Abstract
Chronic rhinosinusitis (CRS) can lead to serious long-term adverse sequelae, particularly if left untreated. The aim of this study was to describe a series of intracranial mucoceles (ICMs) that arose in the context of longstanding CRS combined with a review of the pertinent literature. A retrospective chart review was performed on all patients who developed ICMs in association with CRS between 2003 and 2012. The clinical presentation, radiographic features, surgical approach, intraoperative findings, and patient outcome were examined in the context of a literature review. Sixty-five cases of mucoceles were identified in patients with a history of CRS, of which seven (10.8%) were intracranial. Five patients were men and two were women with a mean age of 42.1 years. Headache, facial pressure, retro-orbital pain, and visual disturbances were the most common presenting symptoms. Five of the seven had previously undergone sinonasal surgery. Imaging studies showed ICMs involving the anterior cranial fossa, two of which were bilateral. Latency between onset of CRS and ICM detection ranged from 3 to 19 years (mean, 9.4 years). All patients underwent endoscopic transnasal drainage with three also requiring a concurrent, open neurosurgical procedure to access the intracranial component. There were no postoperative complications, and no recurrences were observed after a mean follow-up of 2.7 years. ICMs presenting as delayed complications of CRS are uncommon and constitute a surgical challenge. Open, external skull base approaches used in conjunction with transnasal endoscopic drainage procedures may be necessary to achieve successful management of this rare condition.
Keywords: Chronic rhinosinusitis, complications, intracranial mucoceles
Chronic rhinosinusitis (CRS) can lead to intracranial complications with serious adverse neurological sequelae when disease spreads beyond the confines of the paranasal sinuses.1 Intracranial mucocele (ICM) formation is an uncommon, but potentially devastating, long-term complication of CRS.1 Mucoceles are benign, slow-growing, expansile lesions composed of a respiratory epithelium-lined cavity filled with mucoid secretions.2 Development is gradual, with peak incidence between the third and fourth decades of life.3,4 No gender predilection has been reported.3,4 Primary mucoceles are postulated to arise from the obstruction of a minor salivary gland duct within the lining of the paranasal sinus.5 Also known as mucus retention cysts, these are commonly found within the maxillary sinus.5 No predisposing conditions can usually be identified.3 Secondary mucoceles stem from the blockage of sinus ostia.5 The frontal sinuses are the most frequently involved (60–65%) followed by the ethmoid (20–30%), maxillary (10%), and sphenoid sinuses (2–3%).6 The pathogenesis of secondary mucocele formation is multifactorial.3 Mucosal hyperplasia, allergy, inflammation, trauma, fibrosis, scarring, fibrous dysplasia, or neoplasms (benign or malignant) can impede ventilation and distort sinus outflow drainage pathways.2,3,7 Sequestration and long-term retention of residual mucosa within a confined space have also been cited as contributing factors.3 Continued mucus production with accumulation of mucinous material results in gradual erosion and remodeling of the bony walls, with subsequent distention of the obstructed sinus.6 Prior surgery, craniofacial trauma, nasal polyposis (NP), and CRS have all been reported to precede secondary mucocele formation.3,5
In the intracranial cavity, mucoceles typically originate from the paranasal sinuses.8 Although uncommon, when sinus mucocele growth is left unchecked, pressure-induced necrosis can lead to destruction of adjacent bony structures and progressive intracranial extension (ICE). Signs and symptoms are often subtle with an insidious onset.8 However, compression of vital neurovascular structures due to mass effect or infection of the mucocele (mucopyocele) with intracranial rupture can cause catastrophic neurological consequences.9 Therefore, a high index of suspicion and timely recognition are pivotal in avoiding inordinate delays in diagnosis and averting potentially life-threatening consequences of intracranial expansion or infection.9 We present a case series of ICMs that arose in the context of longstanding CRS, which were successfully treated using combined external and/or endoscopic drainage procedures with reconstruction of the skull base defect. The clinical presentation, radiographic features, surgical approach, intraoperative findings, and patient outcome are discussed.
MATERIALS AND METHODS
A retrospective chart review was performed on all patients with a history of CRS that developed ICMs between 2003 and 2012 as detected by imaging and histology. This study was approved by the Southern California Permanente Medical Group Institutional Review Board. The requirement for informed consent was waived. Data collected included age, gender, clinical presentation, site of involvement, imaging studies, operative findings, culture results, treatment, complications, recurrence, and follow-up.
RESULTS
Sixty-five patients with a history of CRS who developed mucoceles were identified during the study period, seven (10.8%) of whom exhibited ICE and comprised the primary focus of the series. The frontal (3/7) and ethmoid (3/7) sinuses were the most frequent sites of involvement, followed by the sphenoid sinus (2/7). In six cases, ICMs arose as extensions of paranasal sinus mucoceles, while in the remaining patient the ICM likely developed as a complication of endoscopic cerebrospinal fluid (CSF) leak repair. The details of that particular case are described later in text.
Patient results are summarized in Table 1. There were five men and two women, with a mean age of 42.1 years (range, 15–72 years). Headache (n = 7), facial pain/pressure (n = 4), and orbital complaints (n = 5) were the most common presenting symptoms. With respect to the latter, diplopia (n = 4), retro-orbital pain (n = 3), ptosis (n = 2), and diminished visual acuity (n = 2) were reported. Vertigo was also noted in one patient with sphenoid sinus involvement. All intracranial cases were secondary mucoceles, with five having a history of prior sinus surgery and four having undergone multiple sinonasal procedures. Latency between the onset of CRS and mucocele detection ranged from 3 to 19 years (mean, 9.4 years). All patients met the criteria for diagnosis of CRS as defined by the 2007 American Academy of Otolaryngology–Head and Neck Surgery Foundation Clinical Practice Guidelines on adult rhinosinusitis, with three showing CRS with NP. No patients reported a history of facial trauma.
Table 1.
Demographic data and outcome
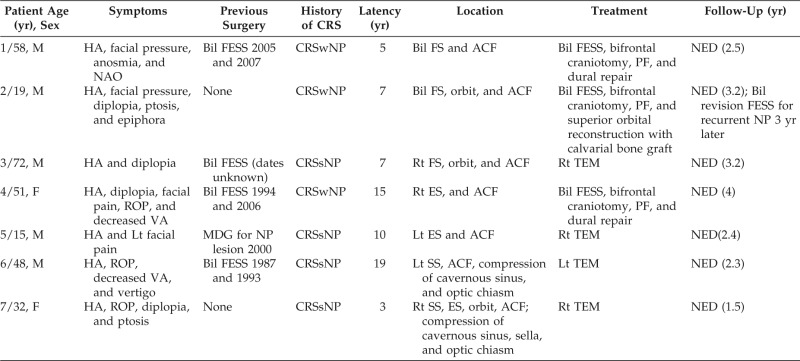
M = male; F: female; HA = headache; Lt = left; Rt: right; VA = visual acuity; ROP = retro-orbital pain; Bil = bilateral; FESS = functional endoscopic sinus surgery; MDG = midface degloving; NP = nasopharyngeal; CRSwNP = chronic rhinosinusitis with nasal polyposis; CRSsNP = chronic rhinosinusitis without nasal polyposis; FS = frontal sinus; ES = ethmoid sinus; SS = sphenoid sinus; ACF = anterior cranial fossa; PF = pericranial flap; TEM = transnasal endoscopic marsupialization; NED = no evidence of recurrent disease.
Computed tomography (CT) and magnetic resonance imaging (MRI) studies revealed expansion of the affected sinus with erosion of the posterior table (n = 3) or skull base (n = 6) and extension of the mucocele into the anterior cranial fossa (ACF). All seven cases were managed by transnasal, endoscopic procedures with three requiring additional external approaches (bifrontal craniotomy) in conjunction with neurosurgery to access the intracranial component (Figs. 1–8). A pericranial flap was used to reconstruct the anterior skull base defect in three patients, and split calvarial bone graft was used for reconstruction of the orbital rim in patient 2. There were no postoperative complications and no evidence of mucocele recurrence 1.5–4 years (mean, 2.7 years) after surgery. However, one patient required bilateral revision endoscopic sinus surgery for recurrent NP 3 years after mucocele excision.
Figure 1.
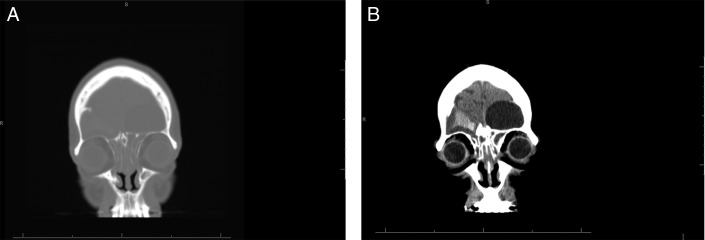
Patient 1. Preoperative coronal CT images with (A) bone and (B) soft tissue windows show bilateral, expansile lesions of the frontal sinuses with thinning and erosion of the sinus walls. Extension into the intracranial vault is evident with mass effect upon the bifrontal lobes. CT, computed tomography.
Figure 2.
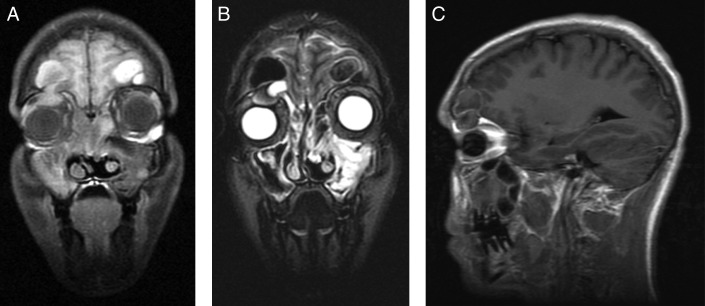
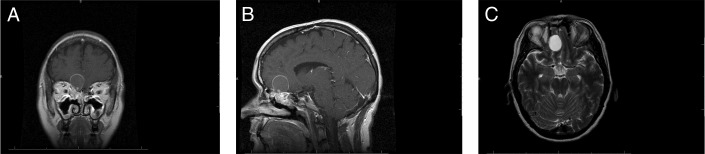
Patient 1. Preoperative (A) coronal T1-MRI postcontrast, (B) coronal T2-MRI, and (C) axial T2-MRI show 4.5 × 4.4-cm cystic lesions arising from the frontal sinuses bilaterally with expansion into the anterior cranial fossa and compression of the frontal lobes. Mild peripheral enhancement is present. MRI, magnetic resonance imaging.
Figure 3.
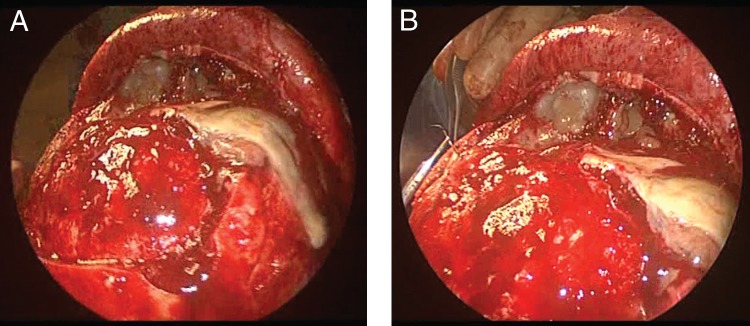
Patient 1. Intraoperative photographs of (A) bifrontal craniotomy with (B) dural repair. Note mucopurulent drainage observed on exposure of the frontal sinus.
Figure 4.
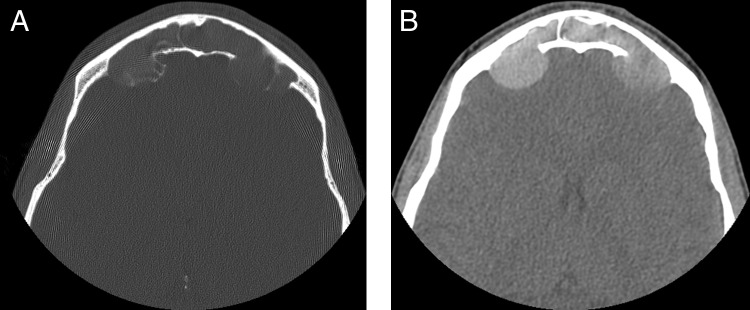
Patient 2. Preoperative axial CT images with (A) bone and (B) soft tissue windows show bilateral, expansile, smoothly marginated lesions of the frontal sinuses with erosion of the posterior table and extension into the anterior cranial fossa. CT, computed tomography.
Figure 5.
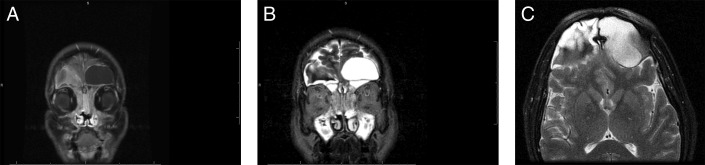
Preoperative (A) coronal T1-MRI postcontrast, (B) coronal T2-MRI, and (C) sagittal T1-MRI postcontrast show bilateral expansile lesions of variable density within the frontal sinuses measuring 2.3 cm on the right and 2.1 cm on the left. Extension into the bilateral anterior cranial fossa and the superior aspect of the right orbit are present with inferior displacement of the right globe. MRI, magnetic resonance imaging.
Figure 6.
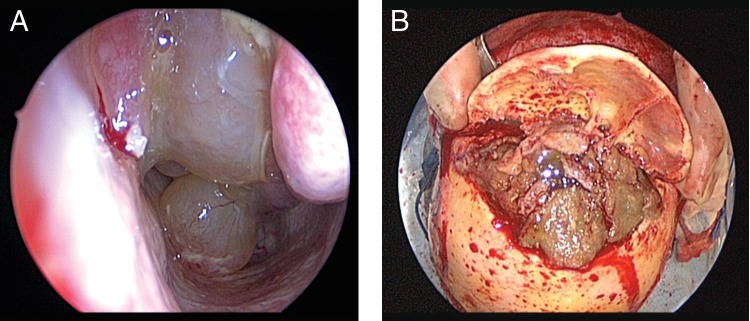
Patient 2. Intraoperative (A) nasal endoscopy showed polyps extending from the roof of the nasal cavity to the floor, and (B) bifrontal craniotomy revealed thick, inspissated mucinous secretions within the frontal sinuses bilaterally.
Figure 7.
Preoperative (A) coronal, (B) sagittal T1-MRI postcontrast, and (C) axial T2-MRI depict a 2.6 × 2.4 × 2.4-cm spherical, well-circumscribed extra-axial lesion centered to the right of midline arising from the ethmoid roof. Extension into the olfactory groove and intracranial compartment are evident with displacement of the right frontal lobe. Note that the mass is isointense and hyperintense on T1- and T2-weighted images, respectively, with a peripheral rim of smooth enhancement. An additional focal area of nodular enhancement involving the inferior aspect of the lesion is also present. MRI, magnetic resonance imaging.
Figure 8.
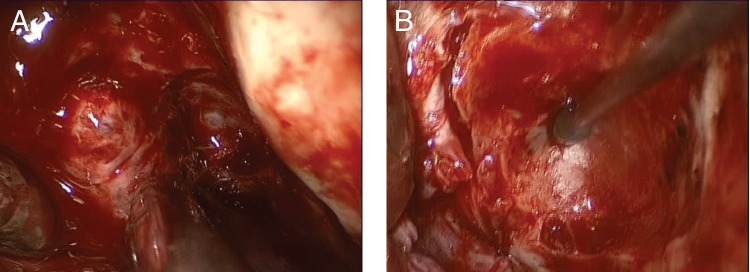
Intraoperative photographs of bifrontal craniotomy. (A) The frontal lobes have been retracted to expose the roof of the mucocele. (B) View of skull base after evacuation and resection of intracranial mucocele. Note scalloped indentation of underlying bone secondary to erosion from overlying mucocele.
Macroscopically, thin, straw-colored, yellow–brown fluid was typically encountered on drainage of the mucocele. The only exception was found in patient 2, in which extremely thick, viscous, gold–brown, mucinous debris was observed filling both frontal sinuses and the intracranial component of the mucocele (Fig. 6 B). Microscopically, the histological features characteristic of a mucocele were seen, although without a radiographic correlation, they are very nonspecific. Respiratory epithelium with ciliated columnar cells was present along with areas of fibrosis immediately adjacent to bone fragments (Fig. 9). The strips of respiratory epithelium associated with a very thin submucosal fibrosis is quite characteristic, although not unique to mucocele. Aerobic, anaerobic, and fungal cultures from the material within the mucoceles were obtained in four cases, all of which were negative.
Figure 9.
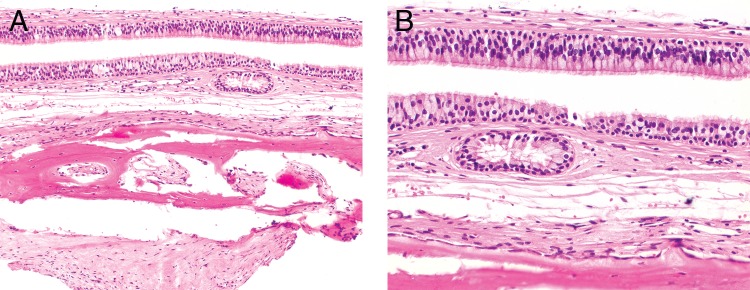
(A) Histology shows two layers of respiratory epithelium subtended by a loose connective tissue stroma overlying bone. There are easily identified cilia, a finding that belies the incredible pressure that can be seen in mucocele. (B) High power shows the very even stratification of the nuclei with luminal cilia.
Case 4
A 51-year-old woman was referred to the rhinology clinic complaining of worsening right-sided headache, retro-orbital pain, diplopia, and blurred vision for 2 years. Her prior medical history was significant for CRS with NP, managed with bilateral functional endoscopic sinus surgeries (FESS) in 1994 and 2006, each at a different facility. The 2006 procedure was complicated by an intraoperative CSF leak with violation of the right cribriform plate, which was repaired using a free mucosal graft from the inferior turbinate. The patient denied CSF rhinorrhea at the time of the current presentation.
On nasal endoscopy, synechiae and inflammatory polyps were appreciated within the ethmoid sinuses bilaterally. No clear discharge was visualized in the right nasal cavity or in the nasopharynx. A CT scan showed evidence of prior surgery and opacification of the bilateral maxillary, ethmoid, frontal, and sphenoid sinuses. MRI revealed a 2.6 × 2.4 × 2.4-cm spherical, well-circumscribed extra-axial lesion centered to the right of midline, arising from the ethmoid roof. Extension into the olfactory groove and intracranial compartment was evident with displacement of the right frontal lobe. The mass was isointense and hyperintense on T1- and T2-weighted images, respectively, with a peripheral rim of smooth enhancement. There was a focal area of nodular enhancement involving the inferior aspect of the lesion (Fig. 7).
The patient underwent a combined transnasal endoscopic subfrontal approach to the ACF for mucocele excision and extirpation of concurrent sinonasal disease. Triplanar stereotactic imaging was obtained in preparation for computer-assisted surgical navigation. Bilateral revision maxillary antrostomies, ethmoidectomies, sphenoidotomies, and frontal sinusotomies were performed. The area of prior skull base repair could be seen endoscopically, with the inferior portion of the mucocele eroding through the ethmoid roof. A bifrontal craniotomy was then performed with neurosurgery for drainage and removal of the mucocele. The capsule was found to be densely adherent to the dura of the right frontal lobe, and a dural defect created by the mucocele was repaired primarily. After mucocele resection, the underlying bone was found to be scalloped and indented, likely secondary to pressure necrosis from the mucocele (Fig. 8). The anterior skull base was then reconstructed using a vascularized pericranial flap. The final pathology showed characteristic features of a mucocele. The patient experienced complete resolution of her symptoms postoperatively and at last follow-up (3 years) had no evidence of mucocele recurrence or CSF leak.
DISCUSSION
ICM formation is a rare, but potentially devastating complication of longstanding CRS.1 Clinical symptoms are usually subtle and insidious in onset, with patients often asymptomatic until significant orbital and/or intracranial expansion has occurred.10 Frontal pain and headache are the most common presenting symptoms.4,8 However, proptosis, retro-orbital pain, diplopia, and visual disturbances may also be present.10 Orbital symptoms have been reported in up to 70% of frontoethmoid mucoceles.2 Nasal obstruction may also be noted in cases with concurrent sinus disease.10 Previous surgery has been associated with paranasal sinus mucocele development, with a reported incidence of 35–66%, in line with 71% of patients in this series.2,11,12 Latency between initial surgery and mucocele clinical presentation ranged from 3 to 15 years (mean, 7.5 years), comparable with time intervals observed in other studies (2.8–24 years).13,14
Physical examination may be normal or can reveal frontal tenderness, forehead bossing, periorbital swelling, exophthalmos, altered visual acuity, or restricted extraocular movements.4,15 Similarly, nasal endoscopy may be normal or show NP, mucopurulent discharge, CSF leak, or mucosal edema.4,10 Imaging studies, particularly thin cut axial/coronal CT and MRI, help to delineate the shape, size, location, and extent of the mucocele. Distention of the affected sinus is usually evident with thinning of the bony walls and displacement of adjacent neurovascular structures.8 On CT, mucoceles are typically hypodense or isodense with the brain.5,8 There is no enhancement initially, but density of the mucocele contents may increase as the mucus becomes more inspissated.5 On MRI, the appearance of mucoceles is variable from hyperintensity, isointensity, or hypointensity on T2-weighted images, depending on the degree of hydration and protein composition (Fig. 5 B).5 The lower the water content, the lower the signal intensity.5 On T1-weighted scans with fat suppression, gadolinium enhancement is usually not present, apart from the lining of the mucocele, which can help differentiate them from solid neoplasms. Axial views show the anterior–posterior dimensions of the mucocele and degree of expansion into the ACF. Histologically, mucoceles are lined with a normal respiratory mucosa (pseudostratified ciliated columnar epithelium) with a background of chronic inflammation and fibrosis. Rare cases may show metaplastic squamous epithelium, but the ciliated respiratory mucosa is the most frequently found lining.10,16,17
Traditionally, evacuation and complete excision of the capsule was considered the treatment of choice for sinonasal mucoceles to minimize the risk of recurrence.8 The type of surgical approach was dictated by the site, extent, and associated complications with the goals of providing maximum exposure but still optimizing cosmesis.8,15 To that end, open obliterative approaches were initially the most commonly used.18 However, after the development of advanced endoscopic techniques and instrumentation, transnasal marsupialization emerged as a more effective, less invasive method. With successful outcomes and low incidence of recurrence (0.9–17%), endoscopic drainage procedures ultimately supplanted open, ablative surgeries as the gold standard in the surgical treatment of paranasal sinus mucoceles.2,17 However, in terms of ICMs, choice of surgical approach is still contingent on degree of cranial fossa infiltration and drainage accessibility of the intracranial component. Consequently, external procedures continue to play a role in their management. In cases of frontal mucoceles with posterior table erosion but minimal protrusion into the epidural space of the ACF, transnasal endoscopic marsupialization has been successfully used.15,17 As long as the mucocele is widely opened, removal of the mucosal lining from the dura is not usually indicated and is avoided to reduce the risk of complications.15 The same paradigm can be applied to patients with sphenoethmoid mucoceles (SEMs) with skull base erosion but minimal ICE.19 However, in cases where significant intracranial involvement is present along with frontal lobe compression, open transcranial surgery may still be necessary to eradicate the lesion and adequately drain the intracranial portion of the mucocele.8 The type of external approach can be adapted according to the degree of exposure needed.10 Osteoplastic flaps are sufficient in most cases, but the addition of a limited craniotomy may be required if more lateral or posterior extension is required.10 If the capsule of the mucocele is loosely adherent, it may be detached without violation of the dura. In the event that dural defects are present or tears are created, these can be repaired primarily with sutures and fibrin glue.8 A pericranial flap is typically used to reconstruct the skull base defect and floor of the ACF.4 Emergent surgical intervention for mucoceles are seldom necessary.19 However, rare infection of the mucocele can initiate life-threatening meningitis or brain abscess, in which case prompt surgical intervention is vital to prevent adverse neurological sequelae.19 Long-term follow-up is needed because of the indolent nature of mucocele formation and protracted latency (months–years) often observed between the inciting episode and time of recurrence.19
The incidence of skull base erosion secondary to paranasal sinus mucoceles has been reported to range from 10 to 35%.8,20 However, comprehensive review of the literature revealed few studies that specifically detailed the management of invasive sinus mucoceles with ICE (Table 2).10 Unlike strictly paranasal sinus mucoceles in which transnasal endoscopic marsupialization is considered to be the gold standard, open approaches still play a major role in the treatment of ICM, particularly those of frontal sinus origin. Weitzel et al., presented five cases of extensive fronto-orbital mucoceles, three of which showed ICE.10 Four were ascribed to recurrent sinusitis and one had a history of facial trauma. The period between the precipitating event and mucocele development ranged from 1 month to 16 years. All were managed via an open, bicoronal approach with frontal sinus cranialization. Dural repair was required in two patients, and split thickness calvarial bone grafts were used to reconstruct the orbitocranial skeleton.10 Likewise, Suri et al.21 reported four cases of giant frontal mucoceles managed with an external approach. These are defined as mucoceles of the frontal sinuses with orbital, extracranial, and ACF extension. All were pediatric patients (range, 10–16 years) who presented with orbital (proptosis) and/or neurological manifestations as well as craniofacial disfigurement (forehead swelling).21 A unilateral fronto-orbital craniotomy was performed in all cases to drain the mucocele, and the frontal sinus obliterated with temporalis muscle. A pericranial flap was then used to reconstruct the defect. The postoperative course was complicated by meningitis in one patient and a CSF leak in two others, which were managed conservatively with antibiotics and lumbar drainage, respectively.21 In this study, two patients fulfilled criteria for giant frontal mucoceles but unlike the series of Suri et al.,21 both also had concomitant CRS with NP. Consequently, a transnasal, endoscopic procedure was also needed to address the concurrent sinonasal disease in addition to the open transcranial approach used to access the intracranial portion of the mucocele.
Table 2.
Literature summary of case series of ICM
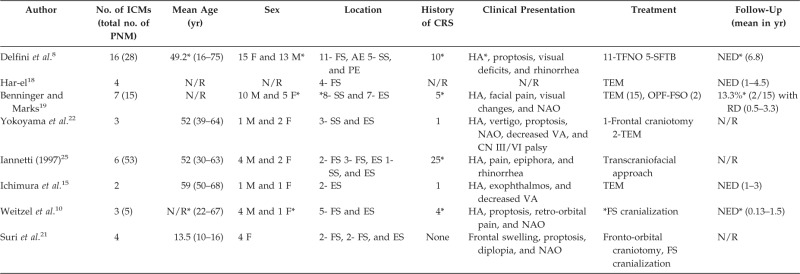
*Refers to entire patient cohort, not just those with intracranial extension.
ICM = intracranial mucoceles; PNM = paranasal sinus mucoceles; M = male; F = female; AE = anterior ethmoid; PE = posterior ethmoid; ES = ethmoid sinus; FS = frontal sinus; SS = sphenoid sinus; CRS = chronic rhinosinusitis; HA = headache; NAO = nasal airway obstruction; VA = visual acuity; CN = cranial nerve; TFNO = transfrontonaso-orbital; SFTB = subfrontal transbasal; TEM = transnasal endoscopic marsupialization; OPF = osteoplastic flap; FSO = frontal sinus obliteration; N/R = not reported; NED = no evidence of disease; RD = recurrent disease; CSF = cerebrospinal fluid.
The largest case series of ICMs was reported by Delfini et al.8 Sixteen of 28 patients identified from 1959 to 1991 with paranasal sinus mucoceles displayed intracranial involvement; 5 originated from the posterior ethmoid and 11 from the anterior ethmoid cavity and/or frontal sinus. Ten (35%) of the 28 had either a history of allergic rhinitis or sinusitis, although it was not specified if these were the same patients with ICE. Transfrontonasoorbital (n = 11) and subfrontal transbasal (n = 5) procedures were used for anterior- (anterior ethmoid, frontal sinus) and posterior (posterior ethmoid, sphenoid sinus)-based ICMs, respectively. Frontal sinuses were obliterated with bone wax, galea, and fibrin glue to seal it from the epidural space. The specific outcome of those patients with intracranial involvement was not given. However, collectively, 2 of 28 developed CSF rhinorrhea, which required subsequent repair and an additional 2 had recurrent mucocele which resulted in revision surgery. After these secondary procedures, 27 did well without evidence of recurrence after a mean follow-up of 6.8 years (range, 0.5–20 years).8 Axiomatic, however, this series predated the advent of advanced endoscopic instrumentation, which may be why combined approaches were not performed to address any concurrent sinonasal disease.
SEMs with ICE are even more uncommon than those that originate from the frontal sinus.19 However, they have been shown to be more amenable to a purely endoscopic approach.19 Yokoyama et al. presented three patients with SEM with invasion into the anterior (n = 1) or middle cranial fossa (n = 2).22 One patient was drained via frontotemporal craniotomy, and the other two patients were drained via transnasal sphenoidotomy. Likewise, Benninger described seven of 15 SEMs with ICE.19 All 15 underwent transnasal endoscopic drainage, with two further managed with an osteoplastic flap with frontal sinus obliteration; 13.3% developed recurrence after a mean follow-up of 20 months. Specific outcomes and management for those cases with ICE were not reported. However, the authors advocated endoscopic drainage for all SEMs even with orbital or ICE.19 Similarly, in this series, three patients with ICM secondary to sphenoid or ethmoid mucoceles were successfully treated with endoscopic marsupialization.
ICM formation after endoscopic CSF fistula repair has been previously documented only twice in the literature.23,24 Eloy et al. described a patient who developed an ethmoid mucocele that extended into the ACF 5 years after undergoing FESS and concurrent repair of an iatrogenic CSF leak with a middle turbinate mucosal autograft.23 Mucocele excision was accomplished via a combined endoscopic transnasal and subcranial procedure. A vascularized pericranial flap with multilayered, autogenous fascia lata were used to reconstruct the skull base defect. There was no clinical or radiographic evidence of recurrence 2 years after surgery.23 Wang et al., presented a patient who was found to have an expansile, anterior cranial base mass 2 years after FESS and intraoperative CSF leak repair of the posterior cribriform using grafts from the middle turbinate. The lesion was removed via a right frontal craniotomy, and histology showed a mucocele. No follow-up information was reported.24
In both cases, ICM formation was attributed to improper graft positioning or dislodgement, with the mucosal surface facing superiorly toward the ACF instead of inferiorly toward the nasal cavity. Entrapped, traumatized mucosa with sequestered mucinous glands induces development of mucus retention cysts, which, with continued growth, ultimately evolve into mucoceles. Placement of the mucosal graft below the skull base can initially cause an intranasal, extracranial mucocele to develop. Progressive enlargement with adjacent bony pressure erosion results in ICE. Alternatively, inappropriate graft deployment into the ACF can directly lead to ICM formation with the associated complications, as seen in our patient (case 4). Precision and attention to detail are critical during skull base defect repair to ensure proper graft positioning and avoidance of such complications.
CONCLUSION
Although uncommon, ICMs can develop in the setting of longstanding CRS, particularly in patients with a history of prior sinonasal surgery. Mucoceles may originate as extensions of paranasal sinus mucoceles or, rarely, as complications of CSF leak repair. Irrespective of origin, progressive growth can lead to adverse neurological sequelae secondary to compression of vital neurovascular structures. Although initial symptoms are often subtle and insidious in onset; timely diagnosis and prompt intervention with surgical drainage are paramount to avoid complications. The type of approach is determined by the areas affected and degree of intracranial involvement, with a combined endoscopic/open procedure often needed in cases with substantial cranial fossa extension.
Footnotes
Submitted for presentation to the American Rhinologic Society Fall Meeting, Vancouver, Canada, September 28, 2013
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Young WF, Rosenwasser RH. Intracranial extension of a frontoethmoidal sinus mucocele: A delayed complication of chronic sinusitis. NY State J Med 8:163–164, 1991 [PubMed] [Google Scholar]
- 2. Obeso S, Llorento JL, Rodrigo JP, et al. Paranasal sinus mucoceles. Our experience in 72 patients. Acta Otorrinolaringol 60:332–339, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Fu CH, Chang KP, Lee TJ. The difference in anatomical and invasive characteristics between primary and secondary paranasal sinus mucoceles. Otolaryngol Head Neck Surg 136:621–625, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Stiernberg CM, Bailey BJ, Calhoun KH, et al. Management of invasive frontoethmoid sinus mucoceles. Arch Otolaryngol Head Neck Surg 112:1060–1063, 1986 [DOI] [PubMed] [Google Scholar]
- 5. Epstein VA, Kern RC. Invasive fungal sinusitis and complications of rhinosinusitis. Otolaryngol Clin North Am 41:497–524, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Perugini S, Pasquini U, Menichelli F, et al. Mucoceles in the paranasal sinuses involving the orbit: CT signs in 43 cases. Neuroradiology 23:133–139, 1982 [DOI] [PubMed] [Google Scholar]
- 7. Pool JL, Potanos JN, Krueger EG. Osteomas and mucoceles of the frontal paranasal sinuses. J Neurosurg 19:130–135, 1962 [DOI] [PubMed] [Google Scholar]
- 8. Delfini R, Missori P, Iannetti G, et al. Mucoceles of the paranasal sinuses with intracranial and intraorbital extension: Report of 28 cases. Neurosurgery 32:901–906, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg 137:S1–-S31, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Weitzel EK, Hollier LH, Calzada G, et al. Single stage management of complex fronto-orbital mucoceles. J Craniofacial Surg 13:739–745, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Bockmuhl U, Kratzsch B, Benda K, Draf W. Surgery for paranasal sinus mucoceles: Efficacy of endonasal micro–endoscopic management and long–term results of 185 patients. Rhinology 44:62–67, 2006 [PubMed] [Google Scholar]
- 12. Serrano E, Klossek JM, Percodani J, et al. Surgical management of paranasal sinus mucoceles: A long–term study of 60 cases. Otolaryngol Head Neck Surg 131:133–140, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Rombaux P, Bertrand B, Eloy P, et al. Endoscopic endonasal surgery for paranasal sinus mucoceles. Acta Otorhinolaryngol Belg 54:115–122, 2000 [PubMed] [Google Scholar]
- 14. Moriyama H, Hesaka H, Tachibana T, Honda Y. Mucoceles of ethmoid and sphenoid sinus with visual disturbance. Arch Otolaryngol Head Neck Surg 118:142–146, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Ichimura K, Ohta Y, Maeda YI, Sugimura H. Mucoceles of the paranasal sinuses with intracranial extension-postoperative course. Am J Rhinol 15:243–247, 2001 [PubMed] [Google Scholar]
- 16. Stankiewicz JA, Newell DJ, Park AH. Complications of inflammatory diseases of the sinuses. Otolaryngol Clin North Am 26:639–655, 1993 [PubMed] [Google Scholar]
- 17. Har-El G. Endoscopic management of 108 sinus mucoceles. Laryngoscope 111:2131–2134, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Har-el G. Telescopic extracranial approach to frontal mucoceles with intracranial extension. J Otolaryngol 24:98–101, 1995 [PubMed] [Google Scholar]
- 19. Benninger MS, Marks S. The endoscopic management of sphenoid and ethmoid mucoceles with orbital and intracranial extension. Rhinology 33:157–161, 1995 [PubMed] [Google Scholar]
- 20. Hurley DB, Javer AR, Kuhn FA, Citardi MJ. The endoscopic management of chronic frontal sinusitis associated with frontal sinus posterior table erosion. Am J Rhinol 14:113–120, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Suri A, Mahapatra AK, Gaikwad S, Sarkar C. Giant mucoceles of the frontal sinus: A series and review. J Clin Neurosci 11:214–218, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Yokoyama T, Inoue S, Imamura J, et al. Sphenoethmoidal mucoceles with intracranial extension—Three case reports. Neurol Med Chir 36:822–828, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Eloy JA, Fatterpekar GM, Bederson JB, Shohet MR. Intracranial mucocele: An unusual complication of cerebrospinal fluid leakage repair with middle turbinate mucosal graft. Otolaryngol Head Neck Surg 137:350–352, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Wang L, Kim Ju, Heilman CB. Intracranial mucocele as a complication of endoscopic repair of cerebrospinal fluid rhinorrhea: Case report. Neurosurgery 45:1243, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Iannetti G, Cascone P, Valentini V, et al. Paranasal sinus mucocele: Diagnosis and treatment. J Craniofac Surg 8:391–398, 1997 [DOI] [PubMed] [Google Scholar]