Abstract
Tumor angiogenesis, tumor cell proliferation, and tumor cell migration result from an accumulation of oncogenic mutations that alter protein expression and the regulation of various signaling cascades. Epsins, a small family of clathrin-mediated endocytic adaptor proteins, are reportedly upregulated in a variety of cancers. Importantly, loss of epsins protects against tumorigenesis, thus supporting an oncogenic role for epsins in cancer. Although a clear relationship between epsins and cancer has evolved, the importance of this relationship with regards to cancer progression and anti-cancer therapies remains unclear. In this review, we summarize epsins’ role as endocytic adaptors that modulate VEGF and Notch signaling through the regulated internalization of VEGFR2 and trans-endocytosis of Notch receptors. As both VEGF and Notch signaling have significant implications in angiogenesis, we focus on the newly identified role for epsins in tumor angiogenesis. In addition to epsins’ canonical role in receptor-mediated endocytosis, and the resulting downstream signaling regulation, we discuss the non-canonical role of epsins as regulators of small GTPases and the implications this has on tumor cell proliferation and invasion. Given epsins’ identified roles in tumor angiogenesis, tumor cell proliferation, and tumor cell invasion, we predict that the investigative links between epsins and cancer will provide new insights into the importance of endocytic adaptors and their potential use as future therapeutic targets.
Keywords: Epsin, Cancer, VEGF, Notch, Angiogenesis
EPSIN: A MULTIVALENT, MULTI-FUNCTIONING ENDOCYTIC ADAPTOR
Epsins are a small family of proteins originally identified as endocytic adaptor proteins that facilitate clathrin-mediated internalization of ubiquitinated cell surface receptors [1–5]. Epsins are an evolutionarily conserved family of proteins consisting of yeast epsins (Ent1 and Ent2), Drosophila melanogaster epsins (known as Liquid Facets) and mammalian epsins (Epn1, Epn2, and Epn3). Mammalian epsins 1 and 2 are ubiquitously expressed to varying degrees in a variety of tissues with the highest expression occurring in the brain [1, 2, 6]. In contrast, mammalian epsin 3 expression is spatially and temporally expressed in migrating keratinocytes of the epidermis [7] and parietal cells of the stomach [8].
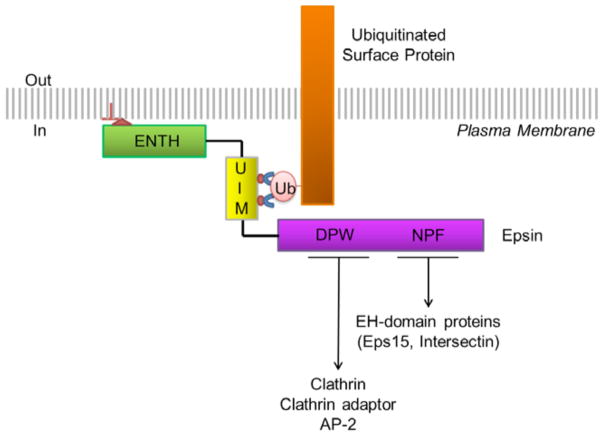
Epsins are multivalent proteins consisting of several different interaction motifs that function cooperatively to target epsins to the plasma membrane and facilitate their interactions with and recruitment of cell surface receptors to clathrin-coated pits for internalization [4, 5, 9–13] (Figure 1). The epsin NH2-terminal homology (ENTH) domain, composed primarily of alpha helices, is the most conserved epsin domain [4]. Its specificity for phosphatidyl-inositol (4,5) bisphosphate (PI(4,5)P2) mediates the recruitment of epsin to the inner leaflet of the plasma membrane [14] and facilitates membrane curvature necessary for clathrin-coated pit formation [15]. The clathrin-, AP2-, and EH (Eps15 homology)-binding domains are largely unstructured and are cooperatively responsible for recruiting epsin, and its ubiquitinated cargo, to clathrin coated pits for subsequent internalization (see review [4]). Lastly, epsin contains two alpha helical ubiquitin interacting motifs (UIM) that reside between the ENTH domain and the unstructured carboxyl-terminal tail [11–13]. It is generally accepted that the UIMs provide the specificity with which epsins interact with and target ubiquitinated receptors for internalization. UIM-mediated interactions between epsins and cell surface receptors, as well as the subsequent clathrin-mediated internalization, has been established as an important regulatory mechanism to modulate signaling events, including those involved in angiogenesis, ion transport, cell proliferation, differentiation, and death [6, 8, 16–20].
Figure 1. Epsin mediated endocytosis of ubiquitinated cell surface receptors.
ENTH, epsin NH2-terminal homology domain; UIM, ubiquitin interacting motif; DPW, aspartate-proline-tryptophan containing central region housing clathrin- and AP-2-binding domains; NPF, asparagine-proline-phenylalanine containing carboxyl-terminal region housing Eps-15 homology (EH) protein binding domains; Ub, ubiquitin.
In addition to its classical role as an endocytic adaptor, several lines of evidence suggest epsins play an additional role in regulating the activation of GTPases, such as the Rho GTPases responsible for actin remodeling [21, 22]. Specifically, epsins reportedly interact with and inhibit various GTPase activating proteins (GAPs) recruited to and/or localized at the plasma membrane, resulting in the accumulation of GTP-loaded GTPases and the formation of polarized edges. These findings provide an alternative mechanism by which epsins may regulate cell polarity and migration.
As a result of epsins’ involvement in cell proliferation, differentiation, and migration, there is an increasing interest in epsins’ potential role in carcinogenesis. Proteomic and transcriptomic analyses of basal cell carcinoma, lung and breast adenocarcinomas reported elevated expression of epsins in tumor cell lines and tumor tissues [7, 23, 24]. Recently, our lab has identified two distinct roles for epsins in carcinogenesis. First, tightly regulated endothelial cell epsins are necessary to modulate vascular endothelial growth factor (VEGF) signaling and functional tumor angiogenesis, thereby ensuring tumor growth [19]. Secondly, epsins are an important oncogenic protein that is both upregulated in and required for the progression of prostate cancer [25]. Cumulative results from these studies have placed an emphasis on further understanding and characterization of the potential roles for epsins in carcinogenesis. In this review, we will focus on epsins’ roles in tumor angiogenesis, tumor cell proliferation, and the implications these findings have on migration. Furthermore, we will provide new insights into the potential usefulness of epsins as novel therapeutic targets.
EPSIN: A REGULATOR OF TUMOR ANGIOGENESIS
Tumor angiogenesis is an important driving force of carcinogenesis (see review [26]). Enhancing the network of tumor vasculature ensures adequate oxygen and nutrient delivery to the largely hypoxic and energy demanding tumor cells, thereby promoting continual tumor growth and development. It has long been understood that tumor cells secrete growth factors, such as VEGF, and promote endothelial cell overexpression of key receptors, including VEGFR2 and Notch. VEGF-induced signaling through VEGFR2 and the juxtacrine Delta-like 4 (Dll4) ligand-induced Notch signaling function to promote endothelial cell proliferation, migration, and differentiation while also promoting stabilization of the tumor vascular network [27–30].
Originally characterized for their fundamental roles in embryonic angiogenesis and organogenesis, the roles VEGF and Notch signaling play in post-natal angiogenesis, specifically tumor angiogenesis, have made them favored targets for anti-cancer therapies [26, 27, 31, 32]. Although both anti-VEGF and anti-Notch therapies have had some success in impairing tumor growth by inhibiting or stabilizing tumor angiogenesis, respectively, and enhancing chemotherapeutic sensitivity, many types of cancer develop strong resistance. Given the important role of tumor angiogenesis in carcinogenesis, and the increasing resistance to current anti-VEGF and anti-Notch therapies, it remains extremely important to continue identifying new potential drug targets involved in these two signaling pathways.
Our studies suggest that epsin is a potentially novel and unique drug target to alter tumor angiogenesis because of its implicated roles in regulating both Notch and VEGF signaling. In 2009, our group reported that global deletion of epsins 1 and 2 (DKO) in mice, but not single deletion of either epsin 1 or epsin 2 (SKO), resulted in embryonic lethality at embryonic day 10 (E10) [6]. Upon further examination of the DKO embryos, we discovered profound vascular defects reminiscent of defects caused by loss of Notch genes in the embryo proper, placenta, and yolk-sac [33]. Further investigation confirmed that loss of epsins impaired Notch signaling in E9 embryos [6].
Although the mechanism(s) and cell type(s) involved were unclear, our findings suggested that epsins play a fundamental role in regulating Notch signaling and promoting angiogenesis required for proper embryonic development. Notch signaling activation is dependent on the proteolytic cleavage and release of the Notch receptor intracellular domain (NICD). Cleavage is dependent on the trans-endocytosis of the Notch receptor extracellular domain (NECD) by the Notch ligand, Dll4 [16]. Furthermore, trans-endocytosis is dependent on the ubiquitination of Dll4. Therefore, we proposed a mechanism in which epsins facilitate the internalization and proteolytic processing of Notch required for Notch signal activation (Figure 2). We speculated that ligand-dependent ubiquitination of Dll4 provides a docking site for the epsin UIM binding domain, thereby providing a ligand-dependent mechanism for trans-endocytosis of the Notch NECD domain and Notch activation. The fact that anti-Notch ligand therapies have had success in impairing tumor progression [34], and given that loss of epsins impairs Notch activation with some specificity [6], our data suggests that targeting epsin function may provide a novel, alternative, and downstream therapeutic target for hindering carcinogenesis.
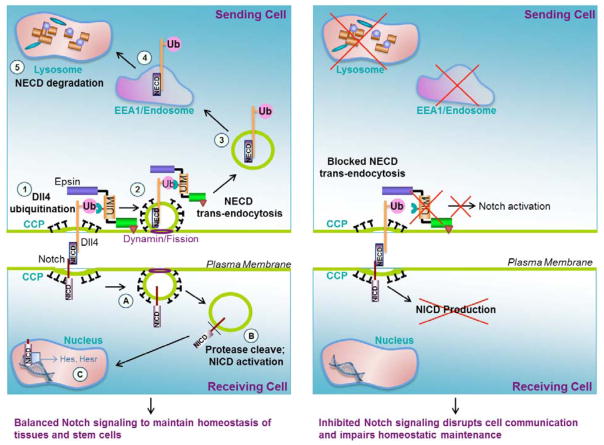
Figure 2. A model for the regulation of Notch Signaling by epsin-mediated Notch ligand endocytosis.
Left panel: in step 1, Notch ligand, Dll4, on the sending cell engages Notch receptor, via its Notch extracellular domain (NECD), on the receiving cell thereby inducing ubiquitination (Ub) of Dll4. Epsin interacts with Dll4 through its ubiquitin interacting motif (UIM) and recruits Dll4 to nascent clathrin-coated pits (CCP). In step 2, dynamin, an essential GTPase for membrane fission, liberates the CCP containing the ubiquitinated Dll4 and the associated NECD. Trans-endocytosis of the NECD by the sending cell is a crucial step in Notch activation in the receiving cell. In step 3, clathrin and epsin dissociate from the CCP creating a free vesicle containing Dll4 and NECD. The free vesicle fuses with the early endosome (step 4) and is eventually targeted to the lysosome for degradation (step 5). In the receiving cell, the remaining membrane-associated Notch intracellular domain (NICD) of the Notch receptor is targeted to CCPs and internalized via a dynamin-dependent mechanism (step A). In step B, the NICD is proteolytically cleaved to release the NICD into the cytoplasm. The free NICD is shuttled to the nucleus where it induces the expression of Notch genes (step C). Balanced Notch signaling is important to maintain the homeostasis of several tissues and stem cells.
Right panel: Loss of epsins in the sending cell prevents the trans-endocytosis of NECD. Failure to separate the NECD from the remaining Notch receptor subsequently impairs the proteolytic activation and release of the NICD in the receiving cell. Collectively, loss of epsins inhibits Notch signaling in the receiving cell thereby disrupting cell communications and impairing homeostatic maintenance.
Importantly, the epsin DKO embryonic phenotype is significantly more severe than any single or double Notch deletion, suggesting other signaling pathways involved in angiogenesis, such as VEGF signaling, may also be altered and contribute to the phenotype. Given the severity of the global epsin DKO phenotype, the various roles of Notch signaling in different cell types, and the potential role of other angiogenic signaling cascades, we generated an endothelial cell-specific epsin 1 and 2 DKO (EC-DKO) mouse model to further investigate the role of epsins in angiogenesis. Surprisingly, both constitutive and tamoxifen-inducible EC-DKO models displayed no gross vascular abnormalities under physiologic conditions indicating the embryonic lethality of the global epsins 1 and 2 DKO embryos was due, in large part, to epsins role in regulating Notch signaling in other cell types [19]. In contrast, under carcinogenic conditions the EC-DKO mice exhibited severe defects in tumor angiogenesis resulting in disorganized vascular structures and exaggerated vascular permeability. These defects resulted in aberrant, dysfunctional tumor vascular networks and impaired tumorigenesis. While we did find impaired Notch signaling in the endothelial cells from EC-DKO mice, the addition of activated NICD, failed to prevent defective tumor angiogenesis, further suggesting the enhanced vascular permeability and enlarged vascular structures occur independent of defects in endothelial cell Notch signaling.
We did, however, find that endothelial cell-specific epsin depletion significantly enhanced and prolonged VEGF signaling, an important signaling pathway involved in vascular permeability [19]. Specifically, epsin depletion increased both VEGF-dependent phosphorylation and total protein levels of its receptor, VEGFR2. Furthermore, we found that the enhanced total and phosphorylated VEGFR2 in the epsin-deficient endothelial cells was due to impaired VEGFR2 internalization and degradation.
Mechanistically, VEGF stimulation induced the ubiquitination of VEGFR2 [19]. Epsin was found to facilitate VEGFR2 internalization by binding, via its UIM, the ubiquitin moieties on VEGFR2. Although counterintuitive to the anti-VEGF therapies that attempt to hinder VEGF-dependent tumor angiogenesis, our data suggests that enhanced VEGF signaling and exaggerated tumor angiogenesis may also effectively hinder tumor growth. Although unsure of the mechanism, we hypothesize that the resulting aberrant tumor vasculature may fail to perfuse blood properly, thereby impairing oxygen and nutrient delivery. Importantly, our study emphasizes the importance of balanced VEGF signaling in tumor angiogenesis and provides a potential alternative approach for treating cancers resistant to anti-VEGF therapies.
In summary, the combined efforts of our lab and others have identified epsins as potential regulators of carcinogenesis. Specifically, our data suggests epsins play important roles in regulating tumor angiogenesis through ligand-mediated ubiquitination and internalization of VEGFR2 and trans-endocytosis of Notch NECD. Furthermore, our findings suggest that, while involved in Notch signaling, epsins’ role in regulating VEGF signaling may be more pronounced in endothelial cells and tumor angiogenesis. However, because epsin utilizes its UIM to interact with both Notch ligand and VEGFR2, it is likely that targeted interference of this domain will provide a very specific therapeutic advantage to adversely affect both pathways implicated in carcinogenesis.
EPSIN: A REGULATOR OF TUMOR CELL PROLIFERATION
While tumor angiogenesis plays an important supporting role in developing tumors by providing oxygen and nutrients, carcinogenesis is initiated and progresses because tumor cells continuously gain permanent oncogenic changes that promote unchecked cell proliferation and avoid programmed cell death [35, 36]. How a cell responds to its external environment, i.e. proliferate, migrate, or die, is often regulated, either directly or indirectly, by the specific activation and subsequent inaction of various cell surface receptors and their downstream signaling pathways. Signaling pathways, such as the VEGF and Notch pathways discussed above, utilize several mechanisms including receptor expression, internalization, and degradation to ensure homeostasis is re-established and/or maintained in an ever-changing environment. Genetic alterations that alter the homeostatic balance often result in pathological conditions such as cancer.
As with its role in signal balance and tumor angiogenesis, epsins’ importance in re-establishing proliferative homeostasis after signaling events are initiated has become increasing clear. It interacts with and facilitates the internalization of several activated and ubiquitinated cell surface receptors [5, 6, 11, 17, 37]. Some, such as VEGFR2 described previously, require internalization to downregulate signaling while others, such as Notch and epidermal growth factor receptor (EGFR), are dependent on internalization for sufficient signal activation. The majority of studies relating epsins, growth factors receptors, and tumor cell proliferation have focused on EGFR and the role epsins play in its sustained activation. Specifically, EGF-stimulation induces EGFR ubiquitination and subsequent interaction with epsin [17]. Epsins facilitate the recruitment of EGFR to clathrin-coated pits for internalization. EGFR kinase activity is maintained during clathrin-mediated endocytosis and traffic through the early endosomes [38]. Defects that prevent epsin from interacting with EGFR significantly alter EGF signaling.
Similar to EGFR, several other growth factor receptors are upregulated in tumor cells to promote proliferation during tumorigenesis including IGFR, FGFR, PDGFR, Notch, and Wnt receptors [24, 39]. In correlation with these increases, epsins are also reportedly overexpressed in several types of cancer including prostate, breast, lung, and skin cancers [7, 23–25]. Therefore, we hypothesize, and are currently investigating, the potential relationships between epsins and these receptors as they relate to tumorigenesis. In light of our previous publications that continuously suggest epsins act as oncogenic proteins to promote tumor cell proliferation, we speculate that interfering with epsin function will protect against cancer progression. In support of this, we recently reported, using the spontaneous TRAMP mouse model for prostate cancer, that epsin deficiency provided significant protection against tumor cell proliferation and carcinogenesis [25].
EPSINS: A REGULATOR OF TUMOR CELL MIGRATION AND INVASION
The final stage in carcinogenesis is invasion, when tumor cells switch from benign proliferative tumors to malignant spreading tumors [36]. Tumor cell invasion depends heavily on whether tumor cells gain the ability to spread and invade surrounding tissues. In the final process of invasion, migrating tumor cells enter circulation and ultimately disseminate to other distant organs. Because cancer invasion contributes significantly to poor survival outcomes, the mechanisms involved in tumor cell migration to and invasion of distant tissues remains an intensely studied area.
Given epsin’s role in regulating VEGF and Notch signaling with regards to angiogenesis [6, 19], a process dependent on immature endothelial cell migration, and epsins’ role in epithelia wound healing via the regulation of immature keratinocyte migration [7], it is likely that epsins may also facilitate tumor cell invasion. Cell migration depends heavily on a functioning actin network to promote cell polarity and regulate the formation and retraction of lamellipodia. In 2010, Coon, et al. identified a non-canonical role for epsins in the process of actin remodeling and cell migration, independent of receptor endocytosis [22]. Instead, they reported that the NH2-terminal ENTH domain of epsins interacts with and inhibits RalBP1, a GTPase-activating protein (GAP), ultimately resulting in the activation of GTPases, Rac1 and Arf6, responsible for actin remodeling and cell migration. In isolated fibrosarcomas, the enhanced Rac1 and Arf6 promoted cell polarity, migration, and invasion. Similar reports implicating epsins as important regulators of cell polarity in mitosis, via regulation of GTPase effectors, have also been reported [20, 21]. These findings, and others like them, illustrate the multi-faceted nature of epsins and their potential involvement in pathological conditions, especially cancer.
CONCLUSIONS AND PERSPECTIVES
In summary, epsins have both canonical and non-canonical functions implicated in the initiation and progression of several cancer types. The fact that epsin expression is upregulated in several types of cancer further supports its role as an oncogenic protein. While epsins do reportedly alter several seemingly different signaling cascades, it is important to emphasize the specificity with which they function to regulate the internalization of ligand-dependent ubiquitinated receptors. Furthermore, it is important to note that epsins have been implicated in the three major changes responsible for carcinogenesis: tumor cell proliferation, tumor cell migration/invasion, and tumor angiogenesis.
Most drug therapies, including the anti-VEGF and anti-Notch ligand therapies discussed above, focus on targeting a single cell surface receptor and, therefore, a single signaling cascade. While this approach has the benefit of selectivity and reduced risk of off-target effects, it also enhances the probability that tumor cells will develop compensatory mechanisms responsible for drug resistance. Given that epsins are involved in several overlapping signaling cascades involved in tumorigenesis downstream of signaling receptors, suggests they may provide a favorable target for future therapeutic development. Furthermore, using a mouse model in which epsins 1 and 2 are depleted post-development, our lab has reported very few, if any adverse effects on normal physiologic functions [19, 25]. These findings, along with the findings reviewed herein on epsins’ roles in tumor angiogenesis, tumor cell proliferation, and tumor cell migration/invasion, provide strong evidence to support further investigation into the roles of epsins, as they pertain to cancer, as well as the development of future epsin-inhibitory drug therapies.
Acknowledgments
This work was supported in part by NIH grants R01HL-093242, P20 RR018758, a grant from the Oklahoma Center for Advanced Science and Technology (OCAST) HR09-116, and a grant from Department of Defense W81XWH-11-1-00226 to H. Chen, and a grant from Oklahoma Center for Advanced Science and Technology (OCAST) AR11-043 to Y. Dong.
Footnotes
CONFLICTS OF INTEREST
The authors have declared no conflicts of interest.
References
- 1.Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, et al. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394(6695):793–7. doi: 10.1038/29555. Epub 1998/09/02. http://dx.doi.org/10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal JA, Chen H, Slepnev VI, Pellegrini L, Salcini AE, Di Fiore PP, et al. The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. J Biol Chem. 1999;274(48):33959–65. doi: 10.1074/jbc.274.48.33959. Epub 1999/11/24. http://dx.doi.org/10.1074/jbc.274.48.33959. [DOI] [PubMed] [Google Scholar]
- 3.Shih SC, Katzmann DJ, Schnell JD, Sutanto M, Emr SD, Hicke L. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat Cell Biol. 2002;4(5):389–93. doi: 10.1038/ncb790. Epub 2002/05/04. http://dx.doi.org/10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- 4.Wendland B. Epsins: adaptors in endocytosis? Nat Rev Mol Cell Biol. 2002;3(12):971–7. doi: 10.1038/nrm970. Epub 2002/12/04. http://dx.doi.org/10.1038/nrm970. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, De Camilli P. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc Natl Acad Sci USA. 2005;102(8):2766–71. doi: 10.1073/pnas.0409719102. Epub 2005/02/11. http://dx.doi.org/10.1073/pnas.0409719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Ko G, Zatti A, Di Giacomo G, Liu L, Raiteri E, et al. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice. Proc Natl Acad Sci USA. 2009;106(33):13838–43. doi: 10.1073/pnas.0907008106. Epub 2009/08/12. http://dx.doi.org/10.1073/pnas.0907008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spradling KD, McDaniel AE, Lohi J, Pilcher BK. Epsin 3 is a novel extracellular matrix-induced transcript specific to wounded epithelia. J Biol Chem. 2001;276(31):29257–67. doi: 10.1074/jbc.M101663200. Epub 2001/05/22. http://dx.doi.org/10.1074/jbc.M101663200. [DOI] [PubMed] [Google Scholar]
- 8.Ko G, Paradise S, Chen H, Graham M, Vecchi M, Bianchi F, et al. Selective high-level expression of epsin 3 in gastric parietal cells, where it is localized at endocytic sites of apical canaliculi. Proc Natl Acad Sci USA. 2010;107(50):21511–6. doi: 10.1073/pnas.1016390107. Epub 2010/12/01. http://dx.doi.org/10.1073/pnas.1016390107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann K, Falquet L. A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends Biochem Sci. 2001;26(6):347–50. doi: 10.1016/s0968-0004(01)01835-7. Epub 2001/06/19. http://dx.doi.org/10.1016/S0968-0004(01)01835-7. [DOI] [PubMed] [Google Scholar]
- 10.De Camilli P, Chen H, Hyman J, Panepucci E, Bateman A, Brunger AT. The ENTH domain. FEBS Lett. 2002;513(1):11–8. doi: 10.1016/s0014-5793(01)03306-3. Epub 2002/03/26. http://dx.doi.org/10.1016/S0014-5793(01)03306-3. [DOI] [PubMed] [Google Scholar]
- 11.Polo S, Sigismund S, Faretta M, Guidi M, Capua MR, Bossi G, et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416(6879):451–5. doi: 10.1038/416451a. Epub 2002/03/29. http://dx.doi.org/10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 12.Polo S, Confalonieri S, Salcini AE, Di Fiore PP. EH and UIM: endocytosis and more. Science’s STKE: Signal Transduction Knowledge Environment. 2003;2003(213):re17. doi: 10.1126/stke.2132003re17. Epub 2003/12/18. [DOI] [PubMed] [Google Scholar]
- 13.Reider A, Wendland B. Endocytic adaptors--social networking at the plasma membrane. J Cell Sci. 2011;124(Pt 10):1613–22. doi: 10.1242/jcs.073395. Epub 2011/05/04. http://dx.doi.org/10.1242/jcs.073395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 2001;291(5506):1047–51. doi: 10.1126/science.291.5506.1047. Epub 2001/02/13. http://dx.doi.org/10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- 15.Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, et al. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419(6905):361–6. doi: 10.1038/nature01020. Epub 2002/09/28. http://dx.doi.org/10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 16.Tian X, Hansen D, Schedl T, Skeath JB. Epsin potentiates Notch pathway activity in Drosophila and C. elegans. Development. 2004;131(23):5807–15. doi: 10.1242/dev.01459. Epub 2004/11/13. http://dx.doi.org/10.1242/dev.01459. [DOI] [PubMed] [Google Scholar]
- 17.Kazazic M, Bertelsen V, Pedersen KW, Vuong TT, Grandal MV, Rodland MS, et al. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic. 2009;10(2):235–45. doi: 10.1111/j.1600-0854.2008.00858.x. Epub 2008/12/05. http://dx.doi.org/10.1111/j.1600-0854.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 18.Csikos G, Lippai M, Lukacsovich T, Juhasz G, Henn L, Erdelyi M, et al. A novel role for the Drosophila epsin (lqf): involvement in autophagy. Autophagy. 2009;5(5):636–48. doi: 10.4161/auto.5.5.8168. Epub 2009/03/24. http://dx.doi.org/10.4161/auto.5.5.8168. [DOI] [PubMed] [Google Scholar]
- 19.Pasula S, Cai X, Dong Y, Messa M, McManus J, Chang B, et al. Endothelial epsin deficiency decreases tumor growth by enhancing VEGF signaling. J Clin Investig. 2012;122(12):4424–38. doi: 10.1172/JCI64537. Epub 2012/11/29. http://dx.doi.org/10.1172/JCI64537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosse C, L’Hoste S, Offner N, Picard A, Camonis J. RLIP, an effector of the Ral GTPases, is a platform for Cdk1 to phosphorylate epsin during the switch off of endocytosis in mitosis. J Biol Chem. 2003;278(33):30597–604. doi: 10.1074/jbc.M302191200. Epub 2003/05/31. http://dx.doi.org/10.1074/jbc.M302191200. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee D, Coon BG, Edwards DF, 3rd, Hanna CB, Longhi SA, McCaffery JM, et al. The yeast endocytic protein Epsin 2 functions in a cell-division signaling pathway. J Cell Sci. 2009;122(Pt 14):2453–63. doi: 10.1242/jcs.041137. Epub 2009/06/18. http://dx.doi.org/10.1242/jcs.041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coon BG, Burgner J, Camonis JH, Aguilar RC. The epsin family of endocytic adaptors promotes fibrosarcoma migration and invasion. J Biol Chem. 2010;285(43):33073–81. doi: 10.1074/jbc.M110.124123. Epub 2010/08/17. http://dx.doi.org/10.1074/jbc.M110.124123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Dai Z, Sadee W, Hancock WS. A pharmacoproteomics study of the cancer cell line EKVX using capillary-LC/MS/MS. Mol Pharmaceut. 2006;3(5):566–78. doi: 10.1021/mp060002b. Epub 2006/10/03. http://dx.doi.org/10.1021/mp060002b. [DOI] [PubMed] [Google Scholar]
- 24.Pawlowski KM, Krol M, Majewska A, Badowska-Kozakiewicz A, Mol JA, Malicka E, et al. Comparison of cellular and tissue transcriptional profiles in canine mammary tumor. J Physiol Pharmacol: An Official J Polish Physiol Soc. 2009;60(Suppl 1):85–94. Epub 2009/07/23. [PubMed] [Google Scholar]
- 25.Tessneer KL, Pasula S, Cai X, Dong Y, Liu X, Yu L, et al. Endocytic Adaptor Protein Epsin Is Elevated in Prostate Cancer and Required for Cancer Progression. ISRN Oncol. 2013;2013:8. doi: 10.1155/2013/420597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerbel RS. Tumor angiogenesis. New Engl J Med. 2008;358(19):2039–49. doi: 10.1056/NEJMra0706596. Epub 2008/05/09. http://dx.doi.org/10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folkman J. Tumor angiogenesis: therapeutic implications. New Engl J Med. 1971;285(21):1182–6. doi: 10.1056/NEJM197111182852108. Epub 1971/11/18. http://dx.doi.org/10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exper Med. 1971;133(2):275–88. doi: 10.1084/jem.133.2.275. Epub 1971/02/01. http://dx.doi.org/10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67(23):11244–53. doi: 10.1158/0008-5472.CAN-07-0969. Epub 2007/12/07. http://dx.doi.org/10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 30.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104(9):3219–24. doi: 10.1073/pnas.0611206104. Epub 2007/02/14. http://dx.doi.org/10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sainson RC, Harris AL. Anti-Dll4 therapy: can we block tumour growth by increasing angiogenesis? Trends Mol Med. 2007;13(9):389–95. doi: 10.1016/j.molmed.2007.07.002. Epub 2007/09/08. http://dx.doi.org/10.1016/j.molmed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2(10):727–39. doi: 10.1038/nrc905. Epub 2002/10/03. http://dx.doi.org/10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 33.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA. 2004;101(45):15949–54. doi: 10.1073/pnas.0407290101. Epub 2004/11/03. http://dx.doi.org/10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7(5):327–31. doi: 10.1038/nrc2130. Epub 2007/04/26. http://dx.doi.org/10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 35.Foulds L. The experimental study of tumor progression: a review. Cancer Res. 1954;14(5):327–39. Epub 1954/06/01. [PubMed] [Google Scholar]
- 36.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33(1):49–54. doi: 10.1038/ng1060. Epub 2002/12/07. http://dx.doi.org/10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Traub LM, Weixel KM, Hawryluk MJ, Shah N, Edinger RS, et al. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. J Biol Chem. 2006;281(20):14129–35. doi: 10.1074/jbc.M512511200. Epub 2006/04/01. http://dx.doi.org/10.1074/jbc.M512511200. [DOI] [PubMed] [Google Scholar]
- 38.Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21(6):737–48. doi: 10.1016/j.molcel.2006.02.018. Epub 2006/03/18. http://dx.doi.org/10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Bache KG, Slagsvold T, Stenmark H. Defective downregulation of receptor tyrosine kinases in cancer. EMBO J. 2004;23(14):2707–12. doi: 10.1038/sj.emboj.7600292. Epub 2004/07/02. http://dx.doi.org/10.1038/sj.emboj.7600292. [DOI] [PMC free article] [PubMed] [Google Scholar]