperfusion process (27, 28). Increased IL-6 is associated with poor prognosis in patients with myocardial infarction or heart failure (29) and with life-threatening events, such as shock, decreased myocardial contractility and arrhythmias (30, 31). As with IL-6, IL-1β is another marker of severe cardiac injury after ischemia and reperfusion (26, 32). IL-1β is toxic for the cardiac myocyte and inhibition of its activity affords cardioprotective effects as demonstrated by studies in patients with myocardial infarction and animal models of ischemia and reperfusion (33-35). Additional production of inflammatory cytokines and chemokines takes place through endothelial dysfunction and direct recruitment of inflammatory cells to the site of injury, which amplifies the local inflammatory response (26). In experimental models of ischemia and reperfusion cardiac mast cells, macrophages and endothelial cells are main sources of TNFα (36), while IL-10 appears to be mainly secreted by T-lymphocytes (37). The chemokine KC (or the human homologue IL-8) is produced by activated neutrophils to mediate their intra-parenchymal migration (38-40). MIP-1α levels are elevated during acute coronary syndromes (41) and are produced by endothelial cells (42) as well as macrophages, dendritic cells, and lymphocytes (43). In our study, PPARγ−/− mice displayed a significant increase in plasma levels of IL-6 and IL-1β after myocardial ischemia and reperfusion when compared to PPARγ+/+ mice. Considering the important role of both IL-6 and IL-1β in myocardial ischemia and reperfusion, our study therefore suggests that the combined elevation of these cytokines can significantly contribute to tissue damage. Our hypothesis is supported by previous studies demonstrating that pro-inflammatory cytokines, including IL-6 and IL-1β, are secreted by cardiomyocytes during inflammation and act synergistically to cause cardiac contractile dysfunction (44). Interestingly, the expression of other established inflammatory markers, such as TNFα, KC, MIP-1α and IL-10 were not significantly different in PPARγ+/+ mice and cardiomyocyte-specific PPARγ−/− mice, in which the expression of PPARγ remains intact in the endothelium and inflammatory cells. Therefore, the differential effect on plasma cytokine levels between the two genotypes clearly reflects the different cell source in response to the ischemia and reperfusion challenge. Our findings, in fact, indicate that PPARγ activity in the myocardium is required for the regulation of IL-6 and IL-1β synthesis. Our data also indicate that endothelial cells, infiltrated neutrophils, macrophages and other immunocompetent cells can maintain a sustained production of other important inflammatory cytokines and chemokines including TNFα, KC, MIP-1α and IL-10. This differential cytokine response may also explain the enhanced leukosequestration in damaged tissue in cardiomyocyte-specific PPARγ−/− mice when compared to PPARγ+/+ mice. Neutrophils infiltrate into the myocardium following ischemia and reperfusion and are important players in the development of irreversible tissue injury. Among chemokine and cytokines, IL-6 and IL-1β appear important factors to drive migration of neutrophils into myocardium (45, 46). For example, it has been shown that IL-6 receptor inhibition suppressed infiltration of neutrophils, thereby reducing MPO activity in the infarct area and the border zone (45). Similarly, IL-1 receptor deficient mice exhibited markedly reduced neutrophil infiltration of the infarcted myocardium (46). Thus, our study suggests that cardiomyocyte PPARγ is an important contributor to the modulation of myocardial neutrophil infiltration through specific regulation of inflammatory cytokines.
As a nuclear transcription factor, PPARγ binds to recognition sites on PPAR response elements in the promoter region of target genes, many of which serve to maintain metabolic homeostasis (5). Similar to other nuclear receptors, PPARγ is also able to bind to co-repressors or other transcription factors and cause transrepression (6). Previously we have shown that the cardioprotective effects of PPARγ ligands are in part due to their ability to downregulate the pro-inflammatory transcription factor NF-κB (7), thus suggesting a mechanism of transrepression of the inflammatory response induced by reperfusion injury. In the present study, we further confirmed that PPARγ in cardiomyocytes is crucial for the negative regulation of NF-κB, as cardiac DNA binding of NF-κB was markedly enhanced in cardiomyocyte-specific PPARγ−/− mice when compared to PPARγ+/+ mice. The ability of the cardiomyocyte PPARγ to negatively regulate NF-κB activation may extend beyond the setting of myocardial ischemia-reperfusion. For example, rat cardiomyocytes pretreated with PPARγ ligands showed reduced NF-κB DNA binding when stimulated with lipopolysaccharide (47). In our study, there was also a modest but significant increase in DNA binding of NF-κB, which was associated with an increase in neutrophil infiltration under basal sham conditions in cardiomyocyte-specific PPARγ−/− mice when compared to PPARγ+/+ mice. Thus, our data also support that PPARγ plays a critical role in maintaining the physiological homeostasis of cell phenotype and suggest that even acute deficiency of PPARγ function may induce a pathological imbalance rendering cardiac tissue susceptible to sham surgical procedures, such as thoracotomy and balloon positioning.
In conclusion, by means of cell type-specific gene knockout technology, the current study provides conclusive data that PPARγ expression in cardiomyocytes functions as a protective factor against reperfusion injury. As the thiazolidinedione class of PPARγ ligands has remained under close scrutiny in the clinical arena due to a potential risk of adverse cardiovascular events (48-50), further insight into the exact biological mechanism and site of action of PPARγ within the cardiovascular system may help to provide better clarity as to the local function of PPARγ and the proper clinical use of these medications.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of General Medical Sciences under Award Number R01 GM067202 (to Dr. Basilia Zingarelli) and the National Institute of Aging under Award Number R01 AG027990 (to Dr. Basilia Zingarelli) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Source of Funding: Supported by the National Institutes of Health (grants R01 GM-067202 and R01 AG-27990 to Dr. Basilia Zingarelli).
Footnotes
Conflict of interest: None declared
REFERENCES
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: A report from the american heart association. Circulation. 2010;121(7):948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53(1):31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 3.Zingarelli B. Ischemia and reperfusion injury. In: Wheeler DS, Wong HR, Shanley TP, editors. Science and Practice of Pediatric Critical Care Medicine. Springer-Verlag London Limited; 2009. pp. 181–192. [Google Scholar]
- 4.Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-γ is a new therapeutic target in sepsis and inflammation. Shock. 2005;23(5):393–399. doi: 10.1097/01.shk.0000160521.91363.88. [DOI] [PubMed] [Google Scholar]
- 5.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95(6):568–578. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 6.Guan Y, Zhang Y, Breyer MD. The role of PPARs in the transcriptional control of cellular processes. Drug News Perspect. 2002;15(3):147–154. doi: 10.1358/dnp.2002.15.3.840011. [DOI] [PubMed] [Google Scholar]
- 7.Zingarelli B, Hake PW, Mangeshkar P, O'Connor M, Burroughs TJ, Piraino G, Denenberg A, Wong HR. Diverse cardioprotective signaling mechanisms of peroxisome proliferator-activated receptor-γ ligands, 15-deoxy-Δ12,14-prostaglandin J2 and ciglitazone, in reperfusion injury: Role of nuclear factor-κB, heat shock factor 1, and AKT. Shock. 2007;28(5):554–563. doi: 10.1097/shk.0b013e31804f56b9. [DOI] [PubMed] [Google Scholar]
- 8.Zingarelli B, Sheehan M, Wong HR. Nuclear factor-κB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31(1 Suppl):S105–S111. doi: 10.1097/00003246-200301001-00015. [DOI] [PubMed] [Google Scholar]
- 9.Wayman NS, Hattori Y, McDonald MC, Mota-Filipe H, Cuzzocrea S, Pisano B, Chatterjee PK, Thiemermann C. Ligands of the peroxisome proliferator-activated receptors (PPARγ and PPARα) reduce myocardial infarct size. FASEB J. 2002;16(9):1027–1040. doi: 10.1096/fj.01-0793com. [DOI] [PubMed] [Google Scholar]
- 10.Khandoudi N, Delerive P, Berrebi-Bertrand I, Buckingham RE, Staels B, Bril A. Rosiglitazone, a peroxisome proliferator-activated receptor-γ, inhibits the Jun NH2-terminal kinase/activating protein-1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes. 2002;51(5):1507–1514. doi: 10.2337/diabetes.51.5.1507. [DOI] [PubMed] [Google Scholar]
- 11.Zhu P, Lu L, Xu Y, Schwartz GG. Troglitazone improves recovery of left ventricular function after regional ischemia in pigs. Circulation. 2000;101(10):1165–1171. doi: 10.1161/01.cir.101.10.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang XJ, Xiong ZB, Tang AL, Ma H, Ma YD, Wu JG, Dong YG. Rosiglitazone-induced myocardial protection against ischaemia-reperfusion injury is mediated via a phosphatidylinositol 3-kinase/AKT-dependent pathway. Clin Exp Pharmacol Physiol. 2010;37(2):156–161. doi: 10.1111/j.1440-1681.2009.05232.x. [DOI] [PubMed] [Google Scholar]
- 13.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible cre protein. Circ Res. 2001;89(1):20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 14.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, Khuchua Z. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem. 2011;286(2):899–908. doi: 10.1074/jbc.M110.171439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPARγ is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4(4):585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 16.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-γ both induce cardiac hypertrophy in mice. Circ Res. 2005;97(4):372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 17.Ding G, Fu M, Qin Q, Lewis W, Kim HW, Fukai T, Bacanamwo M, Chen YE, Schneider MD, Mangelsdorf DJ, Evans RM, Yang Q. Cardiac peroxisome proliferator-activated receptor-γ is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc Res. 2007;76(2):269–279. doi: 10.1016/j.cardiores.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Hallen J, Buser P, Schwitter J, Petzelbauer P, Geudelin B, Fagerland MW, Jaffe AS, Atar D. Relation of cardiac troponin I measurements at 24 and 48 hours to magnetic resonance-determined infarct size in patients with ST-elevation myocardial infarction. Am J Cardiol. 2009;104(11):1472–1477. doi: 10.1016/j.amjcard.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Gallegos RP, Swingen C, Xu XJ, Wang X, Bianco R, Jerosch-Herold M, Bolman RM. Infarct extent by MRI correlates with peak serum troponin level in the canine model. J Surg Res. 2004;120(2):266–271. doi: 10.1016/j.jss.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Metzler B, Hammerer-Lercher A, Jehle J, Dietrich H, Pachinger O, Xu Q, Mair J. Plasma cardiac troponin T closely correlates with infarct size in a mouse model of acute myocardial infarction. Clin Chim Acta. 325;2002;(1-2):87–90. doi: 10.1016/s0009-8981(02)00296-6. [DOI] [PubMed] [Google Scholar]
- 21.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 22.Jiang C, Ting AT, Seed B. PPARγ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan J, Cook JA, O'Connor M, Zingarelli B. Peroxisome proliferator-activated receptor-γ is required for the inhibitory effect of ciglitazone but not 15-deoxy-Δ12,14-prostaglandin J2 on the NF-κB pathway in human endothelial cells. Shock. 2007;28(6):722–726. doi: 10.1097/SHK.0b013e318055683a. [DOI] [PubMed] [Google Scholar]
- 24.Guyton K, Zingarelli B, Ashton S, Teti G, Tempel G, Reilly C, Gilkeson G, Halushka P, Cook J. Peroxisome proliferator-activated receptor-γ agonists modulate macrophage activation by gram-negative and gram-positive bacterial stimuli. Shock. 2003;20(1):56–62. doi: 10.1097/01.shk.0000070903.21762.f8. [DOI] [PubMed] [Google Scholar]
- 25.Li D, Chen K, Sinha N, Zhang X, Wang Y, Sinha AK, Romeo F, Mehta JL. The effects of PPARγ ligand pioglitazone on platelet aggregation and arterial thrombus formation. Cardiovasc Res. 2005;65(4):907–912. doi: 10.1016/j.cardiores.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94(12):1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 27.Kaminski KA, Kozuch M, Bonda T, Wojtkowska I, Kozieradzka A, Dobrzycki S, Kralisz P, Nowak K, Prokopczuk P, Winnicka MM, Musial WJ. Coronary sinus concentrations of interleukin 6 and its soluble receptors are affected by reperfusion and may portend complications in patients with myocardial infarction. Atherosclerosis. 2009;206(2):581–587. doi: 10.1016/j.atherosclerosis.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Shu J, Ren N, Du JB, Zhang M, Cong HL, Huang TG. Increased levels of interleukin-6 and matrix metalloproteinase-9 are of cardiac origin in acute coronary syndrome. Scand Cardiovasc J. 2007;41(3):149–154. doi: 10.1080/14017430601164263. [DOI] [PubMed] [Google Scholar]
- 29.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27(5):1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 30.Yang S, Zheng R, Hu S, Ma Y, Choudhry MA, Messina JL, Rue LW, 3rd, Bland KI, Chaudry IH. Mechanism of cardiac depression after trauma–hemorrhage: increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. Am J Physiol Heart Circ Physiol. 2004;287(5):H2183–2191. doi: 10.1152/ajpheart.00624.2003. [DOI] [PubMed] [Google Scholar]
- 31.Leftheriotis DI, Fountoulaki KT, Flevari PG, Parissis JT, Panou FK, Andreadou IT, Venetsanou KS, Iliodromitis EK, Kremastinos DT. The predictive value of inflammatory and oxidative markers following the successful cardioversion of persistent lone atrial fibrillation. Int J Cardiol. 2009;135(3):361–369. doi: 10.1016/j.ijcard.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Deten A, Volz HC, Briest W, Zimmer HG. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res. 2002;55(2):329–340. doi: 10.1016/s0008-6363(02)00413-3. [DOI] [PubMed] [Google Scholar]
- 33.Abbate A, Van Tassell BW, Seropian IM, Toldo S, Robati R, Varma A, Salloum FN, Smithson L, Dinarello CA. Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodelling following acute myocardial infarction in the mouse. Eur J Heart Fail. 2010;12(4):319–322. doi: 10.1093/eurjhf/hfq017. [DOI] [PubMed] [Google Scholar]
- 34.Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW. VCU-ART Investigators: Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction. Am J Cardiol. 2010;105(10):1371–1377. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 35.Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, Biondi-Zoccai GG, Houser JE, Qureshi IZ, Ownby ED, Gustini E, Biasucci LM, Severino A, Capogrossi MC, Vetrovec GW, Crea F, Baldi A, Kukreja RC, Dobrina A. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117(20):2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 36.Reil JC, Gilles S, Zahler S, Brandl A, Drexler H, Hültner L, Matrisian LM, Welsch U, Becker BF. Insights from knock-out models concerning postischemic release of TNFα from isolated mouse hearts. J Mol Cell Cardiol. 2007;42(1):133–41. doi: 10.1016/j.yjmcc.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165(5):2798–2808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 38.Qi X, Li J, Gu J, Li S, Dang Y, Wang T. Plasma levels of IL-8 predict early complications in patients with coronary heart disease after percutaneous coronary intervention. Jpn Heart J. 2003;44(5):451–461. doi: 10.1536/jhj.44.451. [DOI] [PubMed] [Google Scholar]
- 39.Riesenberg K, Levy R, Katz A, Galkop S, Schlaeffer F. Neutrophil superoxide release and interleukin 8 in acute myocardial infarction: distinction between complicated and uncomplicated states. Eur J Clin Invest. 1997;27(5):398–404. doi: 10.1046/j.1365-2362.1997.1270667.x. [DOI] [PubMed] [Google Scholar]
- 40.Shin WS, Szuba A, Rockson SG. The role of chemokines in human cardiovascular pathology: enhanced biological insights. Atherosclerosis. 2002;160(1):91–102. doi: 10.1016/s0021-9150(01)00571-8. [DOI] [PubMed] [Google Scholar]
- 41.de Jager SC, Kraaijeveld AO, Grauss RW, de Jager W, Liem SS, van der Hoeven BL, Prakken BJ, Putter H, van Berkel TJ, Atsma DE, Schalij MJ, Jukema JW, Biessen EA. CCL3 (MIP-1α) levels are elevated during acute coronary syndromes and show strong prognostic power for future ischemic events. J Mol Cell Cardiol. 2008;45(3):446–452. doi: 10.1016/j.yjmcc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Nossuli TO, Frangogiannis NG, Knuefermann P, Lakshminarayanan V, Dewald O, Evans AJ, Peschon J, Mann DL, Michael LH, Entman ML. Brief murine myocardial I/R induces chemokines in a TNFα-independent manner: role of oxygen radicals. Am J Physiol Heart Circ Physiol. 2001;281(6):H2549–H2558. doi: 10.1152/ajpheart.2001.281.6.H2549. [DOI] [PubMed] [Google Scholar]
- 43.Maurer M, von Stebut E. Macrophage inflammatory protein: Int J Biochem Cell Biol. 2004;36(10):1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Maass DL, White J, Horton JW. IL-1β and IL-6 act synergistically with TNF-α to alter cardiac contractile function after burn trauma. Shock. 2002;18(4):360–366. doi: 10.1097/00024382-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Kobara M, Noda K, Kitamura M, Okamoto A, Shiraishi T, Toba H, Matsubara H, Nakata T. Antibody against interleukin-6 receptor attenuates left ventricular remodelling after myocardial infarction in mice. Cardiovasc Res. 2010;87(3):424–430. doi: 10.1093/cvr/cvq078. [DOI] [PubMed] [Google Scholar]
- 46.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173(1):57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takano H, Nagai T, Asakawa M, Toyozaki T, Oka T, Komuro I, Saito T, Masuda Y. Peroxisome proliferator-activated receptor activators inhibit lipopolysaccharide-induced tumor necrosis factor-α expression in neonatal rat cardiac myocytes. Circ Res. 2000;87(7):596–602. doi: 10.1161/01.res.87.7.596. [DOI] [PubMed] [Google Scholar]
- 48.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. PROactive investigators: Secondary prevention of macrovascular events in patients with type 2 diabetes in the proactive study (prospective pioglitazone clinical trial in macrovascular events): A randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 49.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 50.Nissen SE, Wolski K. Rosiglitazone revisited: An updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170(14):1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.