Abstract
Background
Methotrexate (MTX) is an anti-metabolite drug widely used in treatment of neoplastic disorders, rheumatoid arthritis and psoriasis. The ester derivative, ethyl pyruvate (EP) is stable in solution and should function as an antioxidant and energy precursor. This study was conducted to evaluate the protective role of EP on sperm parameters, testosterone level and malondialdehyde (MDA) production in mice treated with MTX.
Methods
32 adult male NMRI mice weighing 26±2 g were divided into 4 groups. Group 1 received 0.1 ml/mice/day of distilled water intraperitoneally for 30 days (ip). Group 2 was treated with methotrexate at a dose of 20 mg/kg once a week (ip) for 30 days. Group 3 was treated with ethyl pyruvate at a dose of 40 mg/kg/daily (ip) for 30 days. Group 4 was treated with methotrexate (20 mg/kg) once a week simultaneously with ethyl pyruvate 40 mg/kg for 30 days. The results were analyzed by oneway ANOVA. A p<0.05 was considered to be significant.
Results
The results showed significant (p<0.05) decrease in sperm count and sperm motility as well as testosterone concentration while sperm with damaged DNA and MDA concentration in mice treated with MTX in comparison with control and MX+EP groups increased significantly (p<0.05). Instead, MTX+EP group caused partial amelioration in all parameters mentioned above.
Conclusion
Based on the present study, it can be concluded that MTX induced toxicity in sperm parameters and serum level of testosterone and increased MDA level. EP with its antioxidant properties could be administrated during treatment with MTX due to its protective effects on sperm parameters, plasma testosterone levels and lipid peroxidation.
Keywords: Ethyl pyruvate, Malondialdehyde, Methotrexate, Mice, Spermatozoa, Testosterone
Introduction
In recent years, natural antioxidant products have gained attention based on their protective effects against drug-induced toxicities especially whenever free radical generation is involved (1). The intake in human diet of antioxidant compounds or compounds that ameliorate or enhance the biological antioxidant mechanisms, can prevent and in some cases help in the treatment of some oxidative-related disorders and organ toxicity events (2). Pyruvate is a key intermediate metabolite of glucose and a potent antioxidant and free radical scavenger (3).
Pyruvate has been shown to afford protection in numerous in vitro and in vivo models including oxidant mediated cellular ones or organ system injury (4). It is a well-known scavenger of hydrogen peroxide and superoxide radicals (5, 6). In the presence of hydrogen peroxide, pyruvate will decarboxylate to yield acetate, water and carbon dioxide (7). Unfortunately, the usefulness of pyruvate as a therapeutic agent is diminished by its very poor stability in solution (8). Ethyl pyruvate (EP), a simple aliphatic ester derived from pyruvic acid, is safer and more stable than pyruvate (8). Like pyruvate, ethyl pyruvate could also rapidly and stoichiometrically scavenge hydrogen peroxide. Supporting this idea, some studies show that treatment with EP provides biochemical evidence for a decrease in oxidative stress both in vitro and in vivo and in models of ischemia/reperfusion injury (5, 9).
Chemotherapy is one of the most effective methods for the treatment of cancer, but is often associated with several short and long-term toxicities (10). MTX is a widely used anti-cancer drug and a well-known immunosuppressant introduced for therapeutic application in 1950 (11). It is used against a broad range of neoplastic disorders including acute lymphoblastic leukaemia, non-Hodgkin's lymphoma, breast cancer and testicular tumours (12, 13). Further, it is effective for the treatment of psoriasis, rheumatoid arthritis and different immunesuppressive propose (14, 15).
The basic principle of its therapeutic efficacy is related to the inhibition of dihydrofolate reductase, a key enzyme in the folic acid metabolism, which converts dihydrofolicacid to tetrahydrofolic acid. The perturbation in the folic acid metabolism leads to depletion of nucleotide precursors like thymidylates and purines, which in turn inhibits DNA, RNA and protein synthesis (16, 17). MTX has narrow therapeutic index and its toxicity has been reported in various organ systems including gastrointestinal, hematologic and central nervous system. Methotrexate may have an effect on hypothalamic-pituitary-gonadal axis or a direct toxic effect on the gonads (18). Studies in animals have shown altered spermatogenesis, cytotoxicity and degeneration of spermatocytes, Sertoli cells, and Leydig cells (19, 20).
Methods
Animals
Thirty two adult male NMRI mice (8-10 weeks old) weighing 26±2 g were purchased from animal house of faculty of science Urmia University. All animals were kept under controlled environmental conditions at room temperature (22±2°C) with humidity of 50±10% and a 12/12 hr photoperiod. The animals were provided with standard diet pellets and water ad libitum.
Experimental design
Animals were randomly divided into four groups, containing 8 mice in each group. Group 1 (control) received distilled water 0.1 ml/mice/day (ip) for 30 day. Group 2 (MTX) received methotrexate at a dose of 20 mg/kg once a week (ip) for 30 days. Group 3 (EP) received ethyl pyruvate at a dose of 40 mg/kg/daily (ip) for 30 days. Group 4 (MTX+EP) received methotrexate at a dose of 20 mg/kg once a week (ip) concomitant with ethyl pyruvate administration at a dose of 40 mg/kg over a week.
Sperm collection
24 hr after the end of the treatment period, animals were sacrificed by dislocation of cervical vertebrae. The caudal epididymis was removed after sacrificing the animals and placed into 1 ml of HTF medium with 4 mg/ml of Bovine Serum Albumin, then minced into small pieces to allow the sperm to swim out and incubated at 37°C/5% Co2 for 30 min.
Sperm count
The obtained sperm suspension was centrifuged at 1000 rpm for 5 min. After centrifugation, 10 µl of the supernatant was taken and the epididymal sperm count was determined using Neubauerhemocytometer (21).
Sperm viability
Sperm viability was evaluated as follows. 20 µl of 0.05% Eosin Y -Nigrosin were added into an equal volume of sperm suspension. After 2 min of incubation at room temperature, slides were viewed by light microscope with magnification of ×400. Dead sperm appeared to be pink and live sperm were not stained. In each sample, 400 sperm were counted and viability percentages were calculated (22).
Sperm motility
The motility was determined by placing 10 µl of the sperm suspersion on a clean pre-warmed microscopic slide, covered with a cover-slip and examined using a light microscope at 400× magnification (Nikon Labophot 2) equipped with a heated stage (37°C). The motility of each spermatozoon was graded as “RPFM” (rapid progressive forward movement), “SPFM” (slowly progressive forward movement) “RM” (residual motion) and “ML” (motionless) and percentages of motile and immotile sperm were obtained (23).
Acridine Orange (AO) staining
Sperm DNA fragmentation has now become a new biomarker for male infertility diagnosis (24). Acridine Orange (AO) staining is used, after challenge at a low pH, to distinguish between denatured, single stranded, native, and double-stranded DNA regions in sperm chromatin (25). The results showed the cytogram patterns of the fluorescence intensity of denatured DNA (red) and native DNA (green). Thick smears were placed in Carnoy's fixative (methanol/ acetic acid 1:3) for 2 hr for fixation (26). The slides were removed from the fixative and allowed to dry for a few minutes. After 5 min at laboratory temperature, the sperm were stained with stock solution consisting of 1 mg of AO in 1000 ml of distilled water and stored in the dark at 4°C. The staining solution was prepared as follows. 10 ml of the stock solution was added to 40 ml of 0.1 M citric acid and 2.5 ml of 0.3 M Na2HPO4 · 7H2O (28). After staining for 5 min, the slides were rinsed with deionized water. The sperm were analyzed by fluorescent light microscopy. Red and green sperm could be observed, green sperm were classified as normal DNA and yellow to red sperm were classified as damaged DNA (26).
Acidic Aniline Blue Staining (AABS)
During histone replacement with protamines and chromatin condensation in spermatogenesis, normal sperm do not stain with aniline blue, but sperm with defective density or immature sperm absorb the stain (28). Sperm samples were air-dried and fixed for 30 min in 3% glutaraldehyde in phosphate buffered saline (pH=7.2). Each smear was stained with 5% acidic aniline blue stain (5 g aniline blue (Sigma-Aldrich, USA), 4% acetic acid in double distilled water, pH=3.5 for 5 min). At least 200 spermatozoa under light microscopy (Olympus Co, Tokyo, Japan) were counted in each slide (29).
Measurement of Malondialdehyde (MDA)
In order to do this, 300 µl of 10% trichloroacetic acid was added to 150 µl of the sample and centrifuged at 1,000 rpm for 10 min at 4°C. 300 µl of supernatant was transferred to a test tube with 300 µl of 67% thiobarbituric acid and incubated at 100°C for 25 min. 5 min after cooling the solution, a pink color appeared because of MDA-TBA reaction and was evaluated using a spectrophotometer at a wavelength of 535 nm (30).
Testosterone assay
After collecting blood, samples were centrifuged to isolate serum and kept at −80°C until biochemical analysis with immuneradiometric technique (WHO/Sigma Asso-RTGC-768/98) for testosterone measurement.
Statistical analysis
Results were shown as mean± standard error of mean (S.E.M.) for each group. For comparison between the groups, the results were analyzed by SPSS 16 software, using oneway ANOVA followed by a Bonferroni post hoc test. A p<0.05 was considered significant.
Results
Sperm parameters
The results revealed that sperm count decreased significantly (p<0.05) in methotrexate group in comparison with the control group. Ethyl pyruvate+methotrexate group revealed significant enhancement (p<0.05) in sperm count which are presented in Table 1. There was no significant (p>0.05) difference in sperm maturity among groups. Treatment with anti-neoplastic drug (MTX) caused a significant decrease in viability and an increase in sperm with damaged DNA compared with the control group while Ethyl pyruvate caused improvement in viability and DNA fragmentation in MTX+EP group (Figures 1, 2, 3; Table 1).
Table 1.
The effect of methotrexate and Ethyl pyruvate on wperm count, sperm viability, sperm maturity and DNA damage in mice (M±SE)
| Group | Sperm count (×106) | Sperm viability (%) | Sperm maturity (%) | Acridine orange+(%) |
|---|---|---|---|---|
| Control | 26.66±1.87 | 88.00±2.88 | 89.00±0.57 | 5.73±0.29 |
| MTX | 7.50± 1.15 a | 52.33±2.33 a | 90.33±1.76 | 12.66±1.4 a |
| EP | 25.94±0.81 b | 81.66±2.33 ab | 90.33±0.33 | 6.26±0.52 b |
| MTX+EP | 15.16±1.74 ab | 63.88±4.13 ab | 96.00±1.15 | 7.00±0.75 b |
Different significant (p<0.05) compared with control group
Different significant (p<0.05) compared with MTX group
Figure 1.
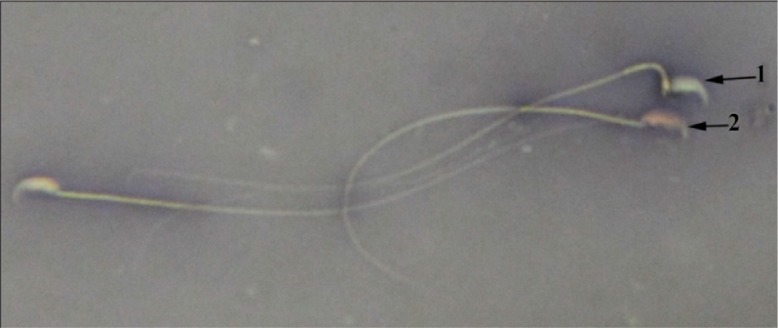
MTX+EP group, live sperm; 1: with uncoloured head and dead sperm; 2: with red head (Eosin Y Nigrosin ×1000)
Figure 2.
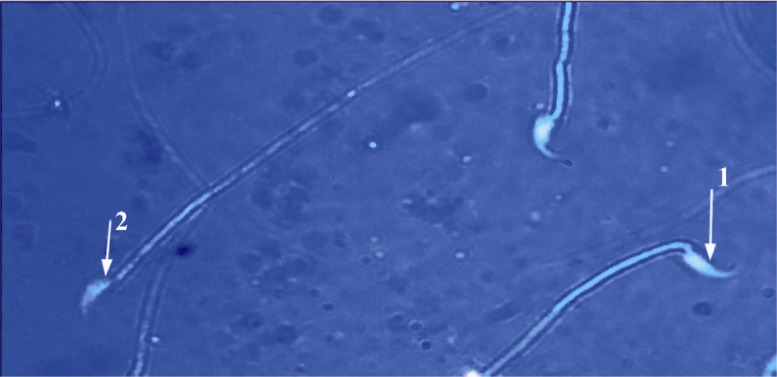
EP group: sperm head with mature nuclei is light blue; 1: and sperm head containing immature nuclear chromatin is dark blue; 2: (Aniline blue ×1000)
Figure 3.
MTX+EP group: sperm with normal DNA integrity had green fluorescence, and those with diminished DNA integrity had orange-red staining (Acridine orange ×400)
In Table 2, we can see that regarding sperm motility, compared with the control group, RPFM decreased significantly (p<0.05) in methotrexate group. However, Ethyl pyruvate caused an increase in RPFM in MTX+EP group which was significantly (p<0.05) higher compared with the MTX group. SPFM results indicated that the difference between groups was not significant. RM and ML in MTX treatment group increased significantly (p<0.05). MTX treatment in EP supplemented animals showed a significant reduction (p<0.05) in RM and ML compared with MTX group alone (Table 2).
Table 2.
Effect of EP on sperm motility in mice treated with MTX (M±SE)
| Group | Sperm motility (RPFM) (%) | Sperm motility (SPFM) (%) | Sperm motility (RM) (%) | Sperm motility (ML) (%) |
|---|---|---|---|---|
| Control | 66.12±0.54 | 14.28±1.00 | 12.10±1.76 | 11.51±1.61 |
| MTX | 26.10±1.82 a | 13.73±1.89 | 39.57±2.60 a | 20.59±0.68 a |
| EP | 61.30±2.13 b | 13.53±.37 | 17.23±3.51 b | 7.93±1.30 b |
| MTX+EP | 49.35±2.92 ab | 16.80±1.10 | 21.58±1.31ab | 11.83±1.24 b |
Different significant (p<0.05) compared with control group
Different significant (p<0.05) compared with MTX group
Biochemical results
The results of biochemical analyses were shown in Table 3. Briefly, the level of MDA, a major degradation product of lipid peroxidation, was significantly increased in MTX treated mice compared with the control group (p<0.05). There was a significant restoration in MDA level in the groups that received EP treatment with MTX (p<0.05).
Table 3.
Comparison of the effect of EP on testosterone level and lipid peroxidation caused by MTX (M±SE)
| Group | MDA (µmol/gr tissue) | Testosterone (ng/ml) |
|---|---|---|
| control | 165.66±4.63 | 0.42±0.02 |
| MTX | 357.33±4.09 a | 0.19±0.02 a |
| EP | 187.00±9.53 ab | 0.58±0.02 ab |
| MTX+EP | 203.66±20.4 ab | 0.49±0.04 b |
Different significant (p<0.05) compared with control group
Different significant (p<0.05) compared with MTX group
The level of testosterone was found to be significantly lower in MTX-treated group when compared with other groups (p<0.005). Ethyl pyruvate administration, after MTX, caused significant increase in testosterone concentrations when compared with MTX and control group alone (p< 0.05). On the other hand, EP+MTX group testosterone level was similar to the control group.
Discussion
Although most of chemotherapeutic agents are mutagenic and carcinogenic (31), they are extensively used for the treatment of various types of cancers, as at times, they cure the disease or at least increase the life expectancy of cancer patients (32).
Also, research shows that there is a significant relationship between ROS production and apoptosis which cause DNA damage in sperm. We reached the same results in our AO assays, observing that MTX caused a significant increase in DNA fragmentation (p<0.05).
Sperm counts are a crude measure of fertility (33, 34). In this study, we observed that sperm count and viability were reduced significantly in MTX-treated animals. Decrease in sperm count often results due to the interference in the spermatogenesis process and the elimination of sperm cells at different stages of development (34, 35).
Sperm motility is also as important as the counts in respect to male fertility. We found that MTX -treated mice had less sperm motility. The significant reduction in sperm motility may be due to the status of oxidative stress observed during MTX administration which is accompanied by increased lipid peroxidation in various tissues (36, 37). Lipid peroxidation products in testis were determined by measuring malondialdehyde (MDA). Malondialdehyde is a good indicator of the degree of lipid peroxidation, which in our study, the level of MDA in MTX-treated rats was significantly higher than the control group (p<0.05). This finding was in agreement with several reports demonstrating MTX-induced oxidative stress in tissues (38, 39).
Whereas EP has been shown to inhibit lipid peroxidation, it is a marker for oxidative stress both in in vitro (40) and in vivo (41) conditions. Tsung et al. have recently shown that EP decreases hepatic lipid peroxidation (42). Interestingly, in our combined EP-MTX treated groups, we observed that MDA value was significantly lower than that of MTX-treated group. Our results in MDA measurement are in agreement with previous studies (41, 43).
Recent advances showed that ROS and hydrogen peroxides are linked with the development of several pathological processes associated with chemotherapy, including adverse effects of anti-tumor drugs. It may be suggested that the cells could be more sensitive to ROS, which subsequently results in reduction of effectiveness of the antioxidant enzyme defense system (44, 45).
On the other hand, EP is unlikely to be harmful to humans, given its close similarity to an endogenous metabolite, its safety profiles in animals, and its common use as a food supplement. EP can react with ROS both via oxidative carboxylation and via formation of hydroxylated adducts at the 3-carbon (46).
In the present study, EP was administered in the form of antioxidant and by a dose of 40 mg/kg which because of its antioxidant and free radical scavenging properties could reduce the side effects of MTX such as DNA damage, low sperm count and decreased viability and motility of sperm in MTX treated mice.
Conclusion
This study clearly showed that MTX treatment in mice induced testicular injury as is evident from the decreased count, viability, motility of sperm and sperm DNA damage. Treatment with EP significantly ameliorated the toxic side effects of MTX. Thus, the results of the present study encourage new experimental and clinical studies to evaluate the efficacy of EP as an adjunctive agent to ameliorate the toxic effects of anti-neoplastic drugs that cause oxidative tissue injury.
Acknowledgement
The authors would like to thank Mr. Eskandar Poorgadim for technical assistance and laboratory equipment supply.
To cite this article: Atashfaraz E, Farokhi F, Najafi Gh. Protective Effect of Ethyl Pyruvate on Epididymal Sperm Characteristics, Oxidative Stress and Testosterone Level in Methotrexate Treated Mice. J Reprod Infertil. 2013;14(4):190-196.
Conflict of Interest
There was no conflict of interest between authors.
References
- 1.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133(10):3275S–84. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 2.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96(2-3):67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 3.Varma SD, Hegde KR, Kovtun S. Oxidative damage to lens in culture: reversibility by pyruvate and ethyl pyruvate. Ophthalmologica. 2006;220(1):52–7. doi: 10.1159/000089275. [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell-Tormey J, Nathan CF, Lanks K, DeBoer CJ, de la Harpe J. Secretion of pyruvate. An antioxidant defense of mammalian cells. J Exp Med. 1987;165(2):500–14. doi: 10.1084/jem.165.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varma SD, Devamanoharan PS, Ali AH. Prevention of intracellular oxidative stress to lens by pyruvate and its ester. Free Radic Res. 1998;28(2):131–5. doi: 10.3109/10715769809065799. [DOI] [PubMed] [Google Scholar]
- 6.Olek RA, Antosiewicz J, Popinigis J, Gabbianelli R, Fedeli D, Falcioni G. Pyruvate but not lactate prevents NADH-induced myoglobin oxidation. Free Radic Biol Med. 2005;38(11):1484–90. doi: 10.1016/j.freeradbiomed.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Constantopoulos G, Barranger JA. Nonenzymatic decarboxylation of pyruvate. Anal Biochem. 1984;139(2):353–8. doi: 10.1016/0003-2697(84)90016-2. [DOI] [PubMed] [Google Scholar]
- 8.Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med. 2001;29(8):1513–8. doi: 10.1097/00003246-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Devamanoharan PS, Henein M, Ali AH, Varma SD. Attenuation of sugar cataract by ethyl pyruvate. Mol Cell Biochem. 1999;200(1-2):103–9. doi: 10.1023/a:1007055503748. [DOI] [PubMed] [Google Scholar]
- 10.Arnon J, Meirow D, Lewis-Roness H, Ornoy A. Genetic and teratogenic effects of cancer treatments on gametes and embryos. Hum Reprod Update. 2001;7(4):394–403. doi: 10.1093/humupd/7.4.394. [DOI] [PubMed] [Google Scholar]
- 11.Peters GJ, van der Wilt CL, van Moorsel CJ, Kroep JR, Bergman AM, Ackland SP. Basis for effective combination cancer chemotherapy with antimetabolites. Pharmacol Ther. 2000;87(2-3):227–53. doi: 10.1016/s0163-7258(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 12.Choudhury RC, Ghosh SK, Palo AK. Cytogenetic toxicity of methotrexate in mouse bone marrow. Environ Toxicol Pharmacol. 2000;8(3):191–196. doi: 10.1016/s1382-6689(00)00041-7. [DOI] [PubMed] [Google Scholar]
- 13.Jensen SB, Mouridsen HT, Reibel J, Brunner N, Nauntofte B. Adjuvant chemotherapy in breast cancer patients induces temporary salivary gland hypofunction. Oral Oncol. 2008;44(2):162–73. doi: 10.1016/j.oraloncology.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Chow M, Koo J, Ng P, Rubin H. Random population-wide genetic damage induced in replicating cells treated with methotrexate. Mutat Res. 1998;413(3):251–64. doi: 10.1016/s1383-5718(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 15.Gisondi P, Girolomoni G. Biologic therapies in psoriasis: a new therapeutic approach. Autoimmun Rev. 2007;6(8):515–9. doi: 10.1016/j.autrev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Novakovic T, Dordevic OM, Grujicic D, Marinkovic D, Jankovic S, Arsenijevic S. Effect of intratumoral application of methotrexate in vivo on frequency of micronuclei in peripheral blood lymphocytes. Arch Oncol. 2003;11:1–4. [Google Scholar]
- 17.Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65(3):168–73. [PubMed] [Google Scholar]
- 18.Padmanabhan S, Tripathi DN, Vikram A, Ramarao P, Jena GB. Methotrexate-induced cytotoxicity and genotoxicity in germ cells of mice: intervention of folic and folinic acid. Mutat Res. 2009;673(1):43–52. doi: 10.1016/j.mrgentox.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Saxena AK, Dhungel S, Bhattacharya S, Jha CB, Srivastava AK. Effect of chronic low dose of methotrexate on cellular proliferation during spermatogenesis in rats. Arch Androl. 2004;50(1):33–5. doi: 10.1080/01485010490250533. [DOI] [PubMed] [Google Scholar]
- 20.Shrestha S, Dhungel S, Saxena AK, Bhattacharya S, Maskey D. Effect of methotrexate (MTX) administration on spermatogenesis: an experimental on animal model. Nepal Med Coll J. 2007;9(4):230–3. [PubMed] [Google Scholar]
- 21.Padmanabhan S, Tripathi DN, Vikram A, Ramarao P, Jena GB. Cytotoxic and genotoxic effects of methotrexate in germ cells of male Swiss mice. Mutat Res. 2008;655(1-2):59–67. doi: 10.1016/j.mrgentox.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Wyrobek AJ, Gordon LA, Burkhart JG, Francis MW, Kapp RW, Jr, Letz G, et al. An evaluation of the mouse sperm morphology test and other sperm tests in nonhuman mammals. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1983;115(1):1–72. doi: 10.1016/0165-1110(83)90014-3. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. WHO Laboratory manual for the examination of human semen and sperm – cervical mucus interaction; Cambridge: Cambridge University press; 1992. [Google Scholar]
- 24.Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis: what is the link? Hum Reprod Update. 2007;13(3):313–27. doi: 10.1093/humupd/dml057. [DOI] [PubMed] [Google Scholar]
- 25.Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19(6):1401–8. doi: 10.1093/humrep/deh280. [DOI] [PubMed] [Google Scholar]
- 26.Rezvanfar M, Sadrkhanlou R, Ahmadi A, Shojaei-Sadee H, Rezvanfar M, Mohammadirad A, et al. Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics, and DNA damage by an herbal source; evidence for role of free-radical toxic stress. Hum Exp Toxicol. 2008;27(12):901–10. doi: 10.1177/0960327108102046. [DOI] [PubMed] [Google Scholar]
- 27.Erenpreiss J, Bars J, Lipatnikova V, Erenpreisa J, Zalkalns J. Comparative study of cytochemical tests for sperm chromatin integrity. J Androl. 2001;22(1):45–53. [PubMed] [Google Scholar]
- 28.Meistrich ML, Brock WA, Grimes SR, Platz RD, Hnilica LS. Nuclear protein transitions during spermatogenesis. Fed Proc. 1978;37(11):2522–5. [PubMed] [Google Scholar]
- 29.Talebi AR, Moein MR, Tabibnejad N, Ghasemzadeh J. Effect of varicocele on chromatin condensation and DNA integrity of ejaculated spermatozoa using cytochemical tests. Andrologia. 2008;40(4):245–51. doi: 10.1111/j.1439-0272.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- 30.Hosseinzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8(3):394–9. [PubMed] [Google Scholar]
- 31.Anders MW, Bull RJ, Cantor KP, Chakraborti D, Chen CJ, DeAngelo AB, et al. Monographs on the evaluation of carcinogenic risks to humans: pharmaceutical drugs; Lyon, France: IARC Scientific Publication; 1990. p. 415. [Google Scholar]
- 32.Choudhury RC, Ghosh SK, Palo AK. Cytogenetic toxicity of methotrexate in mouse bone marrow. Environ Toxicol Pharmacol. 2000;8(3):191–196. doi: 10.1016/s1382-6689(00)00041-7. [DOI] [PubMed] [Google Scholar]
- 33.Padmanabhan S, Tripathi DN, Vikram A, Ramarao P, Jena GB. Cytotoxic and genotoxic effects of methotrexate in germ cells of male Swiss mice. Mutat Res. 2008;655(1-2):59–67. doi: 10.1016/j.mrgentox.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Abdel Aziz AH, Shouman SA, Attia AS, Saad SF. A study on the reproductive toxicity of erythrosine in male mice. Pharmacol Res. 1997;35(5):457–62. doi: 10.1006/phrs.1997.0158. [DOI] [PubMed] [Google Scholar]
- 35.Schimenti KJ, Hanneman WH, Schimenti JC. Evidence for cyclophosphamide-induced gene conversion and mutation in mouse germ cells. Toxicol Appl Pharmacol. 1997;147(2):343–50. doi: 10.1006/taap.1997.8292. [DOI] [PubMed] [Google Scholar]
- 36.Tabassum H, Parvez S, Rehman H, Dev Banerjee B, Siemen D, Raisuddin S. Nephrotoxicity and its prevention by taurine in tamoxifen induced oxidative stress in mice. Hum Exp Toxicol. 2007;26(6):509–18. doi: 10.1177/0960327107072392. [DOI] [PubMed] [Google Scholar]
- 37.Jahovic N, Cevik H, Sehirli AO, Yegen BC, Sener G. Melatonin prevents methotrexate-induced hepatorenal oxidative injury in rats. J Pineal Res. 2003;34(4):282–7. [PubMed] [Google Scholar]
- 38.Jahovic N, Sener G, Cevik H, Ersoy Y, Arbak S, Yegen BC. Amelioration of methotrexate-induced enteritis by melatonin in rats. Cell Biochem Funct. 2004;22(3):169–78. doi: 10.1002/cbf.1071. [DOI] [PubMed] [Google Scholar]
- 39.Sener G, Ekşioglu-Demiralp E, Cetiner M, Ercan F, Sirvanci S, Gedik N, et al. L-Carnitine ameliorates methotrexate-induced oxidative organ injury and inhibits leukocyte death. Cell Biol Toxicol. 2006;22(1):47–60. doi: 10.1007/s10565-006-0025-0. [DOI] [PubMed] [Google Scholar]
- 40.Song M, Kellum JA, Kaldas H, Fink MP. Evidence that glutathione depletion is a mechanism responsible for the anti-inflammatory effects of ethyl pyruvate in cultured lipopolysaccharide-stimulated RAW 264.7 cells. J Pharmacol Exp Ther. 2004;308(1):307–16. doi: 10.1124/jpet.103.056622. [DOI] [PubMed] [Google Scholar]
- 41.Tawadrous ZS, Delude RL, Fink MP. Resuscitation from hemorrhagic shock with Ringer's ethyl pyruvate solution improves survival and ameliorates intestinal mucosal hyperpermeability in rats. Shock. 2002;17(6):473–7. doi: 10.1097/00024382-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Tsung A, Kaizu T, Nakao A, Shao L, Bucher B, Fink MP, et al. Ethyl pyruvate ameliorates liver ischemia-reperfusion injury by decreasing hepatic necrosis and apoptosis. Transplantation. 2005;79(2):196–204. doi: 10.1097/01.tp.0000151681.07474.2e. [DOI] [PubMed] [Google Scholar]
- 43.Vardi N, Parlakpinar H, Cetin A, Erdogan A, Cetin Ozturk I. Protective effect of beta-carotene on methotrexate-induced oxidative liver damage. Toxicol Pathol. 2010;38(4):592–7. doi: 10.1177/0192623310367806. [DOI] [PubMed] [Google Scholar]
- 44.Babiak RM, Campello AP, Carnieri EG, Oliveira MB. Methotrexate: pentose cycle and oxidative stress. Cell Biochem Funct. 1998;16(4):283–93. doi: 10.1002/(SICI)1099-0844(1998120)16:4<283::AID-CBF801>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 45.Tabassum H, Rehman H, Banerjee BD, Raisuddin S, Parvez S. Attenuation of tamoxifen-induced hepatotoxicity by taurine in mice. Clin Chim Acta. 2006;370(1-2):129–36. doi: 10.1016/j.cca.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Ajami AM, Sims CA, Fink MP, investors Pyruvate ester composition and method of use for resuscitation after events of ischemia and reperfusion. United States patent US. 2004;10:116–707. [Google Scholar]



