Abstract
Trigeminal sensory neurons develop from the neural crest and neurogenic placodes, and have been studied as a principle model of sensory neuron formation. While the Notch pathway has been extensively characterized in central nervous system development and other developmental processes, it has not been well characterized in sensory neurogenesis. Here we studied the functional role of Notch signaling in the trigeminal ophthalmic (opV) placode, a prime model of sensory neurogenesis. To establish a good spatiotemporal description of Notch pathway genes in the chick trigeminal placode, a stage-specific expression analysis was conducted, showing that expression of most Notch pathway genes and effectors are expressed in the placode, with expression primarily being confined to ectodermal cells. Expression was highest at stages of peak neuronal differentiation. To test the function of Notch signaling in opV placode cell differentiation, Notch receptor cleavage was blocked using the gamma-secretase inhibitor, DAPT, or signaling was activated by misexpression of the Notch intracellular domain (NICD). Notch activation resulted in a significant reduction in sensory neurogenesis. Cells remained in the ectoderm and did not differentiate. Expression of the opV specification marker Pax3 was also lost in targeted cells. DAPT exposure resulted in a dramatic increase in neurogenesis without increasing proliferation, where many differentiated cells were found in the mesenchyme and, surprisingly, within the ectoderm. This is the first result clearly showing prolific neuronal differentiation in the ectoderm of the trigeminal placodes after experimental manipulation of a molecular signaling pathway, thus identifying Notch signaling as a primary regulator of the sensory neuron fate in the opV placode.
Keywords: Sensory neurogenesis, Notch, Trigeminal ophthalmic placode
Introduction
In vertebrates, the neurogenic placodes and a subset of neural crest cells give rise to all peripheral sensory neurons (D’Amico-Martel and Noden, 1983). Sensory neurons are the sole derivative of the trigeminal placode (Baker and Bronner-Fraser, 2001;Schlosser, 2006), making it an excellent system to investigate a direct path to sensory neurogenesis. The trigeminal placode consists of two molecularly distinct placodes, the ophthalmic (opV) and the maxillomandibular (mmV). The opV and mmV placodes, each contribute neurons to the distal region of their respective ganglionic lobes, while the neural crest contributes proximal neurons, as well as glial cells (Baker and Bronner-Fraser, 2001;D’Amico-Martel and Noden, 1983;Schlosser, 2006). Since identification of Pax3 as a molecular marker of the opV placode (Stark et al., 1997), several studies have followed describing the tissue interactions and key signaling pathways involved in opV development. Baker et al., (1999) characterized the timing and tissue interactions involved in the competence, induction, and specification of the opV placode. Subsequent experiments showed that Pax3 expression in opV placodal ectoderm correlates with specification and commitment to an opV cutaneous sensory neuron fate, suggesting a one-step induction in which neuronal subtype identity is coupled to neuronal differentiation (Baker and Bronner-Fraser, 2000;Baker et al., 2002). These and other early studies also identified important ligands and receptors in opV ectoderm: FGFR4, Frizzled 1, 2, & 7, and Delta1 (Begbie et al., 2002b;Stark et al., 1997;Stark et al., 2000). A functional analysis of the Wnt and Fgf pathways in opV development, as well as PDGF, have recently been described (Canning et al., 2008;Lassiter et al., 2007;Lassiter et al., 2009;McCabe and Bronner-Fraser, 2008). The canonical Wnt signaling pathway was shown to be necessary but not sufficient for opV placode cell fate determination, maintenance, and differentiation (Lassiter et al., 2007). The FGF pathway was also found to be essential for opV placode cell delamination and differentiation, but did not have the ability alone to direct placode development (Lassiter et al., 2009). However, when Wnt and FGF signals were misexpressed in the isthmus, they were found to work in concert to induce trigeminal placode development (Canning et al., 2008). Platelet-derived growth factor (PDGF), via the neural tube, was also shown to be necessary for induction of the opV placode and is sufficient for a moderate increase in the number of opV neurons in the condensing ganglion (McCabe and Bronner-Fraser, 2008).
OpV placode cells become specified coincident with expression of the transcription factor Pax3. These Pax3+ cells quickly become postmitotic and upregulate proneural genes such as Ngn2 and Brn3a (Begbie et al., 2002;Graham et al., 2007;McCabe et al., 2009). The opV neuroblasts then delaminate from surrounding ectoderm and enter the mesenchyme, upregulating neuronal differentiation genes such as Islet1, NeuN, and Neurofilament. Inhibition of the Wnt or FGF pathway prevents opV cells from delaminating from the ectoderm. Once stalled in the ectoderm these cells never continue down the differentiation pathway and even downregulate the opV specification marker Pax3 (Lassiter et al., 2007;Lassiter et al., 2009). Even Pax3 misexpression in the ectoderm only promoted upregulation of FGFR4 and Ngn2 and did not lead to delamination or neuronal differentiation (Dude et al., 2008). Because misexpression of the Wnt, FGF or PDGF pathways did not promote broad ectodermal neurogenesis, and Pax3 misexpression only promoted upregulation of FGFR4 and Ngn2, we hypothesized that additional modulators are necessary for terminal differentiation, with the most likely candidate being Notch/Delta signaling.
The Notch/Delta pathway is important to many developmental processes including neural progenitor maintenance and differentiation in the CNS, somite formation, hematopoesis, angiogenesis, and is also misregulated in certain cancer types (Bolós et al., 2007;Cheung et al., 2006;Fiúza and Arias, 2007;Karamysheva, 2008;Lasky and Wu, 2005;Pourquie, 2001;Weng et al., 2003). Generally, Notch signaling acts to maintain stem cells, laterally inhibit cell fate, and laterally induce boundary formation. The Notch pathway has been shown to regulate avian trunk neural crest cell fate determination in the dorsal root ganglia (Wakamatsu et al., 2000). In the CNS, manipulation of the Notch pathway via Notch1 and RBPjK knockout mice, the gamma-secretase inhibitor DAPT, or dominant-active and dominant-negative Delta, result in premature differentiation of neurons when Notch signaling is blocked and inhibition of the neuronal fate when Notch signaling is activated (Bolós et al., 2007;Yoon and Gaiano, 2005;Abelló et al., 2007;Daudet et al., 2007;Nelson et al., 2007; Kageyama et al., 2005; Lewis, 1998). Recently, studies aimed at deciphering how Notch signaling functions in mammals has challenged and enhanced the classical view of lateral inhibition first described in Drosophila (Heitzler and Simpson, 1991;Muskavitch, 1994). Real-time imaging in the CNS of mouse embryos has revealed a dynamic oscillatory expression of Delta-like ligand 1 (Dll1), Hes1, and Ngn2 (Shimojo et al., 2008). This study suggests that the main function of Notch signaling is to dynamically maintain neural progenitor cells (Kageyama et al., 2008;Shimojo et al., 2008). Thus, while Notch signaling has been well studied in various developmental systems, including neuronal cell fate in the CNS, surprisingly, little is known regarding Notch/Delta signaling in sensory neurogenesis. Only a few Notch pathway components have previously been described as being expressed in the trigeminal placodes. Work on Notch signaling in the peripheral nervous system has been done in the otic placodes, where some functional studies have shown that Notch/Delta acts to regulate the choice between hair-cell and supporting cell, maintain prosensory patches and influence otic placode size (Daudet et al., 2007;Jayasena et al., 2008). Our aims were to identify a functional role for Notch/Delta signaling in opV placode sensory neurogenesis.
In this study, we provide a detailed spatiotemporal description of Notch pathway genes in the trigeminal placodes. During opV placode development, all Notch ligands, receptors, and effectors assayed are confined to the ectoderm, and therefore function prior to delamination. From these data we propose that a key step in neuronal selection is regulated by Notch signaling. We tested this hypothesis by manipulating the Notch pathway using DAPT to block Notch signaling, and the Notch intracellular domain (NICD) to constitutively activate the pathway in the chick head. We show that blocking Notch signaling promotes precocious neurogenesis in all opV specified Pax3+ cells, with an increase in neurons both within the ectoderm where minimal neuronal differentiation typically occurs, and in the mesenchyme pool. In contrast, constitutive Notch signaling completely disrupted development of opV placode cells, preventing neuronal differentiation and cellular delamination.
Materials and Methods
Expression reagents
The NICD-pCIG and control pCIG-GFP constructs were a kind gift from Andy McMahon and Sean Megason. All expression constructs were prepared for electroporation by resuspending at a concentration of 4-6 μg/μl in water with fast green added for visualization.
In ovo electroporation
Fertilized chicken (Gallus gallus) eggs were obtained from local farms and incubated to the desired stage in a humidified incubator at 38°C. The DNA constructs described above were electroporated into 10-12ss chicken embryos using vertical electroporation, where the reference electrode was placed underneath the embryo through a small hole made outside the area opaca, and the driving electrode was placed directly above the area of interest (BTX 820 electroporator from Genetronics: five 10ms pulses of 10V each, one second gap between each pulse). Embryos were then allowed to develop for 30 hours before being harvested.
DAPT head cultures
Head regions above the otic vesicle were dissected from 13-15ss embryos using a micro-scalpel. Tissues were stored in complete medium on ice (10% fetal bovine serum, 2% chick embryonic extract in DMEM) until required, then rinsed in sterile DMEM before transplanting into collagen gels. Collagen matrix gels were prepared as previously described (Groves and Bronner-Fraser, 2000). Briefly, 90 μl of collagen solution and 10 μl of 10X DMEM were combined followed by addition of 4.5 ul of 7.5% sodium bicarbonate to adjust the pH to 7.5. 30 μl drops of the prepared collagen solution were plated and allowed to set. Head regions were then placed on top of collagen mound followed by 20 ul of collagen solution added to cover tissue. DMEM (1 ml) with N2 supplement (GIBCO) and antibiotic were added with either DAPT (100 uM) or DMSO vehicle. Cultures were grown at 37°C and 5% CO2 for 12-24 hours. Cultures were fixed in 4% formaldehyde and prepared for cryosection.
Whole-mount in situ hybridization
Digoxygenin (DIG)-labeled RNA antisense probes were synthesized from plasmids containing fragments or complete cDNA of the following chicken genes: cDelta1, cLunatic Fringe, cNotch1, cSerrate1 (Daudet et al., 2007), cHairy1, and cHairy2 (obtained from D. Henrique, Universidade de Lisboa, Portugal). DIG-labeled RNA antisense probes were synthesized from PCR amplification of chick cDNA of the following chicken genes: cDelta4, cHes5, cNgn2 (Lassiter et al., 2009), and cNotch2. The following primers were used for the respective genes: cDelta4, outer primers (forward 5′-TGTGCCGAACAGAATGGATA-3′ and reverse 5′-TACCTTGACCCACTTGACCT-3′) and inner primers (forward 5′-GAGTGCATCTGTCGTTCTGG-3′ and reverse 5′-TTGAACGACGAGAGTCCACC-3′), cHes5, outer primers (forward 5′-GAGCCAGCTTCGTGCTGA-3′ and reverse 5′-TGTGACCACGTGTAAGGTCT-3′) and inner primers (forward 5′-CTGACAGCAGCTCTCGGATA-3′ and reverse 5′-AGTGGTAGTGGACCTGTGAC-3′), cNotch2, outer primers (forward 5′-ACCGAAGTGGACGTCAGAAC-3′ and reverse 5′-GAACACGGTCCACACAGACA-3′) and inner primers (forward 5′-CCAGGATGGAAATGAAGAACC-3′ and reverse 5′-GAAGGAGCTCTGTGTGGACC-3′). Whole-mount in situ hybridization was performed in chick embryos as described by Henrique et al., (1995). Briefly, formaldehyde-fixed embryos of appropriate developmental stages were buffered and exposed to a DIG-labeled anti-sense RNA probe, which recognized the specific mRNA transcripts. After removal of the non-specifically adhering probe, the embryos were incubated with anti-DIG alkaline-phosphatase (AP) antibody (1/2000; Roche), followed by a chromogenic substrate for AP. Whole-mount embryos stained for specific mRNA transcripts were cryosectioned (12 μm) and mounted for immunohistochemistry and section analysis.
Immunohistochemistry and analysis
The following primary antibodies were used: Pax3 (1:1000, mouse IgG2a; DSHB), Islet-1 (1:200, mouse IgG2b; DSHB), Neurofilament (1:300, mouse IgG1; DSHB), phospho-Histone H3.3 (1:100, rabbit polyclonal IgG; Upstate). The Developmental Studies Hybridoma Bank (DSHB) was developed under the auspices of the NICHD and is maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Appropriately matched Alexa488-, Alexa546- or Alexa633-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies were obtained from Molecular Probes/Invitrogen.
For immunohistochemistry on cryosections, embryos were embedded in gelatin and cryosectioned to generate 10-12 μm sections of the area of interest. Sections were mounted on Superfrost® Plus glass slides and the gelatin removed by treating the slides in phosphate-buffered saline (PBS) at 37°C for 15-20 minutes. The slides were incubated overnight at 4°C in primary antibody, diluted in antibody buffer (PBS, 0.1% bovine serum albumen, 0.1% Tween), followed by incubation for one hour at room temperature in secondary antibodies diluted in antibody buffer. Three 5-10 minute washes in PBS followed each incubation. Slides were mounted in Fluoromount G (SouthernBiotech). Sections were analyzed using epifluorescent microscopy; photographs from different channels were superimposed using Adobe Photoshop or Olympus Microsuite to observe overlapping expression.
For quantitative analysis, cells from five random sections of each opV placode/ganglion were analyzed to minimize variability and bias and averaged to produce a standard mean for each independent placode/ganglion (Lassiter et al., 2007). Embryos displaying obviously unhealthy tissue morphology were excluded from the data set, and only embryos where DAPI stained nuclei were intact without any apparent degradation were included. Positive cells were determined and counted using Olympus Microsuite software to identify cells with minimum color thresholds. Statistical analysis was performed, with p-values calculated using Student’s t-test to compare the standard means of control and experimental samples.
Results
Notch pathway genes are expressed during opV placode development
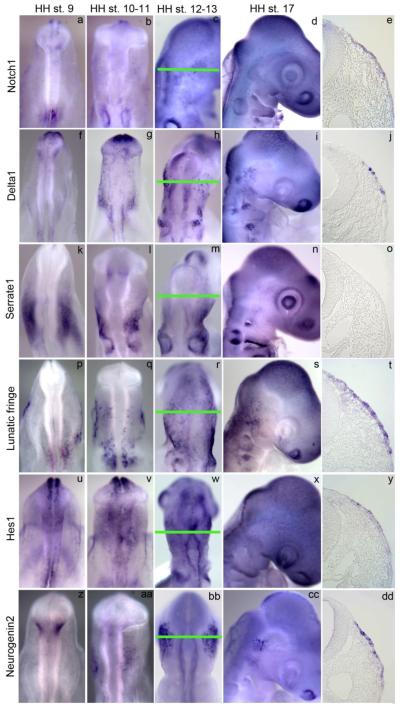
Prior to this study, a spatiotemporal description of the Notch pathway genes in the chick opV placode was not available. A stage-specific analysis of the Notch pathway genes was necessary to better understand this signaling pathway in the opV placode. To accomplish this we compared the mRNA expression of Notch1, Notch2, Delta1, Delta4, Serrate1, Lunatic fringe, Hes1, Hes2, and Hes5 to Ngn2 at different developmental stages (see Table S1). Ngn2 is a proneural cell marker in the opV placode (Begbie et al., 2002) and functions in the Notch signaling pathway. Ngn2 directly induces expression of Delta1 (Castro et al., 2006) and its expression is repressed by the Notch effector, Hes1 (Shimojo et al., 2008). Ngn2 was first expressed in the opV placode at HH stages 10-11 (Fig. 1aa) with more robust staining observed in a broader region by stages 12-13 (Fig. 1bb, 1dd), and expression was less obvious by stage 17 (Fig. 1cc). Other Notch signaling pathway genes were not expressed in the opV placode at stage 9 (Fig. 1). However, by stages 10-11 (Fig. 1b), Notch1 was expressed in the opV domain within the field marked by Ngn2, and became more apparent by stages 12-13 (Fig. 1c, 1e). At stage 17, Notch 1 expression was reduced (Fig. 1 d, S2A). Notch2 was expressed in a similar pattern as Notch1 (Fig. S1a-c) At stages 10-11 (Fig. 1g), Delta1 was highly expressed in a few individual cells. By stages 12-13 expression was diffuse throughout the placode, with strong expression in several individual cells (Fig. 1h, 1j). Expression was less obvious by stage 17 (Fig. 1i, S2C). Lunatic fringe expression was similar to the expression of Delta1 (Fig. 1q-t, S2E). Serrate1 was expressed caudal to the opV placode in the hindbrain ectoderm and never expressed in the trigeminal placode (Fig. 1l-o). Delta4 was not expressed in the opV placode at any of the analyzed stages (Fig. S1d-f). Notch signaling has been shown to activate the transcription of Hes1 and Hes5 (Bailey and Posakony, 1995;Jarriault et al., 1995;Jarriault et al., 1998;Ohtsuka et al., 1999). Hes1 was expressed in the opV placode at stage10-11 (Fig. 1v), expression increased and was scattered by stages 12-13 (Fig. 1w, 1y) and became less obvious by stage 17 (Fig. 1x, S2G). It had a similar pattern as Notch1. Hes2 was expressed in the opV placode at stages 11-12 (Fig. S1h, S1i), stage 13 (data not shown), and stage 17 (data not shown). Hes5 was not expressed in the opV placode at any stage (Fig. S1j-l). A table describing the expression of each gene provides additional detail (Table S1).
Figure 1.
Notch pathway gene expression in trigeminal placode development. Notch1 mRNA is expressed at low levels in ectodermal cells of the midbrain region at HH stage 10-11 (b). Notch1 mRNA expression appears more intense in ectodermal cells (e) at HH stage 12-13 (c) and the expression is faint by HH stage 17 (d). At HH stage 10-11, Delta1 mRNA is expressed at low levels in ectodermal cells of the midbrain region (g). Delta1 mRNA expression levels appear more intense in ectodermal cells (j) at HH stage 12-13 (h) but expression appears slightly fainter by HH stage 17 (i). Serrate1 mRNA is not expressed in the ectodermal cells (k-o) of the trigeminal placode. Lunatic fringe mRNA is expressed at low levels in ectodermal cells of the midbrain region at HH stage 10-11 (q). At HH stage 12-13, Lunatic fringe expression appears more robust in ectodermal cells (r, t) but is faint by HH stage 17 (s). Hes1 mRNA expression was detected at low levels in the midbrain ectoderm at HH stage 10-11 (v). Hes1 expression appears intense in the midbrain ectoderm (y) at HH stage 12-13 (w) and faint expression was detected at HH stage 17 (x). Ngn2 mRNA expression was detected at moderate levels in the trigeminal placode at HH stage 10-11 (aa). Ngn2 mRNA expression levels are most easily detected at HH stage 12-13 (bb, dd) and are more faint by stage 17 (cc). Notch signaling was not detected in the midbrain ectoderm at HH stage 9 (a, f, k, p, u).
Using Pax3 as a marker for the opV placode and ganglion, we show that Pax3 protein expression overlaps the Delta1 domain in the ectoderm, at stages 13 and 17 of chick development (Fig. 2A-F), also individual cells expressing high levels of Delta1 express Pax3 at stage 13 (Fig. A-C, arrow). It is important to note that expression of Notch pathway genes and effectors are confined primarily to the ectoderm, being downregulated upon EMT, with faint expression or no expression in mesenchyme cells. In addition, no Notch pathway gene expression tested was expressed in the opV ganglion (S2A-H). This indicates a distinct difference in the differentiation state between opV ectoderm and mesenchyme cells.
Figure 2.
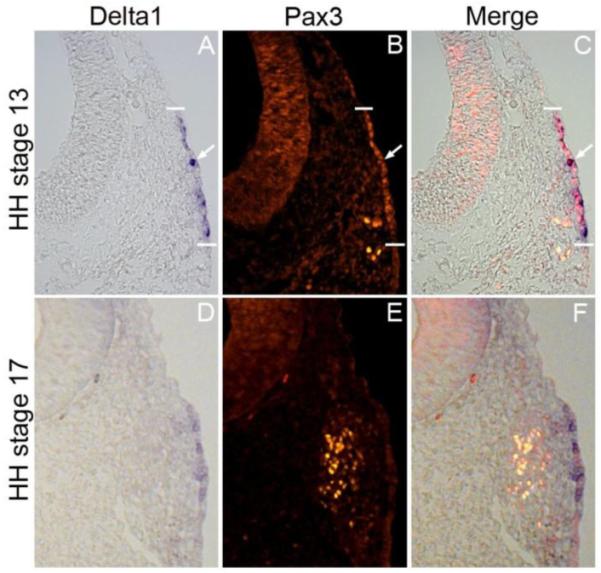
Delta1 gene expression domain overlaps with Pax3 protein expression. At HH stage 13, Delta1 is expressed in the ectoderm (A), and at HH stage 17, residual Delta1 staining is shown in the ectoderm with no obvious expression of Delta1 in the formed ganglion (D). Pax3 protein is expressed in the ectoderm and migrating cells at HH stage 13 (B), and at HH stage 17, most Pax3+cells have left the ectoderm aggregating in the formed ganglion (E). At HH stage 13, individual cells expressing higher levels of Delta 1 also express Pax3 (C, arrow)
Inhibition of Notch signaling leads to premature neuronal differentiation in the opV placode
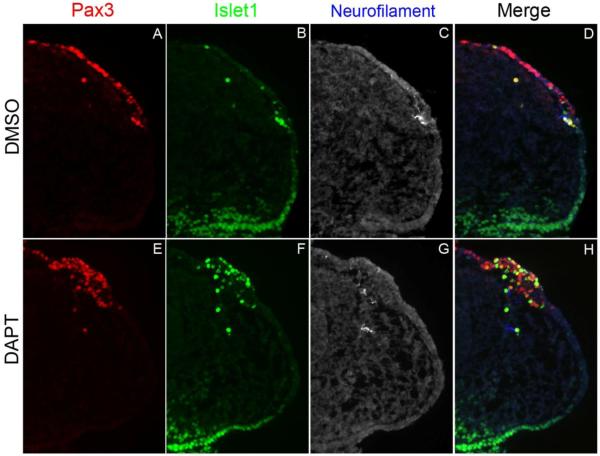
In the opV placode, the vast majority of Pax3+ cells do not express neuronal markers nor do they start to differentiate until they begin delaminating from the ectoderm. The spatiotemporal expression of the Notch pathway genes suggests that Notch signaling may determine EMT initiation and neuronal selection. To test this, we blocked Notch signaling using the gamma-secretase inhibitor, DAPT, in chick head explant cultures (Abelló et al., 2007;Daudet et al., 2007;Nelson et al., 2007). The heads of 13-15 ss chick embryos were removed rostral to the otic vesicle equator and embedded in collagen. These collagen-embedded explants were cultured in DAPT or DMSO for 12 or 24 hr, to about the 20-22 or 30-32 ss, respectively. Embryo heads were then sectioned and immunostained for the opV marker Pax3, the early neuronal marker Islet1, and the later neuronal differentiation marker Neurofilament (NF), which primarily marks axonal projections. In explants incubated for 12 hr, DMSO control embryos showed very few Pax3+ opV cells delaminating into the mesenchyme or differentiating (n=15; Fig. 3A-D), while DAPT treated embryos showed many Pax3+/Islet1+ cells in the ectoderm and mesenchyme (n=16; Fig. 3E-H). Therefore, blocking Notch signaling for 12 hours is sufficient to promote cells toward a sensory neuron cell fate. Importantly, NF expression was rarely observed in the ectoderm of DMSO treated embryos, was observed somewhat in the mesenchyme of DAPT treated embryos, but again only minimally in the ectoderm at this early stage, indicating that most cells are in a state of early neuronal differentiation, expressing only Islet1 and not Neurofilament. Normal NF expression was observed in other tissues such as in the eye and the neural tube. This supports prior studies showing, in the ear for example, that Islet1 precedes neuronal Beta-tubulin (TuJ1) expression, which in a separate study, was shown to precede NF expression (Memberg and Hall 1995; Radde-Gallwitz et al., 2004; Bell et al., 2008).
Figure 3.
Notch inhibition leads to premature neuronal differentiation in the ectoderm. Transverse sections through the ophthalmic trigeminal (opV) placode region of a 20-22 ss embryo. The heads were cultured in either DMSO or DAPT for 12 hrs, harvested, cryosectioned, and immunostained for the opV marker Pax3 (A,E), the early neuronal marker Islet1 (D,F), and the late neuronal marker neurofilament (C,G). DMSO treated embryos showed normal opV placode development (A-D). DAPT treated embryos showed an increase in ectodermal Pax3+cells coexpressing Islet1, and the number of Pax3+/Islet1+ mesenchymal cells was also dramatically increased (E,F,H). DMSO and DAPT treated embryos showed no neurofilament expression in the ectoderm or in the mesenchyme (C,G).
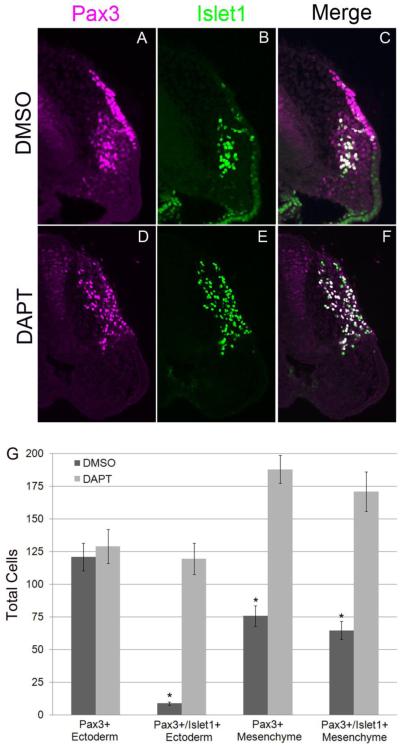
To further characterize the premature neurogenesis in DAPT treated embryos, we performed a more detailed analysis on embryos treated for 24 hours when both experimental and control embryos showed significant cellular differentiation. The number of Pax3+ expressing cells in opV placodal ectoderm at 24 hr did not significantly increase in DAPT treated embryos (129.2 cells, SEM +/− 13.0, n=25; Fig. 4D,G) compared to DMSO controls (121.1 cells, SEM +/− 10.6, n=25; Fig. 4A,G), meaning the placode domain did not expand significantly. However, Notch signaling did appear to greatly affect neuronal differentiation within the opV ectodermal pool, evidenced by a dramatic upregulation of Islet1 expression in opV placodal ectoderm cells in DAPT embryos vs. DMSO control embryos. Control embryos had an average of 9 cells (SEM +/− 1.2, n=25) with co-localization of Pax3 and Islet1, whereas DAPT explants had 119.6 cells (SEM +/− 12.0, n=25) with nearly all Pax3+ ectoderm cells also co-expressing Islet1 (p<0.0000001; Fig. 4). The number of delaminated Pax3+ cells found in the mesenchymal pool did increase considerably, where DMSO controls averaged 75.9 cells (SEM +/− 7.8, n=25; Fig. 4A,G) compared to 188.1 cells in DAPT explants (SEM +/− 10.7, n=25, p<0.00007; Fig. 4D,G). In non-experimental embryos, Islet1 is expressed in only a few ectoderm cells at low levels, but is seen at higher levels in delaminating cells and in mesenchyme cells, where nearly all Pax3+ cells in the mesenchymal pool coexpress Islet1. This mesenchymal expression was also seen in experimental explants but in much greater numbers, with nearly all additional Pax3+ cells also expressing Islet1. Control embryos had 64.9 cells (SEM +/− 6.7, n=25) coexpressing Pax3/Islet1 in the mesenchyme while DAPT embryos had 171.1 Pax3+/Islet1+ cells (SEM +/− 15.0, n=25, p<0.00007; Fig. 4). In DMSO control explants, NF was seen projecting from Pax3+ opV cells in the mesenchyme and was absent from the ectoderm (n=10/10; Fig. 5A-C). Experimental DAPT explants (n=11/11; Fig.5D-F)) showed precocious NF expression projecting from Pax3 cells throughout the ectoderm and mesenchyme. The number of Pax3+ cells in the mesenchyme was increased, as previously seen, and also led to increased NF protein in the mesenchyme when compared to controls. Preventing Notch signaling therefore increases neurogenesis significantly in the opV placode. These data together clearly show that without activation of Notch signaling, Pax3+ opV specified cells will undergo neurogenesis regardless of the ectoderm/mesenchyme location.
Figure 4.
Notch inhibition leads to ectopic neuronal differentiation. Transverse section through the ophthalmic trigeminal (opV) placode region of a 30-32 ss embryo. The 13-15 ss heads were cultured in either DMSO or DAPT for 24 hr, harvested, cryosectioned, and immunostained for the opV marker Pax3 (A,D) and the early neuronal marker Islet1 (B,E). DMSO treated embryos showed normal opV placode development (A-C). DAPT treated embryos showed a significant increase in ectodermal Pax3+ cells coexpressing Islet1, the number of Pax3+/Islet1+ mesenchymal cells was also dramatically increased (D-F). Histogram describing total number of cells for Pax3+ ectoderm, Pax3+/Islet1+ ectoderm, Pax3+ mesenchyme, and Pax3+/Islet1+ mesenchyme in DMSO vs. DAPT cultures (F). Error bars depict SEM.
Figure 5.
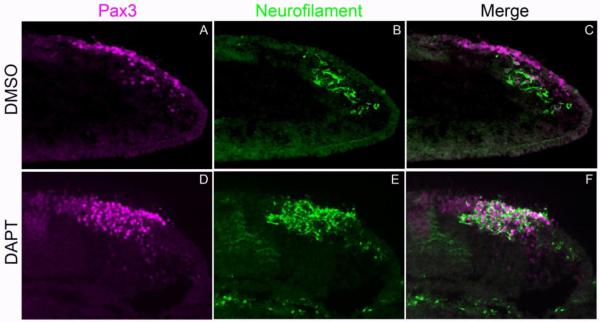
Notch inhibition causes formation of additional differentiated neurons. Transverse section through the ophthalmic trigeminal (opV) placode region of a 30-32 ss embryo. The 13-15ss heads were cultured in either DMSO or DAPT for 24 hr, harvested, cryosectioned, and immunostained for the opV marker Pax3 (A,D) and the late neuronal marker Neurofilament (B,E). DMSO treated embryos showed normal opV placode development (A-C). DAPT treated embryos showed a significant increase in expression of NF in both ectodermal and mesenchymal Pax3+ cells, demonstrating enhanced neuronal differentiation (D-F).
In addition, we also observed precocious neurogenesis caudal to the opV placode in the presumptive mmV placode. While no distinct marker, such as Pax3, has been identified for these cells, many ectodermal and mesenchymal Islet1+ cells were readily visible immediately caudal to the Pax3+ domain (data not shown).
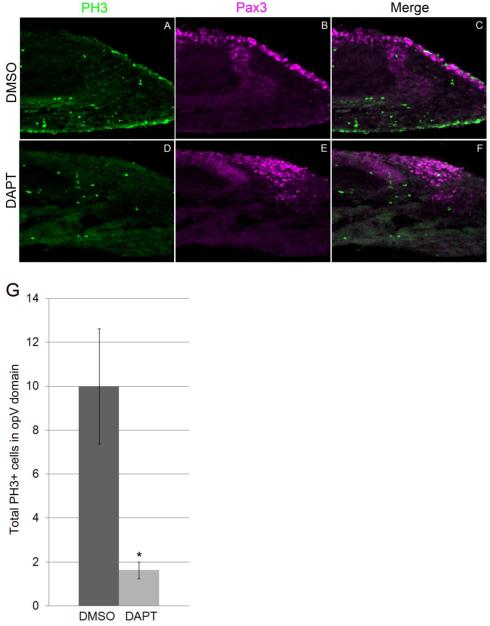
Blocking Notch signaling reduces proliferation in opV ectoderm
To further clarify the previous DAPT result showing more than double the number of Pax3 cells in the mesenchyme, we considered the possibility that increased proliferation could also account for this phenotype. Explant experiments were repeated and immunostained for the mitotic marker phosphohistone-3 (PH3). In DMSO control explants, many cells in the opV ectoderm were dividing, with an average of 10.0 cells expressing PH3 (SEM +/− 2.6, n=5; Fig. 6A-C,G). Whereas, in DAPT explants, an average of only 1.6 cells in the placode domain expressed PH3 (SEM +/− 0.4, n=8; Fig. 6D-G). This demonstrated a statistically significant decrease of proliferating cells in embryos where Notch signaling was inhibited (p<0.025). This supports the idea that blocking Notch activation leads to premature neurogenesis, prompting cells to exit the cell cycle early. Interestingly, ectodermal cells in both control and experimental embryos that did express PH3 did not coexpress Pax3. This confirms previous studies which found that the onset of Pax3 expression is coincident with cells becoming post mitotic neuroblasts (Begbie et al., 2002;Graham et al., 2007;McCabe et al., 2009).
Figure 6.
Notch inhibition results in decreased proliferation in the opV placode. Transverse section through the opV placode region of a 30-32 ss embryo. DMSO/DAPT head cultures were repeated, and immunostained for the mitotic marker Phosphohistone-3 (PH3; A,D) and Pax3 (B,E). DMSO treated embryos showed significantly more PH3+ cells in the opV domain (A-C) vs. DAPT treated embryos which showed significantly fewer PH3+ cells (D-F). Histogram describing total number PH3+ cells in the opV placode domain (G). Error bars depict SEM.
Notch inhibition does not affect Ngn2 proneural gene expression in the opV placode
Neurogenin2 is a proneural transcription factor, expressed in early sensory neurons (Fode et al., 1998;Ma et al., 1998). The ophthalmic trigeminal placode gives rise to sensory neurons of the trigeminal ganglia. The proneural cells of the opV placode are marked by Ngn2 expression at the 10ss, typically localized to the ectoderm (Begbie et al., 2002). Ngn2 is directly repressed by the Notch target gene, Hes1. Upon activation of Notch signaling, Hes1 is transcribed and binds to the promoter of Ngn2, thereby blocking expression. To test whether inhibiting Notch signaling in the chick head could upregulate Ngn2 or expand the domain, we repeated DAPT (n=11) and DMSO (n=9) head cultures and performed whole-mount in situ hybridization for Ngn2 mRNA. Following in situ hybridization, explants were cryosectioned and immunostained for Pax3. Notch inhibition did not result in a significant difference of Ngn2 expression in DMSO vs. DAPT embryos (Fig. S3A-D). Ngn2 expression was not expanded beyond the opV placode domain in experimental vs. control embryos, indicating that placodal specification is necessary for Ngn2 expression.
Constitutive activation of Notch signaling disrupts proper development of opV placode cells
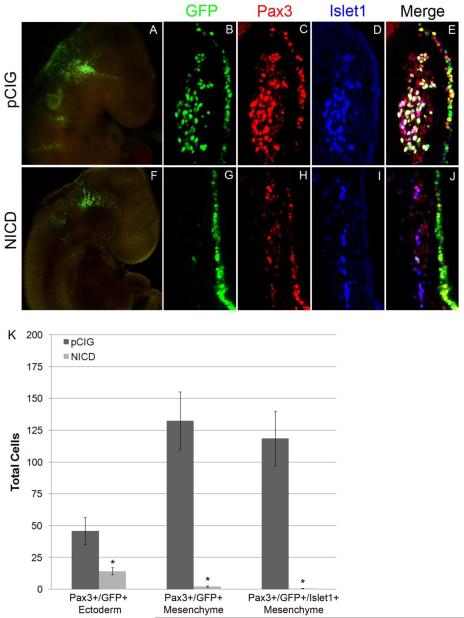
To fully examine how Notch signaling regulates opV cell fate, our subsequent experiments focused on constitutive activation of the Notch pathway through electroporation of the Notch intracellular domain, NICD. In the canonical Notch pathway, NICD translocates to the nucleus, binds to CSL and recruits Mastermind to activate target genes such as Hes1 (Hatakeyama et al., 2004;Hatakeyama et al., 2006;Ohtsuka et al., 1999). Hes1 binds to the Ngn2 promoter blocking its expression thereby preventing neurogenesis. We electroporated NICD into head ectoderm of 10-12 ss embryos and allowed them to develop 30 hr to about the 30-32 ss. The endpoint stage was the same as explant experiments described above, with the starting point being slightly shifted to allow time for transgenic expression after electroporation. Control embryos electroporated with the empty pCIG-GFP vector showed normal development in both whole-mount and embryo sections (Fig. 7A-E). Targeted pCIG-GFP cells coexpressed Pax3 in the ectoderm and mesenchyme; they also delaminated and differentiated accordingly, expressing Islet1 in the mesenchyme. In experimental embryos electroporated with NICD, differentiation of opV placode cells was disrupted (Fig. 7F-J). Targeted NICD cells in the opV domain remained in the ectoderm, failing to delaminate or differentiate; they also did not express Pax3. In control pCIG embryos, a mean of 45.8 cells (SEM +/− 10.5, n=10) in the ectoderm coexpressed Pax3/GFP, whereas only 14.2 (SEM +/− 2.8, n=13) NICD targeted cells colocalized with Pax3 in experimental embryos (p<0.02; Fig.7K). This statistically significant reduction of Pax3 in NICD targeted cells suggests that Notch signaling negatively regulates Pax3 expression. Co-localization of Pax3/GFP in the mesenchyme of experimental embryos showed an even greater decrease due to targeted cells failing to delaminate. Control embryos had an average of 132.3 cells (SEM +/− 22.8, n=10) in the ganglion, coexpressing Pax3/GFP vs. 2.2 cells (SEM +/− 0.6, n=13) in experimental embryos (p<0.0003; Fig. 7K). Therefore, activation of the Notch pathway in the opV placode led to a highly statistically significant reduction of targeted opV cells delaminating and contributing to the ganglion. Of interest, the total number of Pax3+ cells in the opV ganglion of experimental embryos was also significantly diminished (Fig. 7H) compared to pCIG controls, indicating that untargeted ectoderm does not retain placodal potential to compensate for the loss at this stage. Control embryos had an average of 281.6 cells (SEM +/− 39.4, n=10) total Pax3+ cells in the ganglion vs. 103.9 cells (SEM +/− 20.1, n=13) in experimental embryos (p<0.003). As expected, NICD targeted opV cells that were stalled in the ectoderm did not express Islet1. In addition, due to the lack of targeted opV cells delaminating and contributing to the ganglion, targeted Islet1+ placode cells were almost entirely absent in the mesenchyme (Fig. 7I). This further demonstrates that cell-autonomous activation of Notch signaling in opV ectoderm interferes with proper development of the placode and prevents neurogenesis.
Figure 7.
Activation of Notch signaling inhibits opV cell delamination and neuronal differentiation. Chick embryos electroporated at 10-12 ss and incubated for 30 hr. Whole-mount image showing control GFP expression in a pattern similar to wildtype with opV placode cells condensing in the ganglion and extending to the ganglionic branch (A) whereas NICD electroporated embryos show targeted cells scattered only in the ectoderm (F). Transverse sections through the opV placode were immunostained for Pax3 and Islet1 (C,D,H,I). Cells targeted with the pCIG control construct demonstrated normal development; targeted ectoderm cells were Pax3+/Islet1 −, cells also delaminated and entered the mesenchyme with Pax3/Islet1 co-localization, with many cells contributing and differentiating to the ganglion (B-E). In contrast, NICD targeted remain primarily in the ectoderm, indicating they did not delaminate, contribute to the ganglion, or differentiate; NICD ectoderm cells were Pax3−/Islet1− and very few NICD cells ever entered the mesenchyme (G-J). NICD cells remaining in the ectoderm also downregulated Pax3 expression, and the number of untargeted Pax3 cells contributing to the ganglion was greatly reduced (H). Histogram reporting quantitative counts of total cells (K). Error bars depict SEM.
Because cell proliferation was downregulated after blocking Notch signaling we investigated whether increased proliferation would occur following Notch activation. NICD electroporations were repeated and embryos were immunostained for Pax3 and the cell-proliferation marker PH3. Targeted NICD cells remaining in the ectoderm did not show an increase in PH3 expression when compared to pCIG control embryos (Fig. S4). Control embryos had an average of 3.0% (SEM +/− 0.65, n=9; Fig. S4A-D) PH3+ cells in GFP targeted ectoderm and NICD targeted cells had an average of 3.2% (SEM +/− 0.42, n=9, p<0.81; Fig. S4E-I). This finding suggests that Notch signaling may not directly regulate proliferation in the opV placode.
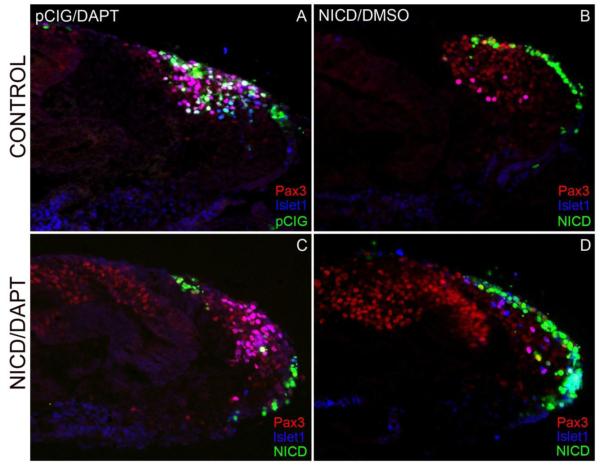
To ensure specificity of both the non-cell autonomous DAPT, and the cell-autonomous NICD construct, we combined these treatments. We wanted confirm that activating the Notch pathway intracellularly, by directly adding NICD, would override the effect of the DAPT gamma-secretase inhibitor which prevents cleavage of the NICD at the cell membrane. We first repeated the NICD/pCIG electroporations in 10-12 ss embryos and let them incubate for 4-6 hr allowing time for the transgene to be transcribed and translated. Embryos were then collected between the 13-15 ss and the heads were dissected and cultured in DAPT or DMSO as described above. Head cultures were harvested after 24 hours, cryosectioned, and immunostained for Pax3 and Islet1. Three separate combined experiments were performed pCIG/DAPT (n=8), NICD/DMSO (n=8), and NICD/DAPT (n=10). Each combination had a distinct phenotype. The pCIG/DAPT controls (Fig. 8A) had many targeted cells in both the ectoderm and mesenchyme showing typical delamination of cells. The GFP+ targeted cells expressed both Pax3 and Islet1 in the ectoderm and mesenchyme, as seen in the previous DAPT head cultures. The second control, NICD/DMSO (Fig. 8B), had a phenotype similar to the NICD electroporations above; targeted cells remained in the ectoderm failing to delaminate. These ectoderm cells did not express Pax3 or Islet1 and there were significantly fewer untargeted Pax3 cells in the mesenchyme. Untargeted ectoderm cells did continue to express Pax3 without differentiating and expressing Islet1. In NICD/DAPT experimental embryos, targeted NICD cells did not delaminate or differentiate, lacking both Pax3 and Islet1 expression (Fig. 8C,D). In areas of the opV placode without targeting, the DAPT phenotype was prevalent, with precocious premature neurogenesis in the ectoderm and mesenchyme illustrated by Pax3/Islet1 co-localization (Fig. 8C). This scenario confirms that NICD cell-autonomous expression in the opV placode will prevent neurogenesis even in the presence of the chemical Notch inhibitor DAPT.
Figure 8.
Notch activation via NICD overrides DAPT inhibition. Embryos were initially electroporated with NICD or pCIG followed by DAPT or DMSO treatment. Transverse sections through the ophthalmic trigeminal (opV) placode region of a 30-32 ss embryo immunostained for Pax3 and Islet1. Both control combinations pCIG/DAPT (A) and NICD/DMSO (B) showed predicted results as seen in separate experiments reported above, with precocious neurogenesis (pCIG/DAPT) and inhibited delamination and differentiation (NICD/DMSO), respectively. The experimental combination of NICD/DAPT showed targeted NICD cells failing to delaminate and Pax3−/Islet1− even in the presence of the Notch inhibitor DAPT (D). In sections from the same embryo, untargeted opV cells are Pax3+/Islet1+, similar to Figure 2, indicating ectopic and increased neurogenesis (C). Magenta cells illustrate co-localization of Pax3+ and Islet1+ cells; white cells depict merge of GFP+/Pax3+/Islet1+ cells.
Discussion
The journey from naïve ectoderm cell to sensory neuron is a complex developmental process that can be directly uncovered through study of the ophthalmic trigeminal placode. Development of the opV placode culminates in the single cell fate choice between sensory neuron and epidermal ectoderm. Previous findings indicate that while several pathways (Wnt, FGF, and PDGF) are needed during the differentiation process, none appear to be sufficient to expand the placode or generate the prolific neurogenesis reported here. Therefore, terminal differentiation of opV cells must be regulated by an independent pathway. We hypothesize that the key step in opV terminal differentiation is determined through juxtacrine signaling of the Notch/Delta pathway in opV ectoderm.
Through a detailed series of gene expression snapshots, we have characterized the spatiotemporal expression pattern of Notch ligands, receptors, and effectors. From these data two things are clear: 1) Notch signaling is active in opV placode cells beginning at stage 10, a developmental timepoint coincident with opV cell fate determination (Baker et al., 1999). 2) The expression of Notch pathway genes (a readout for signaling activity) is confined to the ectodermal pool and downregulated as cells undergo EMT. This is observed in both Notch-active (Notch1, Hes1, etc.) and Notch-inactive (Ngn2, Delta; confirming Begbie et al., 2002a) cells. While candidate Notch/Delta partners are expressed in the placode, the specific molecules involved have not been determined. However, this mRNA expression analysis supports the hypothesis that Notch signaling may regulate neuronal selection and cellular delamination in the placode.
To test this, we utilized the gamma-secretase inhibitor, DAPT, which blocks Notch signaling. Our results show that Notch inhibition resulted in premature and seemingly unrestrained neuronal differentiation in the ectoderm of the opV placode, which has never before been reported. Within 12 hours of DAPT exposure, several opV placode cells have aberrantly delaminated and upregulated the early neuronal marker Islet1. In embryos exposed to DAPT for 24 hours, many additional placode-derived cells are found in the mesenchyme, expressing both Islet1 and Neurofilament. While previous reports have shown that Islet1 is expressed in the placodal ectoderm (Shiau et al., 2008), we observed a profound increase in neuronal differentiation within the ectoderm, with widespread expression of both Islet1 and Neurofilament. Still, the number of Pax3+ ectoderm cells did not increase, and the opV domain did not appear to be expanded. To explain the massive increase in mesenchymal opV neurons, we first eliminated the possibility that blocking Notch signaling increased proliferation in the placode. We actually found a highly significant decrease of proliferative cells in the opV domain, again pointing to premature neurogenesis. We propose that the opV placode domain is tightly regulated in the ectoderm within a subset of specified Pax3+ cells, meaning signaling independent of Notch/Delta acts to retain the placode within a specific domain, or otherwise limit the number of specified (Pax3+) cells that can be regulated by Notch signaling. This model is also supported by our analysis of Ngn2 in DAPT embryos. Blocking Notch signaling did not increase or expand Ngn2 expression laterally beyond the normal placode domain. This may be due to Pax3 directly activating Ngn2 as seen in mice, where Pax3 in the neural tube regulates Ngn2 expression by directly binding to its promoter (Nakazaki et al., 2008). Therefore, in experimental embryos, once neuronal selection occurs and neurons leave the ectoderm, additional opV cells can be specified from nearby competent ectoderm, and they will upregulate Pax3, adopting the potential to undergo neuronal differentiation and delamination. This continual recruitment would be possible because of the accelerated neurogenesis due to global Notch inhibition. One possible scenario in normal development, where differentiation and cellular delamination is only occurring in a select number of cells, is once neuronal selection has occurred, the Delta expressing cell will delaminate thereby allowing the ectodermal field to reset itself and select other Pax3+ cells to differentiate.
To further examine the role of Notch signaling, and to confirm the specificity of DAPT in blocking Notch cleavage rather than another gamma-secretase target, we constitutively activated the Notch pathway through electroporation of the cell-autonomous NICD construct. Notch activation resulted in targeted cells failing to delaminate or differentiate in the ectoderm. NICD electroporations also revealed that Notch signaling negatively regulates Pax3 expression. The loss of differentiated neurons resulted in abnormal opV gangliogenesis, with a significant reduction of Pax3+ neurons contributing to the ganglion. These combined effects are similar to those seen in previous experimental results where the Wnt or FGF pathways were blocked where we have shown that opV specification can be reversed through Pax3 downregulation (Lassiter et al., 2007, 2009). These results indirectly supported the conclusion that DAPT treatment blocked Notch cleavage, but to ensure specificity of the DAPT treatments, performed experiments wherein NICD electroporated embryos were subsequently treated with DAPT in explant culture conditions. In all embryos tested, NICD intracellular activation of the Notch pathway was dominant, meaning targeted cells did not become neurons, whereas untargeted placodal ectoderm cells expressed Islet1 and NF. These findings reaffirmed our individual experimental results and also confirmed that DAPT is blocking Notch signaling, though it may have additional targets.
Evidence for premature differentiation
Examining neuronal differentiation at two distinct time points allowed us to evaluate the effects of DAPT treatment over time. After 24 hours, a large number of additional Islet1+ neurons are present in the mesenchyme, and the vast majority of Pax3+ ectoderm cells also express Islet1. Additionally, NF expression fills the placodal ectoderm and mesenchyme, demonstrating late-stage neurogenesis. This is different than what was observed after 12 hours, where increased Islet1 expression was obvious in the Pax3+ mesenchyme cells and some Pax3+ ectoderm cells, but many Pax3+ ectoderm cells did not yet express Islet1, and NF was largely absent in most sections. Therefore, treatment of the opV placode with DAPT resulted in premature neurogenesis, the early stages being observed at 12 hours where Islet1− and Islet1+ placode cells were prevalent. By the 24 hr time point, neurogenesis had progressed rapidly and had extended into the ectoderm, where most Pax3+ cells also expressed Islet1, and NF expression was widespread. DMSO treated control embryos showed very little Islet1 expression at 12 hours, as very few cells had delaminated from the undifferentiated ectoderm. At 24 hours many Pax3+/Islet1− cells still existed in the ectoderm, indicating significant neurogenesis had yet to occur. While past research has shown that Pax3-expressing placode cells are largely post-mitotic from early stages of differentiation, it is unclear whether a similar number of neurons would ultimately be born in the experimental model presented here. Longer culture techniques that allow for extended embryogenesis and yield good tissue morphology would be required to assess total neuronal output. Future studies will aim to test various culture conditions, and be utilized for more detailed studies of late placode differentiation in experimental and control treatments.
The relationship of delamination and differentiation
The observation that 24 hour DAPT treatment led to precocious neurogenesis within the surface ectoderm relates well to the question of cellular delamination in the process of opV placode differentiation. While ectodermally-localized neurons are occasionally observed in control embryos (McCabe et al., 2009), the vast majority of opV placode cells don’t express Islet1 or Neurofilament until after delamination. Also, the misexpression of Pax3 causes the upregulation of Ngn2 and FGFR4, but cells fail to delaminate and differentiate (Dude et al, 2009). Additionally, blocking FGF signaling led to opV placode cells remaining in the ectoderm, maintaining the expression of Pax3 for a significant length of time, but ultimately failing to delaminate (Lassiter et al., 2009). These observations combined indicate that delamination is a key step for neuronal differentiation. However, in addition to the quantitative results described here, we also saw aberrant delamination and migration patterns in DAPT treated embryos. In the mesenchyme, instead of individual or small groups of cells delaminating and migrating in a stream to condense in the interior mesenchyme as is seen in control embryos, DAPT treated cultures showed mass delamination with neurons scattered beneath the ectoderm. We also observed many differentiated ectoderm cells. The observed ectoderm often appeared fragmented, indicating cellular adhesion was disrupted. It is known that neurogenin regulates the expression of neuronal differentiation genes and cell adhesion genes directly, as shown by studies in the mouse CNS, coupling neurogenesis with cell migration through the bHLH transcription factor Ngn2 (Ge et al., 2006; Nakazaki et al., 2008). While our results could lead to the conclusion that delamination is dispensable, they also support a model wherein Notch signaling regulates both differentiation and delamination. N-cadherin has recently been identified as an important cell adhesion molecule in regulating proper gangliogenesis in the trigeminal ganglion (Shiau and Bronner-Fraser, 2009). Examining cell adhesion changes in experimental and DAPT treated embryos will be an interesting focus of future research.
We showed that preventing Notch signaling promotes precocious neurogenesis in all opV specified Pax3+ cells, with a dramatic increase in the number of neurons in both the ectodermand the mesenchyme. In contrast, constitutive Notch signaling completely disrupted the development of opV placode cells, preventing neuronal differentiation and cellular delamination (see Model; Fig. 9). Altogether the Notch expression, inhibition, and activation data demonstrate that terminal sensory neuron differentiation and delamination are instructed through modulation of the Notch pathway in the opV placode. Current experiments are underway to uncover if and how the Wnt, FGF, and PDGF pathways combine with or regulate Notch signaling in opV placode cell differentiation, to examine changes in cellular adhesion, and to develop strategies to evaluate later stages of gangliogenesis.
Figure 9.
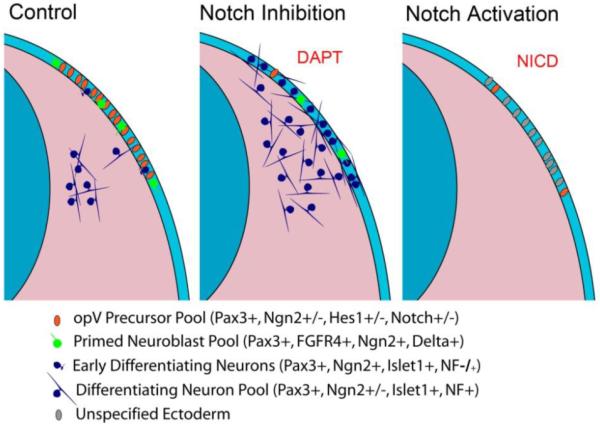
Graphical model summarizing results. Transverse section through the opV region of a chick embryo. Left side of each section represents normal development. Notch inhibition results in prolific neurogenesis in the ectodermal opV pool and an increase in the number of differentiating opV cells in the mesenchyme. Notch activation prevents delamination and differentiation and ultimately results in an ectodermal pool of unspecified cells. Legend depicts differing developmental potential of each color coded cell type.
Supplementary Material
Acknowledgements
We thank Andy McMahon and Sean Megason for kindly providing the NICD-pCIG vector. We thank Nicolas Daudet for generously providing many of the Notch pathway probes. We also thank Kris Marin for his extensive contribution to this work. Thank you to the many undergraduate students who contributed to this work. This research was supported by the following sources: Grant Sponsors: NIH/NICHD #1R01HD046475-01; BYU/ORCA mentored research grant to B.T.W.; BYU graduate mentoring award to J.S.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelló G, Khatri S, Giráldez F, Alsina B. Early Regionalization of the Otic Placode and its Regulation by the Notch Signaling Pathway. Mech. Dev. 2007;124:631–645. doi: 10.1016/j.mod.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Bailey AM, Posakony JW. Suppressor of Hairless Directly Activates Transcription of Enhancer of Split Complex Genes in Response to Notch Receptor Activity. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Establishing Neuronal Identity in Vertebrate Neurogenic Placodes. Development. 2000;127:3045–3056. doi: 10.1242/dev.127.14.3045. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate Cranial Placodes I. Embryonic Induction. Dev. Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Baker CV, Stark MR, Bronner-Fraser M. Pax3-Expressing Trigeminal Placode Cells can Localize to Trunk Neural Crest Sites but are Committed to a Cutaneous Sensory Neuron Fate. Dev. Biol. 2002;249:219–236. doi: 10.1006/dbio.2002.0767. [DOI] [PubMed] [Google Scholar]
- Baker CV, Stark MR, Marcelle C, Bronner-Fraser M. Competence, Specification and Induction of Pax-3 in the Trigeminal Placode. Development. 1999;126:147–156. doi: 10.1242/dev.126.1.147. [DOI] [PubMed] [Google Scholar]
- Begbie J, Ballivet M, Graham A. Early Steps in the Production of Sensory Neurons by the Neurogenic Placodes. Mol. Cell. Neurosci. 2002;21:502–511. doi: 10.1006/mcne.2002.1197. [DOI] [PubMed] [Google Scholar]
- Bell D, Streit A, Gorospe I, Varela-Nieto I, Alsina B, Giraldez F. Spatial and Temporal Segregation of Auditory and Vestibular Neurons in the Otic Placode. Dev. Biol. 2008;1:109–120. doi: 10.1016/j.ydbio.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Bolós V, Grego-Bessa J, de la Pompa JL. Notch Signaling in Development and Cancer. Endocr. Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- Canning CA, Lee L, Luo SX, Graham A, Jones CM. Neural Tube Derived Wnt Signals Cooperate with FGF Signaling in the Formation and Differentiation of the Trigeminal Placodes. Neural Develop. 2008;3:35. doi: 10.1186/1749-8104-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DS, Skowronska-Krawczyk D, Armant O, Donaldson IJ, Parras C, Hunt C, Critchley JA, Nguyen L, Gossler A, Gottgens B, et al. Proneural bHLH and Brn Proteins Coregulate a Neurogenic Program through Cooperative Binding to a Conserved DNA Motif. Dev.Cell. 2006;11:831–844. doi: 10.1016/j.devcel.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Cheung AM, Kwong YL, Liang R, Leung AY. Stem Cell Model of Hematopoiesis. Curr. Stem Cell. Res. Ther. 2006;1:305–315. doi: 10.2174/157488806778226740. [DOI] [PubMed] [Google Scholar]
- D’Amico-Martel A, Noden DM. Contributions of Placodal and Neural Crest Cells to Avian Cranial Peripheral Ganglia. Am. J. Anat. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two Contrasting Roles for Notch Activity in Chick Inner Ear Development: Specification of Prosensory Patches and Lateral Inhibition of Hair-Cell Differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch Signalling is Needed to Maintain, but Not to Initiate, the Formation of Prosensory Patches in the Chick Inner Ear. Development. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Dude CM, Kuan CY, Bradshaw JR, Greene ND, Relaix F, Stark MR, Baker CV. Activation of Pax3 Target Genes is Necessary but Not Sufficient for Neurogenesis in the Ophthalmic Trigeminal Placode. Dev. Biol. 2009;326:314–326. doi: 10.1016/j.ydbio.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiúza U, Arias AM. Cell and Molecular Biology of Notch. J. Endocrinol. 2007;194:459–474. doi: 10.1677/JOE-07-0242. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH Protein NEUROGENIN 2 is a Determination Factor for Epibranchial Placode-Derived Sensory Neurons. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Ge W, He F, Kim KJ, Blanchi B, Coskun V, Nguyen L, Wu X, Zhao J, Heng JI, Martinowich K, et al. Coupling of Cell Migration with Neurogenesis by Proneural bHLH Factors. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1319–1324. doi: 10.1073/pnas.0510419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Blentic A, Duque S, Begbie J. Delamination of Cells from Neurogenic Placodes does Not Involve an Epithelial-to-Mesenchymal Transition. Development. 2007;134:4141–4145. doi: 10.1242/dev.02886. [DOI] [PubMed] [Google Scholar]
- Groves AK, Bronner-Fraser M. Competence, Specification and Commitment in Otic Placode Induction. Development. 2000;127:3489–3499. doi: 10.1242/dev.127.16.3489. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, Guillemot F, Kageyama R. Hes Genes Regulate Size, Shape and Histogenesis of the Nervous System by Control of the Timing of Neural Stem Cell Differentiation. Development. 2004;131:5539–5550. doi: 10.1242/dev.01436. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Sakamoto S, Kageyama R. Hes1 and Hes5 Regulate the Development of the Cranial and Spinal Nerve Systems. Dev. Neurosci. 2006;28:92–101. doi: 10.1159/000090756. [DOI] [PubMed] [Google Scholar]
- Heitzler P, Simpson P. The Choice of Cell Fate in the Epidermis of Drosophila. Cell. 1991;64:1083–1092. doi: 10.1016/0092-8674(91)90263-x. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling Downstream of Activated Mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Le Bail O, Hirsinger E, Pourquie O, Logeat F, Strong CF, Brou C, Seidah NG, Isra l. A. Delta-1 Activation of Notch-1 Signaling Results in HES-1 Transactivation. Mol. Cell. Biol. 1998;18:7423–7431. doi: 10.1128/mcb.18.12.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CS, Ohyama T, Segil N, Groves AK. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development. 2008;135:2251–2261. doi: 10.1242/dev.017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic Notch Signaling in Neural Progenitor Cells and a Revised View of Lateral Inhibition. Nat. Neurosci. 2008;11:1247–1251. doi: 10.1038/nn.2208. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH Genes in Neural Stem Cell Differentiation. Exp. Cell Res. 2005;306:343–348. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Karamysheva AF. Mechanisms of Angiogenesis. Biochemistry (Mosc) 2008;73:751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- Lasky JL, Wu H. Notch Signaling, Brain Development, and Human Disease. Pediatr. Res. 2005;57:104R–109R. doi: 10.1203/01.PDR.0000159632.70510.3D. [DOI] [PubMed] [Google Scholar]
- Lassiter RN, Dude CM, Reynolds SB, Winters NI, Baker CV, Stark MR. Canonical Wnt Signaling is Required for Ophthalmic Trigeminal Placode Cell Fate Determination and Maintenance. Dev. Biol. 2007;308:392–406. doi: 10.1016/j.ydbio.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassiter RN, Reynolds SB, Marin KD, Mayo TF, Stark MR. FGF Signaling is Essential for Ophthalmic Trigeminal Placode Cell Delamination and Differentiation. Dev. Dyn. 2009;238:1073–1082. doi: 10.1002/dvdy.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. Notch Signalling and the Control of Cell Fate Choices in Vertebrates. Semin. Cell Dev. Biol. 1998;9:583–589. doi: 10.1006/scdb.1998.0266. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. Neurogenin1 is Essential for the Determination of Neuronal Precursors for Proximal Cranial Sensory Ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Bronner-Fraser M. Essential Role for PDGF Signaling in Ophthalmic Trigeminal Placode Induction. Development. 2008;135:1863–1874. doi: 10.1242/dev.017954. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Sechrist JW, Bronner-Fraser M. Birth of Ophthalmic Trigeminal Neurons Initiates Early in the Placodal Ectoderm. J. Comp. Neurol. 2009;514:161–173. doi: 10.1002/cne.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memberg SP, Hall AK. Dividing Neuron Precursors Express Neuron-Specific Tubulin. J. Neurobiol. 1995;1:26–43. doi: 10.1002/neu.480270104. [DOI] [PubMed] [Google Scholar]
- Muskavitch MA. Delta-Notch Signaling and Drosophila Cell Fate Choice. Dev. Biol. 1994;166:415–430. doi: 10.1006/dbio.1994.1326. [DOI] [PubMed] [Google Scholar]
- Nakazaki H, Reddy AC, Mania-Farnell BL, Shen Y, Ichi S, McCabe C, George D, McLone DG, Tomita T, Mayanil CSK. Key Basic Helix-Loop-Helix Transcription Factor Genes Hes1 and Ngn2 are Regulated by Pax3 during Mouse Embryonic Development. Dev. Biol. 2008;316:510–523. doi: 10.1016/j.ydbio.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Hartman BH, Georgi SA, Lan MS, Reh TA. Transient Inactivation of Notch Signaling Synchronizes Differentiation of Neural Progenitor Cells. Dev. Biol. 2007;304:479–498. doi: 10.1016/j.ydbio.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as Notch Effectors in Mammalian Neuronal Differentiation. EMBO J. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquie O. The Vertebrate Segmentation Clock. J. Anat. 2001;199:169–175. doi: 10.1046/j.1469-7580.2001.19910169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G. Induction and Specification of Cranial Placodes. Dev. Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Radde-Gallwitz K, Pan L, Gan L, Lin X, Segil N, Chen P. Expression of Islet1 Marks the Sensory and Neuronal Lineages in the Mammalian Inner Ear. J. Comp. Neurol. 2004;4:412–421. doi: 10.1002/cne.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau CE, Lwigale PY, Das RM, Wilson SA, Bronner-Fraser M. Robo2-Slit1 Dependent Cell-Cell Interactions Mediate Assembly of the Trigeminal Ganglion. Nat. Neurosci. 2008;3:269–276. doi: 10.1038/nn2051. [DOI] [PubMed] [Google Scholar]
- Shiau CE, Bronner-Fraser M. N-Cadherin Acts in Concert with Slit1-Robo2 Signaling in Regulating Aggregation of Placode-Derived Cranial Sensory Neurons. Development. 2009;24:4155–4164. doi: 10.1242/dev.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in Notch Signaling Regulate Maintenance of Neural Progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Stark MR, Biggs JJ, Schoenwolf GC, Rao MS. Characterization of Avian Frizzled Genes in Cranial Placode Development. Mech. Dev. 2000;93:195–200. doi: 10.1016/s0925-4773(00)00263-x. [DOI] [PubMed] [Google Scholar]
- Stark MR, Sechrist J, Bronner-Fraser M, Marcelle C. Neural Tube-Ectoderm Interactions are Required for Trigeminal Placode Formation. Development. 1997;124:4287–4295. doi: 10.1242/dev.124.21.4287. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Maynard TM, Weston JA. Fate determination of neural crest cells by NOTCH-mediated lateral inhibition and asymmetrical cell division during gangliogenesis. Development. 2000;127:2811–2821. doi: 10.1242/dev.127.13.2811. [DOI] [PubMed] [Google Scholar]
- Weng AP, Nam Y, Wolfe MS, Pear WS, Griffin JD, Blacklow SC, Aster JC. Growth Suppression of Pre-T Acute Lymphoblastic Leukemia Cells by Inhibition of Notch Signaling. Mol. Cell. Biol. 2003;23:655–664. doi: 10.1128/MCB.23.2.655-664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K, Gaiano N. Notch Signaling in the Mammalian Central Nervous System: Insights from Mouse Mutants. Nat. Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.