Abstract
Upper respiratory tract infections (URIs) represent the most frequent cause of acute asthma exacerbations. It has yet to be determined whether leukotriene receptor antagonist (LTRA) treatment prevents URI-induced acute asthma exacerbations in adults. The objective of the present study was to evaluate the preventive effects of LTRA treatment on URI-induced acute asthma exacerbations. The incidences of URI alone, acute asthma exacerbation without URI, and URI-induced acute asthma exacerbation were determined retrospectively by analyzing diary and medical records of 321 adult asthmatic patients (mean age, 56.3 ± 17.2 years; male/female ratio, 117:204) over 1 year. Results were compared between patients who had been taking an LTRA (n = 137) and those who had never taken any LTRA (n = 184) during the study periods. Significantly fewer URIs alone and acute asthma exacerbations without URI occurred in patients with than in those without prophylactic daily use of LTRA. LTRA treatment significantly reduced the durations of URIs alone and of total acute asthma exacerbations, as well as the incidence of mild exacerbations of asthma. In contrast, in patients with URI-induced acute asthma exacerbations, LTRA treatment failed to significantly reduce the interval between URI onset and acute asthma exacerbation, as well as the duration and severity of both URIs and acute asthma exacerbations. Use of an LTRA for adult asthmatic patients appears to reduce the incidences of URIs alone and acute asthma exacerbations without URI, but it failed to prevent URI-induced acute asthma exacerbations once a URI occurred.
Keywords: Acute asthma exacerbation, bronchial asthma, inhaled corticosteroids, inhaled long-acting beta2-agonist, leukotriene receptor antagonist, montelukast, pranlukast, retrospective cohort study, short-acting beta2-agonist, upper respiratory tract infection
Asthma is one of the most prevalent diseases in the world, with a large socioeconomic impact. The primary objective of therapy for asthma includes not only preventing limitations to routine activities but also reducing the risk of death and the economic impact from hospitalization and absence from work. To date, inhaled corticosteroids (ICSs) represent the most effective treatment for asthma, and they can reduce mortality due to asthma by preventing acute exacerbations. However, a subset of asthma is ICS resistant. These include asthma with obesity,1 smoking,2 and those with exacerbations due to viral respiratory tract infections.3
Viral respiratory tract infections are the most common triggers of an acute exacerbation of asthma in both children and adults.4,5 Although the precise underlying mechanism of virus-induced acute exacerbations of asthma remains unknown, many molecular factors and cells are critically involved.6 Among them, cysteinyl leukotrienes (cysLTs) have received considerable attention, because their levels increase in the airways of asthmatic patients during virus-induced acute exacerbations,7–9 and specific cysLT receptor antagonists (LTRAs) are routinely available in clinics. Thus, because respiratory viral infections increase the amounts of cysLTs in the asthmatic airways, we postulated that LTRA treatment might be able to prevent viral infection–induced acute exacerbations of asthma. Based on patients' asthma diary and medical records, the present study retrospectively evaluated the prophylactic effects of daily LTRA use for preventing upper respiratory tract infection (URI)–induced acute asthma exacerbations.
MATERIALS AND METHODS
Design
A retrospective cohort study was performed in outpatient clinics of four institutes in Nagasaki Prefecture, Japan. This study received ethical approval from the special committee of Nagasaki University Hospital (project registration number 12062548) to proceed between July 2010 and December 2012.
Subjects
Eligible individuals were adults with mild and moderate persistent asthma determined by the Asthma Prevention and Management Guideline 2012, Japan for adults10 who had received a daily fixed dose of medications except during exacerbation periods and recorded their daily symptoms for >1 year during the study period. The asthma diaries, which are being used in the daily outpatient clinics, contain questions not only related to asthma-related symptoms but also to URI-related symptoms, as detailed later in this article. Atopy was defined by a positive skin-prick test using 10 common aeroallergens and/or IgE (radioallergosorbent test). Aspirin-induced asthma was defined either by the clinical history or an oral provocation test with aspirin as described elsewhere.11 A history of otolaryngologist-diagnosed allergic rhinitis and/or sinusitis was determined based on the patients' medical records. A clinical URI-induced acute asthma exacerbation was defined as previously described.12 URI was defined as having at least two of the following symptoms: sneezing, coughing, nasal congestion, runny nose, or fever (>38°C). An asthma exacerbation was defined as an increase in one or more of wheeze, chest tightness, and breathlessness or wheeze during clinical examinations. A URI-induced acute asthma exacerbation was defined as having symptoms of both URI and asthma exacerbation. The duration of a URI and acute asthma exacerbation was determined from the 1st day until the last day before all symptoms had disappeared for at least 2 consecutive days. In each institute, well-trained allergists evaluated the subjects, and exacerbated allergic rhinitis was carefully distinguished from URI. Asthma exacerbation severity was graded according to the Asthma Prevention and Management Guideline 2012, Japan.10 The severity of URI was expressed as the sum of symptoms.
Study Protocol
The incidences of URI alone, asthma exacerbation without URI, and URI-induced asthma exacerbation during the most recent year during the study period were analyzed retrospectively by examination of diary and medical records. In URI-induced asthma, intervals between onset of URI and asthma exacerbation, duration of URI and asthma exacerbation, and severity of URI and asthma exacerbation were also evaluated. These parameters were compared between patients who had been taking an LTRA, pranlukast (ONON; ONO Pharmaceutical Co. Ltd., Osaka, Japan), or montelukast (Singulare; MSD Pharmaceutical Co. Ltd., Tokyo, Japan), denoted as the LTRA+ group, and those who had never taken any LTRA, denoted as the LTRA− group, over the 1-year evaluation period. All participants had to be taking a stable dose of ICS, inhaled long-acting β2-agonists (LABAs), and xanthines, as well as on-demand use of short-acting β2-agonists throughout the study period. Patient adherence to medications was checked orally and confirmed by the diary. Exclusion criteria included pregnancy and/or lactation, history of life-threatening asthma, hospitalization for asthma within 6 months, and oral or parenteral corticosteroids before entry.
Statistics
Results are expressed as numbers or percentages of subjects or means ± SD for continuous variables. Differences between groups were examined for statistical significance using the Mann-Whitney U test and the χ2-test. A value of p < 0.05 denoted the presence of a significant difference.
RESULTS
Enrollment and Baseline Characteristics of the Patients
The present retrospective study included 321 (male/female, 117:204; mean age, 56.3 ± 17.2 years) patients with asthma visiting the outpatient clinics of four institutes in Nagasaki Prefecture, Japan; 137 patients were LTRA+ and 184 patients were LTRA−. Table 1 summarizes the patients' baseline characteristics. Except for sex, baseline demographic characteristics were closely matched, and no parameters differed significantly between the LTRA+ and LTRA−groups. The percentage of female patients was significantly higher in the LTRA+ group than in the LTRA− group.
Table 1.
Patients' characteristics
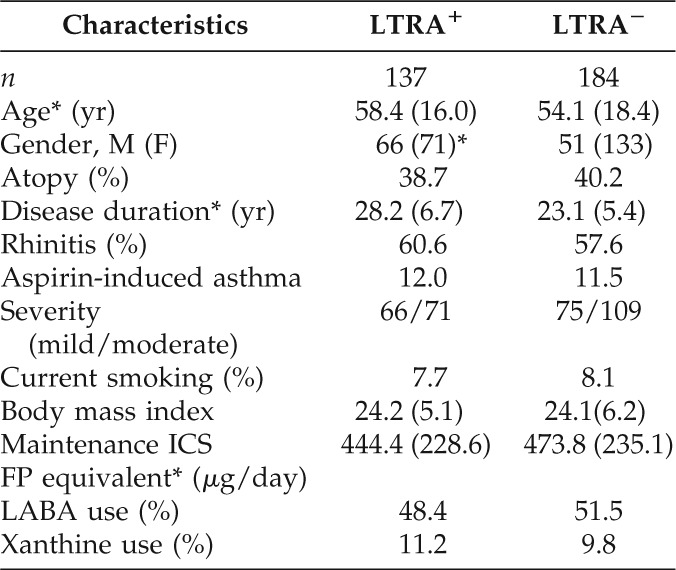
*Values are shown as means (SD; *p < 0.05).
ICS = inhaled corticosteroid; LABA = long-acting β2-antagonist; LTRA = leukotriene receptor antagonist; FP = fluticasone propionate.
Prevention of URI Alone, Acute Asthma Exacerbation without URI, and URI-Induced Acute Asthma Exacerbation by LTRA Treatment
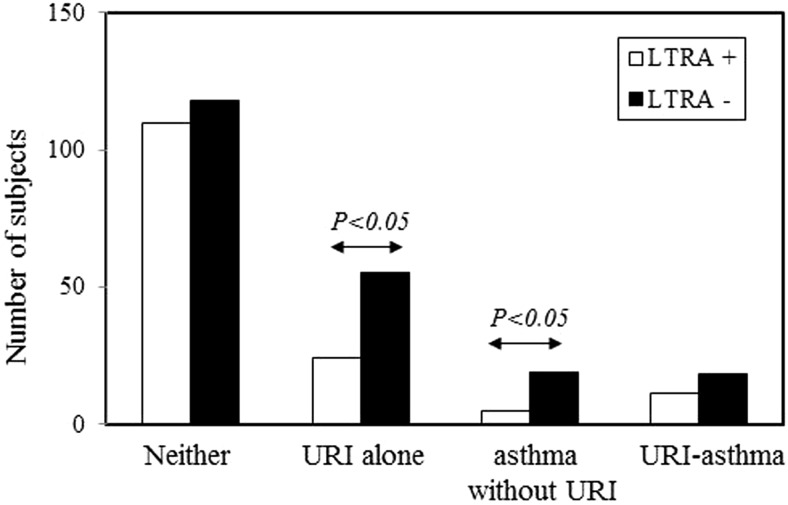
During the study period, 79 patients developed URI alone and 24 patients developed acute asthma exacerbation without URI. The present study found that significantly fewer URIs alone and acute asthma exacerbations without URI occurred in the LTRA+ group than in the LTRA− group (Fig. 1). A URI-induced acute asthma exacerbation occurred in 29 subjects, and LTRAs failed to significantly prevent URI-induced acute asthma exacerbations (Fig. 1). Thus, LTRAs showed the potential to prevent either URIs alone or acute asthma exacerbations without URI, but it did not prevent URI-induced acute asthma exacerbations.
Figure 1.
Prevention of upper respiratory tract infections (URIs) alone, acute asthma exacerbations without URI, and URI-induced acute asthma exacerbations by leukotriene receptor antagonist (LTRA) treatment. Incidences of URIs alone, acute asthma exacerbations without URI, and URI-induced acute asthma exacerbations are compared between the LTRA+ (n = 137) and LTRA−p (n = 184) groups. Bars represent the number of subjects.
Effects of LTRA Treatment on Duration and Severity of URIs Alone and Total Acute Asthma Exacerbations
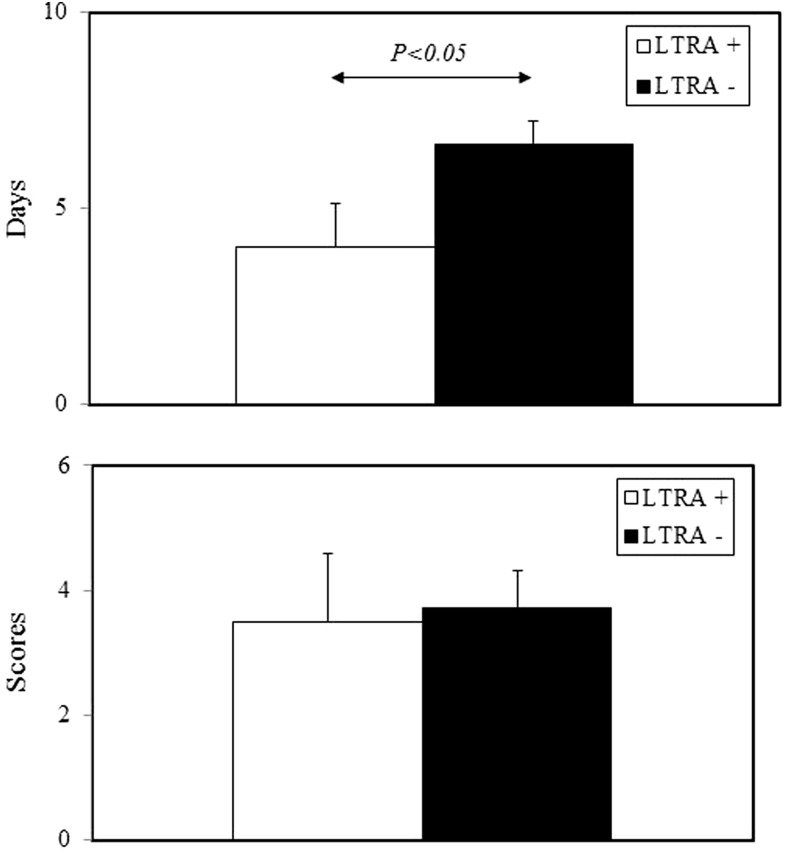
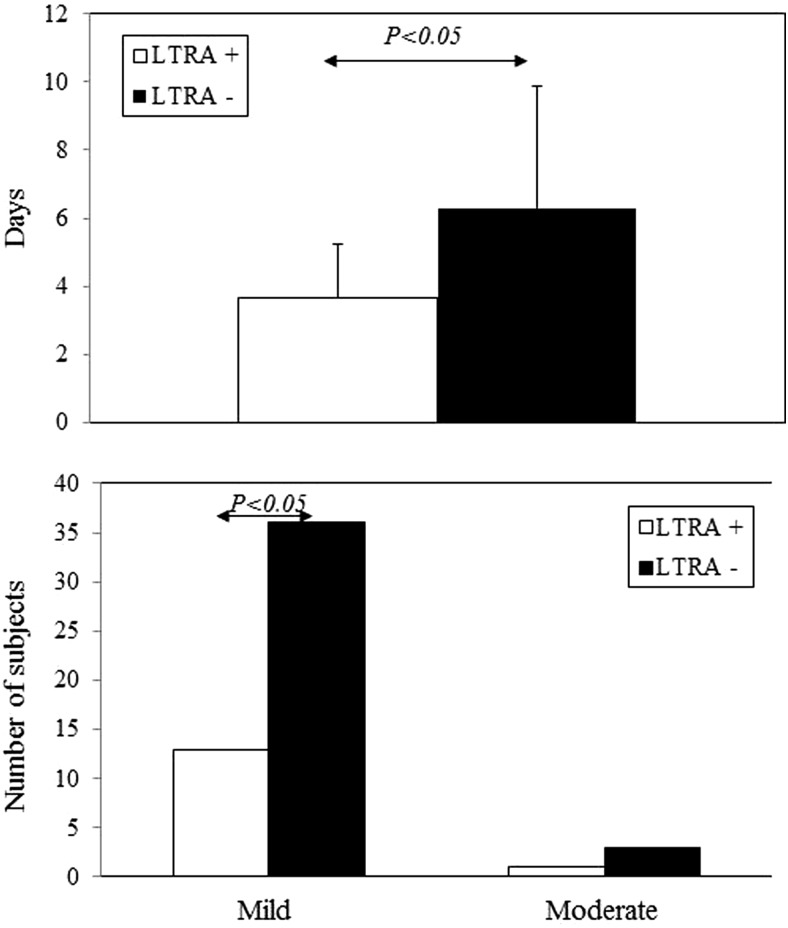
In patients with URI alone (n = 79), LTRA treatment significantly shortened URI duration, but it failed to reduce URI severity (Fig. 2). However, among all patients with acute asthma exacerbations irrespective of URI (n = 53), LTRAs significantly shortened the duration of acute asthma exacerbations and reduced the incidence of mild asthma exacerbations (Fig. 3).
Figure 2.
Effects of leukotriene receptor antagonist (LTRA) treatment on duration (upper) and severity (lower) of upper respiratory tract infection (URI) symptoms. Duration and severity of total URI (n = 79) are compared between the LTRA+ and LTRA− groups. Bars represent means ± SD.
Figure 3.
Effects of leukotriene receptor antagonist (LTRA) treatment on duration (upper) and severity (lower) of acute asthma exacerbation. Among all patients with acute asthma exacerbations irrespective of upper respiratory tract infection (URI; n = 53), the duration and incidences of mild and moderate exacerbations are compared between the LTRA+ and LTRA− groups. Bars represent means ± SD in the upper part and numbers of subjects in the lower part.
Effects of LTRA Treatment on the Clinical Characteristics of URI-Induced Asthma
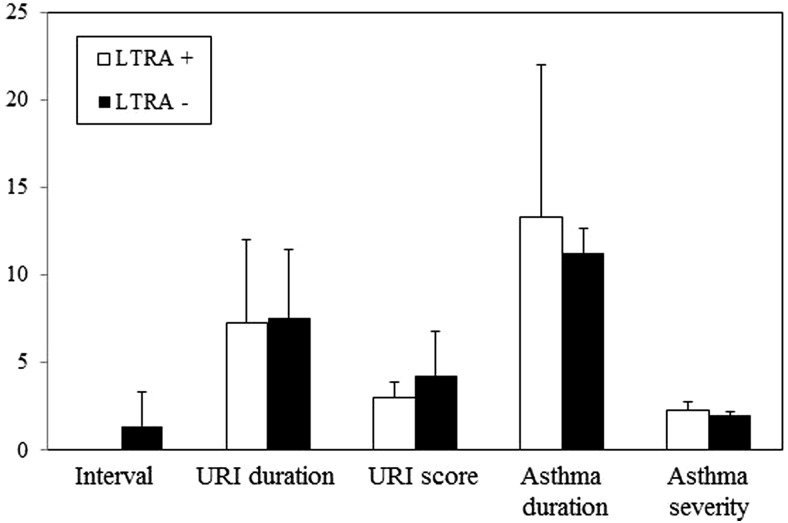
In patients with URI-induced asthma (n = 29), LTRA treatment failed to significantly reduce the interval between URI onset and acute asthma exacerbation, as well as the duration and severity of both URIs and acute asthma exacerbations, as shown in Fig. 4. Thus, although LTRAs decreased the incidences of URIs alone and acute asthma exacerbations alone, it did not prevent URI-induced acute asthma exacerbations once a URI occurred.
Figure 4.
Effects of leukotriene receptor antagonist (LTRA) on clinical characteristics of upper respiratory tract infection (URI)–induced asthma. In patients with URI-induced asthma (n = 29), clinical characteristics are compared between LTRA+ and LTRA− groups. Bars represent means ± SD.
DISCUSSION
The major findings of the present retrospective study were as follows: (i) significantly fewer URIs alone and acute asthma exacerbations without URI occurred in patients with prophylactic daily use of LTRA than in those without prophylactic daily use of LTRA, (ii) prophylactic daily LTRA use significantly shortened the durations of URI alone and acute asthma exacerbation without URI and reduced the incidence of mild exacerbations of asthma, and (iii) prophylactic daily LTRA use did not significantly affect the clinical course of URI-induced acute asthma exacerbations.
Viral respiratory tract infections often exacerbate asthma, which can be significantly reduced by the regular administration of ICSs.13 An additive therapy that would increase the beneficial effects and decrease the toxicity of corticosteroids would be useful. From this viewpoint, cysLTs are appealing because their concentrations increase during respiratory virus-induced acute asthma exacerbations,7–9 and CSs do not inhibit their production.14 In fact, the LTRA montelukast prevents respiratory virus-induced acute asthma exacerbations in children.15–17 We have also reported that pranlukast inhibits respiratory syncytial virus-induced allergic airway inflammation in a murine model of allergic asthma,18 and the combination of an LTRA and CS might be more useful than CS alone for treating URI-induced acute asthma exacerbations and reducing the cumulative CS dose.19
Although LTRAs reduce asthma symptoms or exacerbations in children with colds,15–17 few studies have evaluated their effects on asthma in adults. One study found that LTRAs did not improve symptoms of mild asthma caused by experimental rhinovirus infection in adults.20 Thus, the role of LTRAs in acute asthma exacerbation in adults caused by naturally occurring viral respiratory infection remains unknown. In agreement with the present study, LTRA treatment was associated with a lower incidence of common cold-like symptoms in a retrospective analysis of adult asthma.21 However, contrary to the present results, Kozer et al. reported that montelukast did not reduce the incidence or duration of URI in preschool-aged children who did not have asthma.22 Thus, LTRA treatment may only be effective for asthmatic patients, who have higher LT levels in the airway while stable, to prevent URI symptoms.
It has been reported that LTRA treatment is effective in a subset of asthma, such as aspirin-induced asthma,11 asthma with rhinitis23 and obesity,1 and in current smokers.24 In the present study, the ratio of these subtypes was comparable between the LTRA+ and LTRA− groups. However, there were significantly more female subjects in the LTRA+ group than in the LTRA− group, which could affect the results, because differential responses to LTRAs between girls and boys have been reported.25 Attending physicians at each institute decided to use or not use LTRAs based on their clinical experience. The present study protocol thus could not identify why some patients took LTRAs but others did not during the study period.
Regular use of ICS is the single most effective treatment to reduce the risk of acute exacerbations of asthma. Additional benefit can be obtained with the regular use of combinations of ICS and LABA. Besides LTRA, LABAs also have antiviral as well as bronchodilator effects.26 In the present study, approximately one-half of the patients in both LTRA+ and LTRA− groups had used LABAs, and the incidences of URIs, acute asthma exacerbations without URI, and URI-induced acute asthma exacerbations were comparable between those taking and not taking LABAs (data not shown). A future study should also examine the effects of adding LABAs for URI-induced acute exacerbations of asthma.
The present study had several critical limitations. First, URI was defined based on clinical symptoms without determining causative viruses. Second, asthma-related symptoms were also evaluated based on clinical symptoms, and objective measures of pulmonary function, such as peak expiratory flow or forced expiratory volume in 1 second FEV1.0, were not available. Finally, this was not a prospective, placebo-controlled study. Thus, not all relevant URI and acute asthma exacerbation events could be captured for analysis.
In conclusion, the present findings suggest that LTRAs have the potential to prevent URI and acute asthma exacerbation without URI, but not URI-induced acute asthma exacerbation. Large-scale, placebo-controlled, prospective studies evaluating objective parameters, such as peak expiratory flow and spirometry, are warranted.
Footnotes
Funded in part by a research grant from Ono Pharmaceutical Co., Ltd.
H Matsuse has received lecture fees from AstraZeneca K.K.; Astellas Pharma, Inc.; Abbott Japan Co., Ltd.; Ono Pharmaceutical Co., Ltd.; GlaxoSmithkline K.K.; Novartis Pharma K.K.; and MSD K.K. S Kohno has received research grants from AstraZeneca K.K.; Astellas Pharma, Inc.; Abbott Japan Co., Ltd.; Ono Pharmaceutical Co., Ltd.; GlaxoSmithkline K.K.; Teijin Pharma Limited; Novartis Pharma K.K.; and MSD K.K. and lecture fees from AstraZeneca K.K.; Ono Pharmaceutical Co., Ltd.; and MSD K.K. The remaining authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Peters-Golden M, Swern A, Bird SS, et al. Influence of body mass index on the response to asthma controller agents. Eur Respir J 27:495–503, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Pedersen B, Dahl R, Karlström R, et al. Eosinophil and neutrophil activity in asthma in a one-year trial with inhaled budesonide. The impact of smoking. Am J Respir Crit Care Med 153:1519–1529, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Doull IJ, Lampe FC, Smith S, et al. Effect of inhaled corticosteroids on episodes of wheezing associated with viral infection in school age children: randomised double blind placebo controlled trial. BMJ 315:858–862, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lemanske RF., Jr Viruses and asthma: Inception, exacerbation, and possible prevention. J Pediatr 142:S3–-S7, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ 307:982–986, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tauro S, Su YC, Thomas S, et al. Molecular and cellular mechanisms in the viral exacerbation of asthma. Microbes Infect 10:1014–1023, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dimova-Yaneva D, Russell D, Main M, et al. Eosinophil activation and cysteinyl leukotriene production in infants with respiratory syncytial virus bronchiolitis. Clin Exp Allergy 34:555–558, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Gentile DA, Fireman P, Skoner DP. Elevations of local leukotriene C4 levels during viral upper respiratory tract infections. Ann Allergy Asthma Immunol 91:270–274, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Matsuse H, Kondo Y, Saeki S, et al. Naturally occurring parainfluenza virus 3 infection in adults induces mild exacerbation of asthma associated with increased sputum concentrations of cysteinyl leukotrienes. Int Arch Allergy Immunol 138:267–272, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Ohta K. The essence of “Asthma prevention and management guideline 2012, Japan (JGL 2012)” for adults. Arerugi 62:139–143, 2013 [PubMed] [Google Scholar]
- 11. Obase Y, Shimoda T, Tomari SY, et al. Effects of pranlukast on chemical mediators in induced sputum on provocation tests in atopic and aspirin-intolerant asthmatic patients. Chest 121:143–150, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ 307:982–986, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Venarske DL, Busse WW, Griffin MR, et al. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis 193:1536–1543, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dworski R, Fitzgerald GA, Oates JA, Sheller JR. Effect of oral prednisone on airway inflammatory mediators in atopic asthma. Am J Respir Crit Care Med 149:953–959, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Bisgaard H, Zielen S, Garcia-Garcia ML, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med 171:315–322, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Johnston NW, Mandhane PJ, Dai J, et al. Attenuation of the September epidemic of asthma exacerbations in children: A randomized, controlled trial of montelukast added to usual therapy. Pediatrics 120:e702-–e712, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Robertson CF, Price D, Henry R, et al. Short-course montelukast for intermittent asthma in children: A randomized controlled trial. Am J Respir Crit Care Med 175:323–329, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Matsuse H, Kondo Y, Machida I, et al. Effects of anti-inflammatory therapies on recurrent and low-grade respiratory syncytial virus infections in a murine model of asthma. Ann Allergy Asthma Immunol 97:55–60, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Matsuse H, Fukahori S, Tsuchida T, et al. Effects of a short course of pranlukast combined with systemic corticosteroid on acute asthma exacerbation induced by upper respiratory tract infection. J Asthma 49:637–641, 2012 [DOI] [PubMed] [Google Scholar]
- 20. Kloepfer KM, DeMore JP, Vrtis RF, et al. Effects of montelukast on patients with asthma after experimental inoculation with human Rhinovirus 16. Ann Allergy Asthma Immunol 106:252–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horiguchi T, Ohira D, Kobayashi K, et al. Clinical evaluation of leukotriene receptor antagonists in preventing common cold-like symptoms in bronchial asthma patients. Allergol Int 56:263–267, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Kozer E, Lotem Z, Elgarushe M, et al. RCT of montelukast as prophylaxis for upper respiratory tract infections in children. Pediatrics 129:e285–-e290, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Price DB, Swern A, Tozzi CA, et al. Effect of montelukast on lung function in asthma patients with allergic rhinitis: Analysis from the COMPACT trial. Allergy 61:737–742, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Lazarus SC, Chinchilli VM, Rollings NJ, et al. National Heart Lung and Blood Institute's Asthma Clinical Research Network. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med 175:783–790, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rabinovitch N, Strand M, Stuhlman K, Gelfand EW. Exposure to tobacco smoke increases leukotriene E4-related albuterol usage and response to montelukast. J Allergy Clin Immunol 121:1365–1371, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Singam R, Jena PK, Behera S, et al. Combined fluticasone propionate and salmeterol reduces RSV infection more effectively than either of them alone in allergen-sensitized mice. Virol J 3:32, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]