Abstract
The role of leptin in the mucosal immune response to Clostridium difficile colitis, a leading cause of nosocomial infection, was studied in humans and in a murine model. Previously, a mutation in the receptor for leptin (LEPR) was shown to be associated with susceptibility to infectious colitis and liver abscess due to Entamoeba histolytica as well as to bacterial peritonitis. Here we discovered that European Americans homozygous for the same LEPR Q223R mutation (rs1137101), known to result in decreased STAT3 signaling, were at increased risk of C. difficile infection (odds ratio, 3.03; P = 0.015). The mechanism of increased susceptibility was studied in a murine model. Mice lacking a functional leptin receptor (db/db) had decreased clearance of C. difficile from the gut lumen and diminished inflammation. Mutation of tyrosine 1138 in the intracellular domain of LepRb that mediates signaling through the STAT3/SOCS3 pathway also resulted in decreased mucosal chemokine and cell recruitment. Collectively, these data support a protective mucosal immune function for leptin in C. difficile colitis partially mediated by a leptin-STAT3 inflammatory pathway that is defective in the LEPR Q223R mutation. Identification of the role of leptin in protection from C. difficile offers the potential for host-directed therapy and demonstrates a connection between metabolism and immunity.
INTRODUCTION
Clostridium difficile is a Gram-positive, spore-forming, and toxin-producing anaerobic bacterium that causes antibiotic-associated diarrhea, a leading cause of health care-associated infections (1–3). In the last decade, the incidence of C. difficile infection has markedly increased (4). The epidemic strain of C. difficile (BI/NAP1) predominantly responsible for this increase is associated with an ∼15% 30-day mortality and high rates of recurrence and relapse (5–9). While C. difficile typically causes disease in individuals who are hospitalized or reside in long-term-care facilities (10), there has recently been an increase in community-acquired cases (11). Disruption of the indigenous gut microbiome by antimicrobial or chemotherapeutic agents creates a niche for C. difficile colonization and infection; however, colonization does not always translate to disease (12, 13). The spectrum of clinical disease is wide and ranges from mild diarrheal illness to fulminant colitis and death, suggesting a role for the host immune response in disease pathogenesis.
C. difficile disease is characterized by an intense inflammatory response, including recruitment of neutrophils to the colon (14), peripheral leukocytosis (15), and increased inflammatory cytokine (interleukin-23 [IL-23], IL-6, IL-8, tumor necrosis factor alpha [TNF-α]) (16, 17) and chemokine (CCL5, CCL2) production (16). Leukocytosis is a key feature seen during C. difficile infections with severe colitis, characterized by pseudomembranes composed of neutrophilic exudates and cellular debris (15, 18, 19). The role of this intense innate inflammatory response in providing protection is not clear. Blocking of neutrophil infiltration in mice (14), induction of neutropenia in rats (20), or the use of mice deficient in mast cells (21) was shown to lead to decreased inflammation after toxin A injection. Similarly, blocking of neuroinflammatory mediators (neurotensin and substance P) led to decreased toxin A-induced enteritis (22). Mice that lack inflammatory IL-23 signaling (IL-23p19-knockout mice and anti-IL-23p19 monoclonal antibody-neutralized mice) were protected from C. difficile infection (17). However, some degree of inflammation may be protective, as evidenced by increased gut bacterial translocation and increased mortality upon depletion of neutrophils (24), enhanced protection after treatment with Toll-like receptor 5 (TLR5) agonists (25), and increased disease severity in mice lacking innate immune receptors (TLR4 deficiency and NOD-1 deficiency) (23, 26).
Leptin is an adipocytokine and member of the IL-6 family that was initially discovered because of its role in regulation of metabolism and satiety (27). It is now known that leptin is important in defense against infectious diseases (28–30). Humans with leptin deficiency have an increased incidence of infection (28), and mice with leptin deficiency (ob/ob) or leptin receptor deficiency (db/db) have increased susceptibility to pneumonia, listeriosis, and amebiasis (29, 30, 32, 33). Leptin is also expressed in the colon of patients with Crohn's disease and ulcerative colitis (34, 35). Leptin has been demonstrated to induce inflammatory cytokine and chemokine production, neutrophil chemotaxis, and NK cell cytotoxicity, in part due to its effects on T regulatory cells (36–39). Leptin actions in the gut include stimulation of mucin secretion and maintenance of intestinal barrier function (40, 41).
We previously studied the role of leptin in Entamoeba histolytica infection, demonstrating that a Q-to-R mutation at position 223 in the leptin receptor cytokine receptor homology 1 (CRH1) domain was associated with the increased susceptibility of humans to both amebic colitis and liver abscess (31). Mice lacking a functional leptin receptor (db/db) were susceptible to amebic colitis, and targeted deletion of the leptin receptor from the intestinal epithelium replicated that phenotype (29). Leptin was protective in the murine model via activation of STAT3/SOCS3 and SHP2/mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase (ERK) signaling pathways downstream of the leptin receptor (29). Other investigators have shown an association of the Q223R mutation with the increased susceptibility of patients to bacterial peritonitis (42), and in murine models, leptin has been shown to protect from bacterial pneumonia (30, 32).
The impact of leptin in C. difficile was first studied in a mouse model of toxin A-induced enteritis, where both leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice lacked toxin A-induced ileal fluid secretion (43). We hypothesized that leptin enhancement of inflammation would protect from C. difficile infection. Here we show that the leptin receptor 223R mutation in humans is associated with an increased risk of C. difficile infection. In a murine model, we further show that leptin signaling is protective and the LepRb-STAT3 signaling pathway enhances the inflammatory response.
MATERIALS AND METHODS
Leptin receptor polymorphism.
Cases were identified from patients with C. difficile, and controls were race and sex matched. This list was cross-referenced from the archives of the University of Virginia Health System, and patients with any available tissue sample (for example, tissue biopsy samples, skin specimens) were included. Samples were not linked to protected health information. DNA was extracted from 54 cases of C. difficile colitis and 141 controls using a QIAamp DNA formalin-fixed, paraffin-embedded (FFPE) tissue kit (Qiagen) according to the manufacturer's instructions, with minor modifications. In brief, tissue samples were first removed from FFPE tissue blocks using a sterile needle, and the tissues were then deparaffinized by xylene and washed by ethanol. After removing the residual ethanol, the pellet was resuspended in buffer ATL and proteinase K solution and incubated at 56°C overnight, followed by incubation at 90°C for 1 h to reverse formaldehyde cross-linking. RNase A was then added, and the mixture was incubated for 2 min at room temperature. Buffer AL and 95% ethanol were subsequently added to the samples, and the components were mixed thoroughly. The lysate was transferred to a QIAamp MinElute column and centrifuged. The column was then washed with buffers AW1 and AW2. DNA was finally eluted from the column using buffer ATE. To measure the LEPR Q223R (rs1137101) genotype, the LEPR region was amplified via PCR and an Eclipse assay (Epoch Biosciences) was utilized. PCR was performed on Bio-Rad Tetrad thermal cyclers, and subsequent melt curves were performed on an ABI 7900 apparatus. The research study was approved by the University of Virginia Institutional Review Board Committee.
Statistical analysis.
Associations with C. difficile and the LEPR polymorphism were done using logistic regression adjusted for sex. The statistical package STATA (v.12.0) was used, and a P value of <0.05 was considered significant. Hardy-Weinberg equilibrium was determined using an exact test (P < 0.0001). Statistical analysis for all murine studies was performed using GraphPad Prism software (v.5.0a; GraphPad Software Inc., La Jolla, CA). A paired 2-tailed Student's t test was used to compare the means of two groups, and a P value of <0.05 was considered statistically significant.
Animals.
Six- to 8 week-old leptin receptor-deficient (db/db) male C57BL/6.BKS-Leprdb/J mice (stock number 000697) and respective C57BL/6J control mice were purchased from The Jackson Laboratory, Bar Harbor, ME. S1138 mice, in which the tyrosine 1138 residue in LepRb was changed to serine, resulting in a specific disruption in LepRb → STAT3 signaling, were generated by Bates et al. (44) and were bred and maintained in the University of Virginia vivarium. Mice were bred as heterozygous pairs, and 8- to 14-week-old homozygous mutant and wild-type (WT) littermate control mice were used for infection. Mice were maintained under strict pathogen-free conditions at the University of Virginia, and all protocols were approved by the Institutional Animal Care and Use Committee.
Clostridium difficile culture.
Clostridium difficile strain VPI 10463 (ATCC 43255) was incubated at 37°C for 18 to 20 h in anaerobic chopped meat medium (Anaerobe Systems, Morgan Hill, CA). Bacteria were then subcultured under the same conditions for 5 h, centrifuged at 10,000 rpm for 2 min, and washed with brain heart infusion (BHI) medium two times. Bacteria were resuspended in BHI medium and quantified by spectrophotometry. An optical density value of 1.0 at 490 nm was equivalent to 5 × 107 CFU/ml bacteria, as described previously by Li et al. (45). For sham infection, mice were challenged with BHI medium alone.
Mouse model of C. difficile infection.
The mouse model of C. difficile colitis was as previously described by Chen et al. (46). Briefly, mice were treated with an antibiotic mixture in the drinking water for 3 days (gentamicin, 0.035 mg/ml; colistin, 850 U/ml; vancomycin, 0.045 mg/ml; metronidazole, 0.215 mg/ml). After 3 days, mice were switched to regular water for 2 days. On the day prior to infection, mice were injected once with 32 mg/kg of body weight clindamycin via intraperitoneal injection. Mice were then infected with 104 or 105 CFU/mouse C. difficile VPI 10463 by orogastric gavage. Mice were weighed daily and every 12 h during the peak of infection. The clinical signs of inflammation (clinical score) were assessed by measuring diarrhea, piloerection, activity, posture, and ocular discharge daily and every 12 h during the peak of infection on a scale of from 0 to 4 for activity and a scale of from 0 to 3 for the other parameters, as described previously (45, 47). Animals judged to be in a moribund state on the basis of a total score of ≥14 were euthanized.
Measurement of C. difficile pathogen burden.
C. difficile pathogen burden was quantified by measuring C. difficile toxins and C. difficile-specific glutamate dehydrogenase (GDH) antigen in the cecal contents of mice using a modified protocol for the TechLab C. difficile toxin A/B II and C. diff Chek-60 enzyme-linked immunosorbent assay (ELISA) kits, respectively (TechLab, Blacksburg, VA). After the mice were sacrificed, each cecal stool sample was put in preweighed tubes and stored at −80°C prior to analysis. The samples were thawed, weighed, and diluted with assay diluent. The amount of diluent per sample was normalized to provide the same stool mass-to-diluent ratio for each sample. This normalized diluent-sample mixture was vortexed, and then 1:10, 1:100, and 1:1,000 dilutions were added to precoated wells provided in the kit. In order to generate a standard curve, 2-fold dilutions of the toxin A-B mixture or C. difficile GDH antigen provided in the kit were made with assay diluent, and 100 μl of the undiluted sample and various dilutions was used. One hundred microliters of assay diluent was used as a negative control. One drop of conjugate was added to each well, and the plate was incubated at 37°C for 50 min. Each well was washed four times with the wash buffer provided in the kit, and 2 drops of substrate was added to each well. After 10 min, 1 drop of stop solution was added to each well and the plates were read at 450 nm in an ELISA reader.
Histopathologic scoring.
At 2 days after infection, mice were sacrificed and ceca were harvested. Tissue was fixed for 24 h in Bouin's solution, washed, and then stored in 70% ethanol prior to processing for hematoxylin-eosin (H&E) staining at the University of Virginia Research Histology Core. The histological severity of colonic inflammation was graded in a blinded fashion by scoring for inflammatory cell infiltration, mucosal hyperplasia, epithelial cell destruction (ulceration, disrupted architecture, or loss of integrity), submucosal edema, and vascular congestion, as previously described (45, 47, 48). A score of 0 to 3 was assigned to each of these parameters. The overall score was the sum of each component score.
Flow cytometry.
Single-cell leukocyte suspensions of lamina propria cells were generated from mouse colons as described by Rivollier et al. (49). Briefly, the resected mouse cecum and proximal colon were rinsed by washing in a buffer consisting of Hank's balanced salt solution (HBSS), 5% fetal calf serum (FCS), 25 mM HEPES. The pieces of intestine were then weighed and incubated with prewarmed buffer consisting of HBSS, 15 mM HEPES, 5 mM EDTA, 10% FCS, and 1 mM dithiothreitol in a 37°C water bath with shaking for 15 min (and with vortexing every 5 min) to remove the epithelial cell layer. The colon was then cut into small pieces and digested in prewarmed Iscove's complete medium containing Liberase TL (0.17 mg/ml; Roche) and DNase (30 μg/ml; Roche) for 60 min in a 37°C water bath with shaking. The cells were pelleted and then passed through a 100-μm-mesh-size followed by a 40-μm-mesh-size nylon cell strainer (BD Falcon) and washed with fluorescence-activated cell sorting buffer (phosphate-buffered saline, 2% FCS).
Cell viability was assessed using trypan blue exclusion, and the percentage of live cells ranged from 14 to 40% in all samples. A minimum of 106 live cells were stained for each sample. After blockade of Fc receptors with CD16/32 blocking antibody (eBioscience), leukocytes were stained using monoclonal antibodies to the following cell surface markers: CD45-fluorescein isothiocyanate (FITC), CD45-allophycocyanin (APC)-Alexa 750, CD11b-eflour450, F4/80-APC, SiglecF-phycoerythrin (PE), Ly6G-PE, and Ly6C-FITC (eBioscience and BD Biosciences). Data were acquired using a BD FACSCanto II flow cytometer and BD FACSDiva (v.6.1.2) software (BD Biosciences). A total of 105 events were acquired per sample. Data analysis was done using FlowJo (v.9.2) software (TreeStar Inc., Ashland, OR). Dead cells were excluded on the basis of forward scatter/side scatter (SSC) gating, and data are shown as the number of live cells per gram of tissue.
Tissue chemokine and cytokine levels.
Colonic tissue was weighed and then homogenized by bead beating for 1 min in a buffer consisting of 1 M HEPES and Halt protease inhibitor cocktail (Thermo-Fisher Scientific Inc., Rockford, IL) and then further incubated for a period of 30 min on ice with buffer containing Triton X-100, HEPES, and Halt protease inhibitor cocktail. The lysates were pelleted by centrifugation, and the supernatants were harvested and stored at −80°C until they were analyzed by Luminex assay (leukemia inhibitory factor [LIF], CCL3, CCL4, CXCL10, CXCL1, CXCL2, IL-17A, IL-6, IL-1β, gamma interferon [IFN-γ], and TNF-α; EMD Millipore, St. Charles, MO) and ELISA (mouse IL-23 ELISA; eBioscience). Data were normalized to tissue weight.
RESULTS
The leptin receptor 223R mutation is associated with an increased risk of C. difficile colitis.
We previously studied the association between different single nucleotide polymorphisms (SNPs) present in the leptin receptor (LEPR) gene and susceptibility to amebic colitis. Children with the ancestral A allele at SNP rs1137101 located at amino acid position 223 encoding glutamine (223Q) had a lower risk of amebiasis than children with a mutation to arginine (223R) (31). The homozygous 223R mutation was also associated with susceptibility to amebic liver abscess in an independent cohort of adults (31). Other studies have shown that the same mutation (223R) is linked to an increased risk of death in patients with nonappendicular secondary peritonitis (42). In order to study the association of the LEPR Q223R polymorphism, DNA was extracted from pathology specimens of 54 patients with a diagnosis of C. difficile colitis and 141 healthy controls at the University of Virginia and genotyped for SNP rs1137101. The genotypic distribution of the polymorphism in the control population was in Hardy-Weinberg equilibrium (P = 0.17); however, there was variability in the minor allele frequency on the basis of self-reported ethnic ancestry (African Americans = 0.55, European Americans = 0.40), as expected (see Table S1 in the supplemental material). Thus, we stratified by ethnicity. European Americans with two copies of the 223R mutation were at an increased risk of C. difficile colitis after adjustment for sex (odds ratio, 3.03; P = 0.015) (Table 1). This association trended in the same direction for African Americans; however, it did not reach statistical significance (odds ratio, 3.47; P = 0.07) (Table 1).
TABLE 1.
The leptin receptor 223R mutation is associated with an increased risk of C. difficile colitisa
| Group with rs1137101 polymorphism | No. (%) of subjects with the following genotype: |
Odds ratio | 95% CI | P value | |
|---|---|---|---|---|---|
| QQ/QR | RR | ||||
| European Americans (n = 141) | |||||
| Controls | 89 (86) | 14 (14) | 1.0 | ||
| C. difficile colitis | 26 (68) | 12 (32) | 3.03 | 1.2, 7.5 | 0.015 |
| African Americans (n = 52) | |||||
| Controls | 28 (76) | 9 (24) | 1.0 | ||
| C. difficile colitis | 8 (53) | 7 (47) | 3.47 | 0.9, 13.4 | 0.07 |
Individuals with C. difficile colitis were 3 times more likely to have two copies of the arginine (R) allele than controls. The logistic regression model was adjusted for sex. CI, confidence interval.
Leptin is protective during acute C. difficile infection.
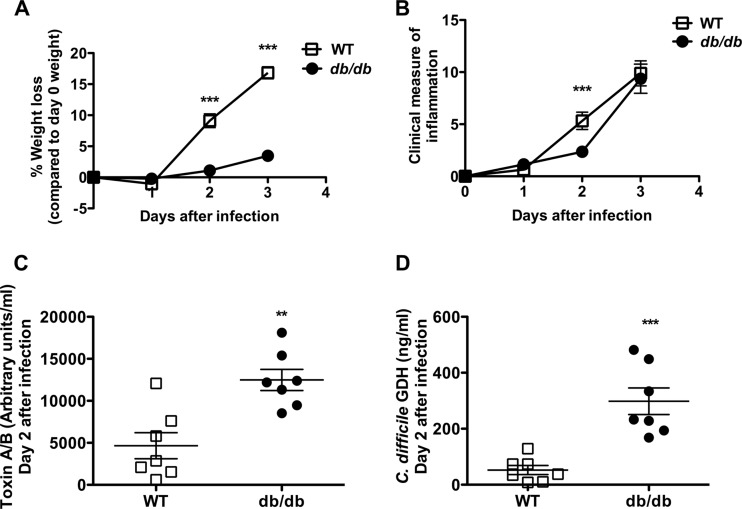
The role of leptin in C. difficile infection was further explored in the mouse model of C. difficile colitis. Leptin receptor-deficient (db/db) and C57BL/6 WT mice were treated with an antibiotic cocktail for 3 days, followed by intraperitoneal injection of clindamycin and challenge with the VPI 10643 strain of C. difficile by orogastric gavage (46). Disease severity was assessed by survival and clinical measures of inflammation (45). WT mice had greater weight loss and earlier onset of clinical signs and symptoms of inflammation than db/db mice but no significant difference in survival from db/db mice (Fig. 1A and B and data not shown). This heightened response to infection was associated with better control of the pathogen burden during acute infection, as measured by the levels of C. difficile toxin A/B and C. difficile-specific glutamate dehydrogenase (GDH) in the cecal contents (Fig. 1C and D).
FIG 1.
Leptin receptor-deficient (db/db) mice have diminished pathogen clearance, weight loss, and clinical scores after C. difficile infection. Age-matched male WT mice and leptin receptor-deficient (db/db) mice were pretreated with antibiotics for 3 days, followed by intraperitoneal treatment with clindamycin, and then infected with 104 CFU C. difficile VPI 10643 by orogastric gavage. (A) Percent weight loss depicted as a percentage of the day 0 weight. Data are from 2 different experiments with a combined 11 to 28 mice. (B) Clinical measure of inflammation assessed by quantifying piloerection, ocular discharge, diarrhea, activity, and posture on a scale of 0 to 4. Data are from 2 different experiments with a combined 11 to 28 mice. (C) Toxin A/B levels determined by ELISA (arbitrary toxin units) for 7 mice. (D) C. difficile-specific GDH levels determined by ELISA (ng/ml) for 7 mice. Two db/db mice had no detectable infection, no detectable toxin or GDH by ELISA, and no histopathologic changes in the colon and were excluded from the analysis. All data shown as the mean ± SEM. **, P < 0.005; ***, P < 0.001 (Student's t test).
Abrogation of LepRb-STAT3 signaling partially recapitulates the phenotype of a complete lack of leptin signaling.
Binding of leptin to its receptor (LepRb) leads to activation of multiple signaling pathways via phosphorylation of different conserved tyrosine residues in the intracellular domain (50, 51). In mouse models of Gram-negative bacterial pneumonia, leptin signaling via the STAT3 pathway (Y1138S-STAT3 mutant mice [S1138 mice]) was protective (32). Studies from our laboratory have also shown that S1138 mice had worse disease in a mouse model of amebic colitis (29). In this study, we were particularly interested to examine the STAT3 pathway because the LEPR 223R polymorphism is associated with lower STAT3 signaling in vitro (52).
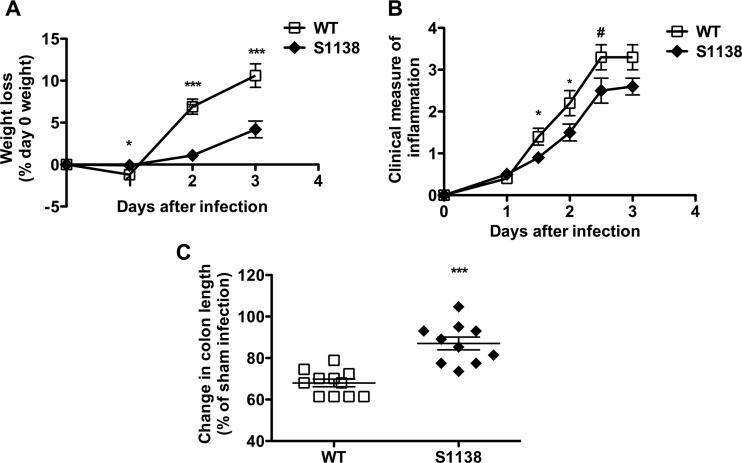
In order to define the contribution of LepRb-STAT3 signaling to leptin-mediated protection from C. difficile infection, S1138 and littermate WT control mice were pretreated with antibiotics as described above and then infected with the VPI 10643 strain of C. difficile by orogastric gavage (46). Weight loss and clinical measures of inflammation were quantified during the course of infection. WT mice had greater weight loss and higher clinical scores than S1138 mice during acute infection (Fig. 2A and B). It should be noted that, perhaps due to differences in the origins of the mice, the WT control mice for db/db mice lost an average of about 16% of their weight by day 3 of infection (Fig. 1A), while the weight loss of the WT littermate controls of S1138 mice was lower (6.8% to 10.6%). The WT control mice (and db/db mice) for all db/db experiments were from The Jackson Laboratory, while the WT littermate controls for S1138 mice were bred at the University of Virginia.
FIG 2.
Decreased inflammation from C. difficile infection in the absence of LepRb-STAT3 signaling. Age-matched WT and leptin receptor STAT3 mutant (S1138) mice were pretreated with antibiotics for 3 days, followed by intraperitoneal treatment with clindamycin, and then infected with 105 CFU C. difficile VPI 10643 by orogastric gavage. (A) Percent weight loss depicted as a percentage of the day 0 weight. Data are from 4 different experiments with a combined 22 to 34 mice. (B) Clinical measure of inflammation assessed by quantifying piloerection, ocular discharge, diarrhea, activity, and posture on a scale of 0 to 4. Data are from 2 different experiments with a combined 8 to 19 mice. (C) Percent colon length shortening (compared to the colon lengths for sham-infected mice) at day 2 after infection for 10 to 11 mice. All data shown as the mean ± SEM. *, P < 0.05; ***, P < 0.001; #, P = 0.06 (Student's t test).
The LepRb-STAT3 signaling axis increases inflammation, colonic chemokine expression, and cellular recruitment.
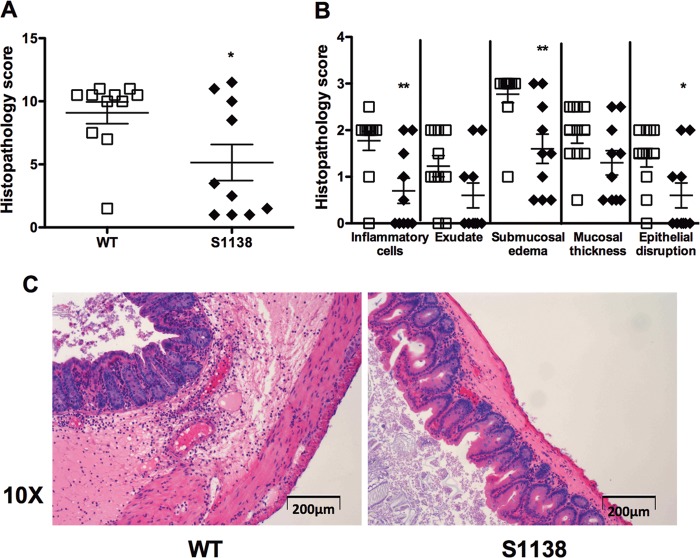
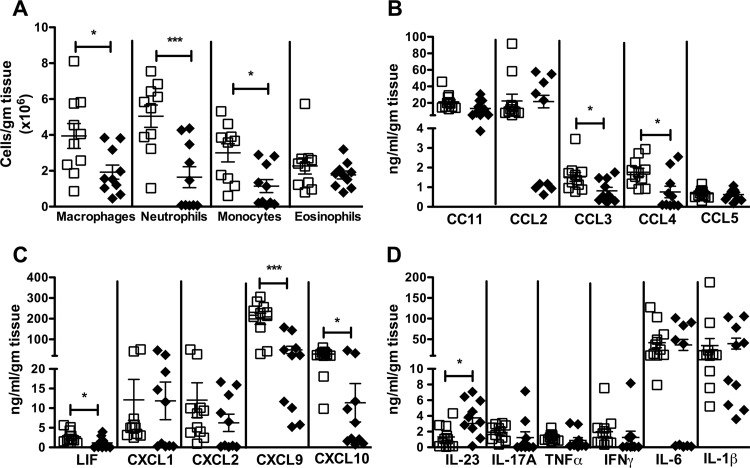
Colon length shortening is a measure of colonic inflammation (53). At baseline, antibiotic-treated, sham-challenged S1138 mice had longer colonic lengths than WT mice of the same age (data not shown). WT mice had higher levels of inflammation, as evidenced by a significant shortening of the colon compared to the length of the colon of S1138 mice on day 2 after infection (Fig. 2C) (data were normalized to those for sham-challenged mice). Microscopically, on day 2, S1138 mice had less inflammatory cell recruitment, submucosal edema, and epithelial cell disruption (Fig. 3A to C). To further define the infiltrating cells recruited to the gut during acute C. difficile infection, the lamina propria cells were harvested and analyzed by flow cytometry. WT mice had an increased number of infiltrating macrophages (CD45+ CD11b+ F4/80+ SiglecF−), neutrophils (CD45+ CD11b+ Ly6Ghi Ly6Cmed), and monocytes (CD45+ CD11b+ Ly6Chi Ly6Gmed) per gram of tissue (Fig. 4A). While leptin has been shown to act as a survival factor for human eosinophils in vitro (54), we did not see any differences in eosinophil (SSChi SiglecF+) numbers between WT and S1138 mice during acute infection (Fig. 4A). Reflective of increased inflammation, WT mice had higher levels of the chemokines leukemia inhibitory factor (LIF), CXCL9, CXCL10, CCL3, and CCL4 (Fig. 4B and C). Of interest because of the pathogenic role of IL-23 in C. difficile (17), the levels of colonic IL-23 were elevated in the absence of LepRb-STAT3 signaling. There was no difference in the levels of the other proinflammatory cytokines, suggesting a potentially protective negative regulation of IL-23 by leptin-STAT3 signaling (Fig. 4D).
FIG 3.
Leptin receptor-STAT3 signaling enhances colonic inflammation during acute C. difficile infection. Age-matched WT mice (open symbols) and leptin receptor STAT3 mutant (S1138) mice (closed symbols) were pretreated with antibiotics for 3 days, followed by intraperitoneal treatment with clindamycin, and then infected with 105 CFU C. difficile VPI 10643 by orogastric gavage. Ceca were harvested 2 days after infection, and inflammation was assessed by scoring for 5 histological criteria, inflammatory cell infiltration, mucosal hypertrophy, luminal exudate and vascular congestion, submucosal edema, and epithelial disruption, in a blinded fashion. (A) Overall histopathology; (B) extent of inflammatory cell recruitment, exudate, submucosal edema, mucosal thickness, and epithelial disruption; (C) representative slide sections for WT and S1138 mice showing inflammatory cell recruitment and submucosal edema. All data are shown as the mean ± SEM for 10 to 11 mice. *, P < 0.05; **, P < 0.005 (Student's t test).
FIG 4.
Leptin receptor-STAT3 signaling enhances inflammatory cell recruitment and colonic chemokine expression during acute C. difficile infection. Age-matched WT mice (open symbols) and leptin receptor STAT3 mutant (S1138) mice (closed symbols) were pretreated with antibiotics for 3 days, followed by intraperitoneal treatment with clindamycin, and then infected with 105 CFU C. difficile VPI 10643 by orogastric gavage. On day 2 after infection, colonic lamina propria cells were harvested and stained with monoclonal antibodies to identify the infiltrating cell populations. Chemokine levels were measured in tissue lysates using a Luminex assay. (A) CD45+ CD11b+ F4/80+ SiglecF− macrophages, CD45+ CD11b+ Ly6Ghi Ly6Cmed neutrophils, CD45+ CD11b+ Ly6Chi Ly6Gmed inflammatory monocytes, and SSChi SiglecF+ eosinophils; (B) CC chemokines; (C) CXC chemokines; (D) proinflammatory cytokines. All data are shown as the mean ± SEM for 10 to 11 mice. *, P < 0.05; ***, P < 0.001 (Student's t test).
DISCUSSION
The most important findings of this study are that a mutation (Q223R) in the leptin receptor present in up to 50% of humans was associated with susceptibility to C. difficile, with mouse studies demonstrating that a leptin-mediated inflammatory response decreased the burden of C. difficile in the gut. Previously, the leptin receptor mutation had been shown to affect susceptibility to amebic colitis and liver abscess and to bacterial peritonitis (31, 42). Here, studies in mice demonstrated that the protective leptin-mediated inflammation was in part via STAT3 signaling from the wild-type leptin receptor. This was of interest because the Q223R leptin receptor mutation is known to diminish leptin-mediated STAT3 signaling (52).
Leptin is produced by adipose tissue (small amounts are produced from placenta and gastric epithelium), while the leptin receptor is expressed not only in the hypothalamus but also on immune and epithelial cells (27). It is thus postulated that leptin acts both as a sensor of metabolism and as a regulator of the inflammatory response on the basis of the nutritional status of the host (39, 55). A lack of leptin signaling or lower leptin levels (as seen in starvation) are associated with an increased risk of infection and decreased immune responses (28, 39). We therefore tested the hypothesis that leptin is protective in C. difficile infection.
The LEPR 223Q allele is protective in amebiasis (31) and is associated with a decreased risk of death in patients with secondary peritonitis (42). This study provides further support for the role of a LEPR polymorphism in controlling susceptibility to infectious colitis due to C. difficile. The LEPR position 223 polymorphism is located in the cytokine receptor homology 1 (CRH1) domain of the leptin receptor. CRH1 is not the ligand-binding domain of the receptor, and at this time its function is unclear. However, we have previously shown in vitro that the LEPR 223Q isoform had higher levels of downstream STAT3 signaling (52). Thus, the role of the LepRb-STAT3 pathway in leptin-mediated inflammation was further investigated using a murine model.
WT mice had an early onset of inflammation and better control of the pathogen burden. The mechanism of leptin-mediated protection was in part by downstream STAT3 signaling. Similar to db/db mice, S1138 mice, which have disrupted leptin-mediated STAT3 signaling, had less weight loss and better clinical scores. Consistent with lower inflammation, S1138 mice also had lower levels of the chemokines LIF, CXCL9, CXCL10, CCL3, and CCL4 and fewer infiltrating neutrophils, macrophages, and monocytes. It is interesting to note that leptin-STAT3 signaling enhances the production of CXCR3 ligands (CXCL9 and CXCL10) and CCR5 ligands (CCL3 and CCL4), which are preferentially expressed on Th1 cells and enhance Th1 inflammation (reviewed in references 56 and 57). This would suggest that leptin-STAT3 signaling enhances Th1 cell recruitment by increasing chemokine secretion. While our current focus has been on the study of polymorphonuclear leukocytes, T cell responses could be playing a role in the leptin-mediated inflammatory response during C. difficile infection, and this can be further examined using our mouse model.
Note that while, on the one hand, leptin-mediated inflammation is manifest as worse disease symptoms (increased weight loss and higher clinical scores in WT mice), on the other hand, this inflammation seems to be beneficial and bactericidal in nature, leading to a better control of the pathogen. One of the possible mechanisms of leptin-mediated bactericidal inflammation could be increased cellular recruitment mediated by the leptin-STAT3 axis; however, other known mechanisms of leptin actions, like increased oxidative species production in neutrophils (36), increased mucin glycoprotein and antimicrobial peptide secretion (41, 58, 59), and maintenance of epithelial integrity (40), could also contribute to leptin-mediated bactericidal inflammation during acute C. difficile infection and will need further investigation. While our study does not directly quantify the numbers of C. difficile CFU, toxin A/B levels are an accurate measure of the critical C. difficile virulence factors and, in combination with C. difficile-specific GDH levels, are a reliable surrogate to monitor bacterial clearance.
Signaling downstream of LepRb leads to activation of multiple signaling pathways, including the JAK2/PI3K-Akt, STAT3/SOCS3, SHP2/mitogen-activated protein kinase (MAPK)-ERK, and STAT5 pathways (51). It is important to note that SOCS3, downstream of STAT3, acts as a negative regulator of the JAK2/PI3K-Akt and SHP2/MAPK-ERK pathways and S1138 mice, which have decreased expression of downstream SOCS3, have enhanced ERK1/2 activation (32, 51). The phenotype of diminished inflammation in S1138 mice could thus be due to a combination of interrupted STAT3-mediated signaling and a lack of SOCS3-mediated negative regulation of other signaling pathways. Studies using inhibitors of the JAK-STAT pathway show that leptin-mediated chemokine production is dependent on activation of the JAK-STAT pathways (60), suggesting that the chemokine expression is likely controlled by STAT3 signaling.
We have recently shown that IL-23 is induced in the colons of humans with C. difficile colitis and causes mortality in the mouse model of C. difficile (17). IL-23 is also implicated in the pathogenesis of inflammatory bowel disease (61). Colonic IL-23 was increased in S1138 mice that lacked the protective function of leptin-mediated STAT3 signaling. Thus, leptin protection may be mediated in part via repression of IL-23. The mechanism of leptin suppression of IL-23 production could be direct, as dendritic cells express leptin receptors and are a major source of IL-23 (62, 63), or indirect via maintenance of intestinal barrier function (diminishing exposure of dendritic cells to C. difficile and/or commensal bacteria).
Leptin can induce chemokine production from both immune (60, 64) and nonimmune cells, including colonic epithelium (65) and endometrial cells (66). The chemokine/cytokine induction and inflammatory cell recruitment seen in our studies thus could be mediated by direct effects of leptin on immune cells or via effects on nonimmune, epithelial, or neuronal cells. Definition of the cellular subsets that lead to leptin-mediated inflammation after C. difficile infection will be enlightening and could be accomplished in future studies by the use of reciprocal bone marrow chimeras between WT and db/db mice and/or S1138 mice, as well as the use of cell type-specific LepRb-deficient mice.
In conclusion, our observations in humans and mice indicate that the LepRb-STAT3 axis has a regulatory role in the inflammatory response to C. difficile infection, with more robust leptin-mediated STAT3 signaling promoting resistance via an enhanced inflammatory response. The study of the role of genetic polymorphisms in defining the susceptibility to infectious diseases in this case not only gives insight into leptin's role in mucosal defense but also provides a potential tool for genetic risk stratification of patients with C. difficile infections as well as a potential avenue for host-targeted therapy.
Supplementary Material
ACKNOWLEDGMENTS
We thank Martin Myers for providing LepRb-STAT3 mutant S1138 mice.
Funding support was from NIH grant R01 AI-26649 to W. A. Petri, Jr.
Footnotes
Published ahead of print 28 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00972-13.
REFERENCES
- 1.Chang TW, Gorbach SL, Bartlett JB. 1978. Neutralization of Clostridium difficile toxin by Clostridium sordellii antitoxins. Infect. Immun. 22:418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. 1978. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology 75:778–782 [PubMed] [Google Scholar]
- 3.Miller BA, Chen LF, Sexton DJ, Anderson DJ. 2011. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect. Control Hosp. Epidemiol. 32:387–390. 10.1086/659156 [DOI] [PubMed] [Google Scholar]
- 4.Lessa FC, Gould CV, McDonald LC. 2012. Current status of Clostridium difficile infection epidemiology. Clin. Infect. Dis. 55(Suppl 2):S65–S70. 10.1093/cid/cis319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, Rene P, Monczak Y, Dascal A. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442–2449. 10.1056/NEJMoa051639 [DOI] [PubMed] [Google Scholar]
- 6.Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Fulop K, Godin D, Bourassa C. 2005. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin. Infect. Dis. 40:1591–1597. 10.1086/430315 [DOI] [PubMed] [Google Scholar]
- 7.Pepin J, Valiquette L, Cossette B. 2005. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 173:1037–1042. 10.1503/cmaj.050978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maroo S, Lamont JT. 2006. Recurrent Clostridium difficile. Gastroenterology 130:1311–1316. 10.1053/j.gastro.2006.02.044 [DOI] [PubMed] [Google Scholar]
- 9.Pepin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. 2007. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am. J. Gastroenterol. 102:2781–2788. 10.1111/j.1572-0241.2007.01539.x [DOI] [PubMed] [Google Scholar]
- 10.Pawar D, Tsay R, Nelson DS, Elumalai MK, Lessa FC, Clifford McDonald L, Dumyati G. 2012. Burden of Clostridium difficile infection in long-term care facilities in Monroe County, New York. Infect. Control Hosp. Epidemiol. 33:1107–1112. 10.1086/668031 [DOI] [PubMed] [Google Scholar]
- 11.Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, Harmsen WS, Zinsmeister AR. 2012. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am. J. Gastroenterol. 107:89–95. 10.1038/ajg.2011.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. 1989. Nosocomial acquisition of Clostridium difficile infection. N. Engl. J. Med. 320:204–210. 10.1056/NEJM198901263200402 [DOI] [PubMed] [Google Scholar]
- 13.Viscidi R, Willey S, Bartlett JG. 1981. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology 81:5–9 [PubMed] [Google Scholar]
- 14.Kelly CP, Becker S, Linevsky JK, Joshi MA, O'Keane JC, Dickey BF, LaMont JT, Pothoulakis C. 1994. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J. Clin. Invest. 93:1257–1265. 10.1172/JCI117080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulusu M, Narayan S, Shetler K, Triadafilopoulos G. 2000. Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea. Am. J. Gastroenterol. 95:3137–3141. 10.1111/j.1572-0241.2000.03284.x [DOI] [PubMed] [Google Scholar]
- 16.Kelly CP, Kyne L. 2011. The host immune response to Clostridium difficile. J. Med. Microbiol. 60:1070–1079. 10.1099/jmm.0.030015-0 [DOI] [PubMed] [Google Scholar]
- 17.Buonomo EL, Madan R, Pramoonjago P, Li L, Okusa MD, Petri WA., Jr 2013. Role of interleukin 23 signaling in Clostridium difficile colitis. J. Infect. Dis. 208:917–920. 10.1093/infdis/jit277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly CP, Pothoulakis C, LaMont JT. 1994. Clostridium difficile colitis. N. Engl. J. Med. 330:257–262. 10.1056/NEJM199401273300406 [DOI] [PubMed] [Google Scholar]
- 19.Fujitani S, George WL, Murthy AR. 2011. Comparison of clinical severity score indices for Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 32:220–228. 10.1086/658336 [DOI] [PubMed] [Google Scholar]
- 20.Qiu B, Pothoulakis C, Castagliuolo I, Nikulasson S, LaMont JT. 1999. Participation of reactive oxygen metabolites in Clostridium difficile toxin A-induced enteritis in rats. Am. J. Physiol. 276:G485–G490 [DOI] [PubMed] [Google Scholar]
- 21.Wershil BK, Castagliuolo I, Pothoulakis C. 1998. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology 114:956–964. 10.1016/S0016-5085(98)70315-4 [DOI] [PubMed] [Google Scholar]
- 22.Castagliuolo I, LaMont JT, Letourneau R, Kelly C, O'Keane JC, Jaffer A, Theoharides TC, Pothoulakis C. 1994. Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology 107:657–665. 10.1016/0016-5085(94)90112-0 [DOI] [PubMed] [Google Scholar]
- 23.Ryan A, Lynch M, Smith SM, Amu S, Nel HJ, McCoy CE, Dowling JK, Draper E, O'Reilly V, McCarthy C, O'Brien J, Ni Eidhin D, O'Connell MJ, Keogh B, Morton CO, Rogers TR, Fallon PG, O'Neill LA, Kelleher D, Loscher CE. 2011. A role for TLR4 in Clostridium difficile infection and the recognition of surface layer proteins. PLoS Pathog. 7:e1002076. 10.1371/journal.ppat.1002076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarchum I, Liu M, Shi C, Equinda M, Pamer EG. 2012. Critical role for MyD88-mediated neutrophil recruitment during Clostridium difficile colitis. Infect. Immun. 80:2989–2996. 10.1128/IAI.00448-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarchum I, Liu M, Lipuma L, Pamer EG. 2011. Toll-like receptor 5 stimulation protects mice from acute Clostridium difficile colitis. Infect. Immun. 79:1498–1503. 10.1128/IAI.01196-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasegawa M, Yamazaki T, Kamada N, Tawaratsumida K, Kim YG, Nunez G, Inohara N. 2011. Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J. Immunol. 186:4872–4880. 10.4049/jimmunol.1003761 [DOI] [PubMed] [Google Scholar]
- 27.Friedman JM, Halaas JL. 1998. Leptin and the regulation of body weight in mammals. Nature 395:763–770. 10.1038/27376 [DOI] [PubMed] [Google Scholar]
- 28.Ozata M, Ozdemir IC, Licinio J. 1999. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J. Clin. Endocrinol. Metab. 84:3686–3695. 10.1210/jc.84.10.3686 [DOI] [PubMed] [Google Scholar]
- 29.Guo X, Roberts MR, Becker SM, Podd B, Zhang Y, Chua SC, Jr, Myers MG, Jr, Duggal P, Houpt ER, Petri WA., Jr 2011. Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol. 4:294–303. 10.1038/mi.2010.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. 2002. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J. Immunol. 168:4018–4024 [DOI] [PubMed] [Google Scholar]
- 31.Duggal P, Guo X, Haque R, Peterson KM, Ricklefs S, Mondal D, Alam F, Noor Z, Verkerke HP, Marie C, Leduc CA, Chua SC, Jr, Myers MG, Jr, Leibel RL, Houpt E, Gilchrist CA, Sher A, Porcella SF, Petri WA., Jr 2011. A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J. Clin. Invest. 121:1191–1198. 10.1172/JCI45294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancuso P, Peters-Golden M, Goel D, Goldberg J, Brock TG, Greenwald-Yarnell M, Myers MG., Jr 2011. Disruption of leptin receptor-STAT3 signaling enhances leukotriene production and pulmonary host defense against pneumococcal pneumonia. J. Immunol. 186:1081–1090. 10.4049/jimmunol.1001470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikejima S, Sasaki S, Sashinami H, Mori F, Ogawa Y, Nakamura T, Abe Y, Wakabayashi K, Suda T, Nakane A. 2005. Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes 54:182–189. 10.2337/diabetes.54.1.182 [DOI] [PubMed] [Google Scholar]
- 34.Sitaraman S, Liu X, Charrier L, Gu LH, Ziegler TR, Gewirtz A, Merlin D. 2004. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J. 18:696–698. 10.1096/fj.03-0422fje [DOI] [PubMed] [Google Scholar]
- 35.Biesiada G, Czepiel J, Ptak-Belowska A, Targosz A, Krzysiek-Maczka G, Strzalka M, Konturek SJ, Brzozowski T, Mach T. 2012. Expression and release of leptin and proinflammatory cytokines in patients with ulcerative colitis and infectious diarrhea. J. Physiol. Pharmacol. 63:471–481 [PubMed] [Google Scholar]
- 36.Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP. 2001. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J. Leukoc. Biol. 69:414–418 [PubMed] [Google Scholar]
- 37.Caldefie-Chezet F, Poulin A, Vasson MP. 2003. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic. Res. 37:809–814. 10.1080/1071576031000097526 [DOI] [PubMed] [Google Scholar]
- 38.Tian Z, Sun R, Wei H, Gao B. 2002. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem. Biophys. Res. Commun. 298:297–302. 10.1016/S0006-291X(02)02462-2 [DOI] [PubMed] [Google Scholar]
- 39.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. 1998. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394:897–901. 10.1038/29795 [DOI] [PubMed] [Google Scholar]
- 40.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G, Martines D. 2007. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 292:G518–G525. 10.1152/ajpgi.00024.2006 [DOI] [PubMed] [Google Scholar]
- 41.El Homsi M, Ducroc R, Claustre J, Jourdan G, Gertler A, Estienne M, Bado A, Scoazec JY, Plaisancie P. 2007. Leptin modulates the expression of secreted and membrane-associated mucins in colonic epithelial cells by targeting PKC, PI3K, and MAPK pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 293:G365–G373. 10.1152/ajpgi.00091.2007 [DOI] [PubMed] [Google Scholar]
- 42.Bracho-Riquelme RL, Loera-Castaneda V, Torres-Valenzuela A, Loera-Castaneda GA, Sanchez-Ramirez JP. 2011. Leptin and leptin receptor polymorphisms are associated with poor outcome (death) in patients with non-appendicular secondary peritonitis. Crit. Care 15:R227. 10.1186/cc10467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mykoniatis A, Anton PM, Wlk M, Wang CC, Ungsunan L, Bluher S, Venihaki M, Simeonidis S, Zacks J, Zhao D, Sougioultzis S, Karalis K, Mantzoros C, Pothoulakis C. 2003. Leptin mediates Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology 124:683–691. 10.1053/gast.2003.50101 [DOI] [PubMed] [Google Scholar]
- 44.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr 2003. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421:856–859. 10.1038/nature01388 [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Figler RA, Kolling G, Bracken TC, Rieger J, Stevenson RW, Linden J, Guerrant RL, Warren CA. 2012. Adenosine A2A receptor activation reduces recurrence and mortality from Clostridium difficile infection in mice following vancomycin treatment. BMC Infect. Dis. 12:342. 10.1186/1471-2334-12-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992. 10.1053/j.gastro.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 47.Warren CA, van Opstal E, Ballard TE, Kennedy A, Wang X, Riggins M, Olekhnovich I, Warthan M, Kolling GL, Guerrant RL, Macdonald TL, Hoffman PS. 2012. Amixicile, a novel inhibitor of pyruvate:ferredoxin oxidoreductase, shows efficacy against Clostridium difficile in a mouse infection model. Antimicrob. Agents Chemother. 56:4103–4111. 10.1128/AAC.00360-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawlowski SW, Calabrese G, Kolling GL, Platts-Mills J, Freire R, AlcantaraWarren C, Liu B, Sartor RB, Guerrant RL. 2010. Murine model of Clostridium difficile infection with aged gnotobiotic C57BL/6 mice and a BI/NAP1 strain. J. Infect. Dis. 202:1708–1712. 10.1086/657086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. 2012. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 209:139–155. 10.1084/jem.20101387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myers MG, Cowley MA, Munzberg H. 2008. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 70:537–556. 10.1146/annurev.physiol.70.113006.100707 [DOI] [PubMed] [Google Scholar]
- 51.Banks AS, Davis SM, Bates SH, Myers MG., Jr 2000. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275:14563–14572. 10.1074/jbc.275.19.14563 [DOI] [PubMed] [Google Scholar]
- 52.Marie CS, Verkerke HP, Paul SN, Mackey AJ, Petri WA., Jr 2012. Leptin protects host cells from Entamoeba histolytica cytotoxicity by a STAT3-dependent mechanism. Infect. Immun. 80:1934–1943. 10.1128/IAI.06140-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. 1990. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98:694–702 [DOI] [PubMed] [Google Scholar]
- 54.Conus S, Bruno A, Simon HU. 2005. Leptin is an eosinophil survival factor. J. Allergy Clin. Immunol. 116:1228–1234. 10.1016/j.jaci.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 55.Matarese G, Procaccini C, De Rosa V. 2012. At the crossroad of T cells, adipose tissue, and diabetes. Immunol. Rev. 249:116–134. 10.1111/j.1600-065X.2012.01154.x [DOI] [PubMed] [Google Scholar]
- 56.Moser B, Loetscher P. 2001. Lymphocyte traffic control by chemokines. Nat. Immunol. 2:123–128. 10.1038/84219 [DOI] [PubMed] [Google Scholar]
- 57.Groom JR, Luster AD. 2011. CXCR3 in T cell function. Exp. Cell Res. 315:620–631. 10.1016/j.yexcr.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plaisancie P, Ducroc R, El Homsi M, Tsocas A, Guilmeau S, Zoghbi S, Thibaudeau O, Bado A. 2006. Luminal leptin activates mucin-secreting goblet cells in the large bowel. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G805–G812. 10.1152/ajpgi.00433.2005 [DOI] [PubMed] [Google Scholar]
- 59.Kanda N, Watanabe S. 2008. Leptin enhances human β-defensin-2 production in human keratinocytes. Endocrinology 149:5189–5198. 10.1210/en.2008-0343 [DOI] [PubMed] [Google Scholar]
- 60.Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S. 2009. Leptin enhances CC-chemokine ligand expression in cultured murine macrophage. Biochem. Biophys. Res. Commun. 384:311–315. 10.1016/j.bbrc.2009.04.121 [DOI] [PubMed] [Google Scholar]
- 61.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. 2006. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116:1310–1316. 10.1172/JCI21404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. 2005. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J. Immunol. 174:6820–6828 [DOI] [PubMed] [Google Scholar]
- 63.Iwakura Y, Ishigame H. 2006. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 116:1218–1222. 10.1172/JCI28508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meier CA, Chicheportiche R, Dreyer M, Dayer JM. 2003. IP-10, but not RANTES, is upregulated by leptin in monocytic cells. Cytokine 21:43–47. 10.1016/S1043-4666(02)00491-X [DOI] [PubMed] [Google Scholar]
- 65.Abolhassani M, Aloulou N, Chaumette MT, Aparicio T, Martin-Garcia N, Mansour H, Le Gouvello S, Delchier JC, Sobhani I. 2008. Leptin receptor-related immune response in colorectal tumors: the role of colonocytes and interleukin-8. Cancer Res. 68:9423–9432. 10.1158/0008-5472.CAN-08-1017 [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez RR, Rueda BR, Ramos MP, Littell RD, Glasser S, Leavis PC. 2004. Leptin-induced increase in leukemia inhibitory factor and its receptor by human endometrium is partially mediated by interleukin 1 receptor signaling. Endocrinology 145:3850–3857. 10.1210/en.2004-0383 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.