Abstract
As an obligate pathogen, the Lyme disease spirochete Borrelia burgdorferi has a streamlined genome that encodes only two two-component signal transduction systems, Hk1-Rrp1 and Hk2-Rrp2 (in addition to CheA-CheY systems). The output of Hk1-Rrp1 is the production of the second messenger cyclic di-GMP (c-di-GMP), which is indispensable for B. burgdorferi to survive in the tick vector. The output of Hk2-Rrp2 is the transcriptional activation of the global regulator RpoS, which is essential for the pathogen to accomplish its tick-mouse transmission and to establish mammalian infection. Although evidence indicates that these two systems communicate with each other, how they are connected is not fully understood. In this study, we showed that the c-di-GMP-binding protein PlzA, a downstream effector of Rrp1, positively modulates the production of RpoS, a global regulator and downstream target of Rrp2. Thus, PlzA functions as a connector that links Hk1-Rrp1 with Hk2-Rrp2. We further showed that PlzA regulates rpoS expression through modulation of another regulator, BosR, at both the transcriptional and the posttranscriptional levels. In addition, PlzA was also capable of regulating rpoS expression independently of Rrp1, suggesting that besides being a c-di-GMP-binding protein, PlzA has other functions. Along with the previous finding of PlzA controlling motility, these studies demonstrate that PlzA is a multifunctional protein. These findings further reinforce the notion that B. burgdorferi utilizes its limited signaling systems and regulators to govern multiple cellular processes during its complex enzootic cycle between ticks and mammals.
INTRODUCTION
Borrelia burgdorferi, the Lyme disease spirochetal pathogen, is maintained in an enzootic cycle involving two markedly different hosts, an arthropod vector and a mammalian host (1–3). As an obligate pathogen, B. burgdorferi has a dramatically reduced genome. Remarkably, B. burgdorferi has evolved by utilizing its limited genomic capabilities to adapt to and survive in these two distinct host environments during its natural cycle (4, 5). In this regard, the B. burgdorferi genome only has two two-component signal transduction systems, Hk1-Rrp1 and Hk2-Rrp2 (in addition to the chemotactic CheA-CheY systems), and two alternative sigma factors, RpoS (σS) and RpoN (σ54).
In the past 10 years, we and others have shown that response regulator Rrp2 activates transcription of rpoS from its RpoN (σ54)-type promoter. The stationary-phase sigma factor RpoS (σS) functions as a global regulator, controlling expression of many virulence genes, such as ospC, dbpB/A, bbk32, etc., prior to spirochetal transmission from ticks to mammals (6–10). This unique RpoN-RpoS (σ54-σS) sigma cascade is essential for B. burgdorferi transmission from ticks to mammals and for the establishment of infection in the mammalian host. Recently, a Fur/PerR-like transcription factor, BosR, was also shown to be essential for rpoS expression (11–16). Although how BosR fits into Rrp2- and RpoN-dependent activation of rpoS remains unclear, in vitro data showed that BosR can directly bind to the promoter region of rpoS (13, 17). In addition, a small RNA-binding protein (DsrA), a ROC-type repressor (BadR), and a plasmid-coded protein (BBI16) have all been shown to be involved in regulation of RpoS level in B. burgdorferi (18, 19).
The second response regulator of B. burgdorferi, Rrp1, is the sole diguanylate cyclase that produces the secondary messenger cyclic di-GMP (c-di-GMP) (20). Degradation of c-di-GMP is carried out by two phosphodiesterases, the EAL domain protein BB0363/PdeA and the HD-GYP domain protein BB0374/PdeB, in B. burgdorferi (21, 22). Recently, three independent groups reported construction of infectious hk1 or rrp1 mutants and demonstrated that while Hk1-Rrp1 is not required for mammalian infection, it is essential for spirochetal survival in the tick vector (23–25). It appears that the defect of the rrp1 mutant in ticks is, in part, due to a defect in motility and its inability to utilize glycerol, chitobiose, and N-acetylglucosamine (23–27). Glycerol is produced by some insects as well as arthropods as a cryoprotective molecule (28), whereas chitobiose (derived from chitin), a major component of the tick cuticle, is an important source of N-acetylglucosamine for cell wall synthesis of B. burgdorferi (29–31). It was found that expression of the glycerol uptake/metabolism operon glpFKD, a chitobiose transporter gene (chbC) (30, 31), and several glucosamine metabolism genes is governed by Rrp1 (23, 25, 32). Furthermore, constitutive expression of glpFKD in the rrp1 mutant or supplementing N-acetylglucosamine in tick midguts partially rescued the rrp1 mutant's survival in ticks or its transmission to the mammalian host (23, 32).
Initial observation of the interplay between Hk1-Rrp1 and Hk2-Rrp2 was reported by Rogers et al., showing that deletion of rrp1 resulted in reduced rpoS and ospC expression (26). More recently, Sze et al. further showed that Rrp1 influences rpoS by modulating bosR transcription, which in turn modulates chbC expression (32). We also reported a nearly 3-fold reduction of ospC expression in the rrp1 mutant by microarray analysis (23). However, how Rrp1 modulates the production of BosR and RpoS remains unclear.
PlzA belongs to a group of c-di-GMP receptors that contain a conserved PilZ domain (33, 34). Unlike most bacteria, which harbor multiple PilZ domain-containing proteins, B. burgdorferi strain B31 has only a single copy of one PilZ domain-containing protein, PlzA (35, 36). Some B. burgdorferi strains have a second copy on the plasmid (36). The B. burgdorferi plzA mutant has a defect in motility with reduced infectivity in mice (35). In ticks, expression of plzA is induced during feeding (35, 36). The plzA mutant showed decreased but not abolished survival, unlike the hk1 and rrp1 mutants (23–25, 35). In this study, we present the first account of a multifunctional PilZ domain c-di-GMP receptor. We provide evidence that Rrp1 influences BosR and RpoS through PlzA. In addition, we show that PlzA is also capable of regulating rpoS expression independently of Rrp1.
MATERIALS AND METHODS
Bacterial strains.
The low-passage, virulent B. burgdorferi strain 5A4NP1 (a gift from H. Kawabata and S. Norris, University of Texas Health Science Center at Houston) was derived from wild-type strain B31 by inserting a kanamycin resistance marker into the restriction modification gene bbe02 on plasmid lp25 (37). B. burgdorferi was cultivated in Barbour-Stoenner-Kelly (BSK-II) medium supplemented with 6% normal rabbit serum (Pel Freez Biologicals, Rogers, AR) at 35°C with 5% CO2. Relevant antibiotics were added to the cultures at the following final concentrations: 300 μg/ml for kanamycin, 100 μg/ml for streptomycin, and 50 μg/ml for gentamicin. The constructed suicide vectors were maintained in Escherichia coli strain TOP10.
Inactivation of plzA.
To construct a suicide vector for generating the plzA mutant by homologous recombination, the 1.2-kb upstream region and the 1.0-kb downstream region of plzA were PCR amplified from strain B31 genomic DNA with primer pairs bb0733-AF/bb0733-AR and bb0733-BF/bb0733-BR (Table 1), respectively. The resulting DNA fragments were cloned upstream and downstream of a streptomycin-resistant cassette (aadA), respectively, in the pCR-XL-TOPO cloning vector (Invitrogen). The resulting suicide vector was confirmed by sequencing and was designated pMH23. pMH23 plasmid DNA was then transformed into B. burgdorferi strain 5A4NP1 using a previously described protocol (38, 39). Kanamycin- and streptomycin-resistant transformants were analyzed by reverse transcription-PCR (RT-PCR) and quantitative RT-PCR to confirm the loss of plzA. Plasmid profiles of the plzA mutant clones were determined by performing PCR with 21 pairs of primers specific for the endogenous plasmids (40, 41).
TABLE 1.
Primers used in this study
| Primer name | Sequence (5′–3′) | Purpose |
|---|---|---|
| bb0733-AF | CAGGTACCAATTCCAAGTATAGCTCCAAAACTTG | Construct pMH23 |
| bb0733-AR | CAGGATCCGAATCCATAGAAATAGAATAAATTACTC C | Construct pMH23 |
| bb0733-BF | CACTGCAGATTTTTAGTTTTGAATTTATAGATGGAG | Construct pMH23 |
| bb0733-BR | CACTCGAGCTCTTAAGATGGCATCAATTAAATTTC | Construct pMH23 |
| KPNflgBp-5 | CAGGTACCATGTTTAAGGTTTATGATTTAG | Construct pMH105 |
| flgBp-733-3 | CCATAATCTCTTATTTTTCTAGATAAAAGCATATGGAAACCTCCCTCATTTAAAATTGC | Construct pMH105 |
| flgBp-733-5 | GCAATTTTAAATGAGGGAGGTTTCCATATGCTTTTATCTAGAAAAATAAGAGATTATGG | Construct pMH105 |
| Pst-Flag-733-3 | CACTGCAGTTACTTGTCGTCATCGTCTTTGTAGTCATTGAAATAATCATGGATCAAC | Construct pMH105 |
| qBB0680F | TTGAGCAAATAGCCTCAGGT | qRT-PCR |
| qBB0680R | TCCACAATGTCTTGCATAGC | qRT-PCR |
| qBB0566F | GCCTGAAGGAGAGCTTGTAA | qRT-PCR |
| qBB0566R | ATCCCCTTCTTTCATTTTCTT | qRT-PCR |
| qBBA73F | AAACAACACAGAAGCGATAA | qRT-PCR |
| qBBA73R | AGATTTTGGGTGCTTACAAT | qRT-PCR |
| qBBA24F | GATACCCCACTACCCGTTTT | qRT-PCR |
| qBBA24R | CATGTGGACTAACAGGAGCA | qRT-PCR |
| qBBA07F | ACGAAGCAGATGCATCATAA | qRT-PCR |
| qBBA07R | AATGTTGCCAATGCTAAACA | qRT-PCR |
| qBB0771F | TAACCCTTTACCCGCATATC | qRT-PCR |
| qBB0771R | TACAAAGAGGCAATGCAAAA | qRT-PCR |
| qBB0733F | ATGCTTTTATCTAGAAAAATAAGAGATTATGG | qRT-PCR |
| qBB0733R | ATTGAAATAATCATGGATCAACATAG | qRT-PCR |
| qBB0844F | CATGCACCTCTGCTTGTATT | qRT-PCR and RT-PCR |
| qBB0844R | ATTAGCGATGGGAGTCTTGA | qRT-PCR and RT-PCR |
| qPCR-flaB-R | CAGCAATAGCTTCATCTTGGTTTG | qRT-PCR and RT-PCR |
| qPCR-flaB-F | ACCAGCATCACTTTCAGGGTCTCA | qRT-PCR and RT-PCR |
| qBB0733F | ATGCTTTTATCTAGAAAAATAAGAGATTATGG | qRT-PCR |
| qBB0733R | ATTGAAATAATCATGGATCAACATAG | qRT-PCR |
| qBBB19F | CGGATTCTAATGCGGTTTTACTTG | qRT-PCR |
| qBBB19R | CAATAGCTTTAGCAGCAATTTCATCT | qRT-PCR |
Construction of a shuttle vector for constitutive expression of plzA.
The shuttle vector pMH105 was constructed by inserting a flgB promoter driving a plzA gene fused with a FLAG tag at the C terminus of PlzA into plasmid pTM61 (kindly provided by George Chaconas, University of Calgary, Canada) (42). This shuttle vector, pMH105, was then transformed into the plzA mutant, wild-type strain 5A4NP1, or the rrp1 mutant (15). The transformants with proper antibiotic resistance were selected and subjected to reverse transcription-PCR (RT-PCR) or quantitative RT-PCR (qRT-PCR) analyses to confirm the plzA expression in these clones.
qRT-PCR.
RNA samples were extracted from either B. burgdorferi cultures or ticks using an RNeasy minikit (Qiagen, Valencia, CA) according to the manufacturer's protocols. For RNA analysis of in vitro-cultivated spirochetes, three independent culture samples were used for each strain. Contaminating DNA in RNA samples was digested using RNase-free DNase I (Promega, Madison, WI), and the absence of DNA was confirmed by PCR amplification using primers specific for the B. burgdorferi flaB gene. For cDNA synthesis, SuperScript III reverse transcriptase and random primers (Invitrogen, Carlsbad, CA) were used. Absolute quantitative PCR was performed in triplicate using the ABI 7000 sequence detection system and SYBR green PCR master mixture (ABI, Pleasanton, CA). A cloning vector containing a related DNA sequence was used as the standard template. A series of 10-fold dilutions of the standard template (concentrations, 100 to 107 copies per μl) was prepared and subjected to qPCR analysis. A standard curve was then generated by plotting the number of copies of the standard template against the cycle threshold (CT) values. The numbers of copies of a specific gene were then extrapolated from the standard curve based on the CT values obtained for each reaction.
SDS-PAGE and immunoblotting.
SDS-PAGE and immunoblotting were performed as previously described (43), with the exception that the images were developed using the chemiluminescent method (Pierce ECL Western blotting substrate; Thermo Scientific, IL). Monoclonal antibodies directed against OspC, RpoS, Rrp1, FlaB, or BosR have been described previously (23, 43–45).
Tick-mouse cycle of B. burgdorferi.
All animal experiments and tick protocols described below were approved by the Institutional Animal Care and Use Committee at Indiana University. Pathogen-free Ixodes scapularis larvae were purchased from the Tick-Rearing Center at Oklahoma State University, Stillwater, OK. The tick-mouse experiments were conducted in the Vector-borne Diseases Laboratory at Indiana University School of Medicine, Indianapolis, IN. Pairs of C3H/HeN SCID mice were first needle infected with B. burgdorferi (105 spirochetes per mouse). Two weeks postinoculation, mouse infection was confirmed by cultivation of ear punch biopsy specimens for spirochete growth. Unfed larvae were then placed on the infected mice, and fully engorged larvae were subjected to qPCR analysis to determine the B. burgdorferi loads.
Statistical analyses.
To determine the statistical significance of differences observed in qRT-PCR and qPCR, values were compared using an unpaired t test. The P values are given in each figure.
RESULTS
Construction of the plzA mutant and the complemented strains.
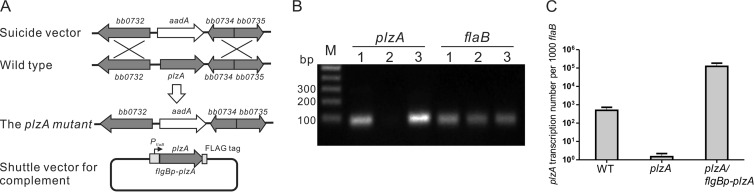
To study the role of plzA in B. burgdorferi, we generated a plzA mutant of the infectious B. burgdorferi strain B31-5A4NP1 (37) by allele exchange as described previously (Fig. 1A) (39). A mutant containing all the same endogenous plasmids as the parental wild-type strain was chosen for further study. To complement the plzA mutant, a pBSV2G-based shuttle vector carrying a constitutively expressed FLAG-tagged PlzA was then transformed into the plzA mutant (Fig. 1A). The plzA mutation and complementation were confirmed by RT-PCR and qRT-PCR (Fig. 1B and C).
FIG 1.
Construction of the plzA mutant and complemented strains. (A) Schematic representation of the genes bb0732 to bb0735 in the Borrelia chromosome and replacement of plzA (bb0733) with an aadA gene cassette (conferring streptomycin resistance) by homologous recombination. A shuttle vector carrying a copy of a constitutive flgB promoter-driven plzA gene encoding a FLAG-tagged PlzA was used for complementation of the plzA mutant (plzA/flgBp-plzA). (B) Confirmation of the plzA mutation and complementation by RT-PCR. flaB serves as a positive control. Lane 1, wild-type B. burgdorferi strain B31-5A4NP1; lane 2, the plzA mutant; lane 3, the plzA mutant carrying flgBp-plzA. (C) qRT-PCR analysis of plzA levels in the plzA mutant and the plzA mutant carrying flgBp-plzA.
The plzA mutant is defective in RpoS and OspC production.
To determine the impact of the plzA deletion on the protein profile of B. burgdorferi, whole-cell lysates harvested from stationary growth phase of the wild-type strain, the plzA mutant, and the complemented clone were subjected to SDS-PAGE analyses (Fig. 2, top). The result showed that production of OspC, the major RpoS-dependent surface lipoprotein and virulence factor (46–51), was dramatically reduced in the plzA mutant in comparison to the wild-type and complemented strains (Fig. 2, top). The defect in OspC and RpoS production was further confirmed by immunoblotting analysis (Fig. 2, bottom). These results were also confirmed using another plzA mutant previously reported by Pitzer et al. (35) (data not shown). Thus, PlzA appears to modulate production of the major virulence factor OspC through RpoS.
FIG 2.
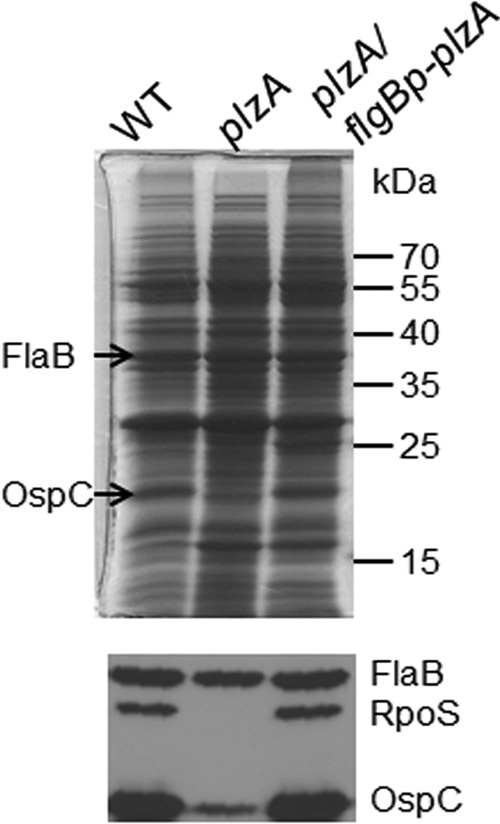
PlzA controls production of RpoS and OspC. Wild-type B. burgdorferi B31-5A4NP1 (WT), the plzA mutant (plzA), and the plzA mutant carrying a constitutive copy of plzA (plzA/flgBp-plzA) were harvested at late logarithmic phase, and the whole-cell lysates were subjected to SDS-PAGE (Coomassie blue stain) (top) and immunoblotting (bottom, using the chemiluminescent method). For immunoblotting, a mix of monoclonal antibodies against FlaB, RpoS, and OspC were used as primary antibodies, and the image was developed by chemiluminescence. FlaB served as a loading control.
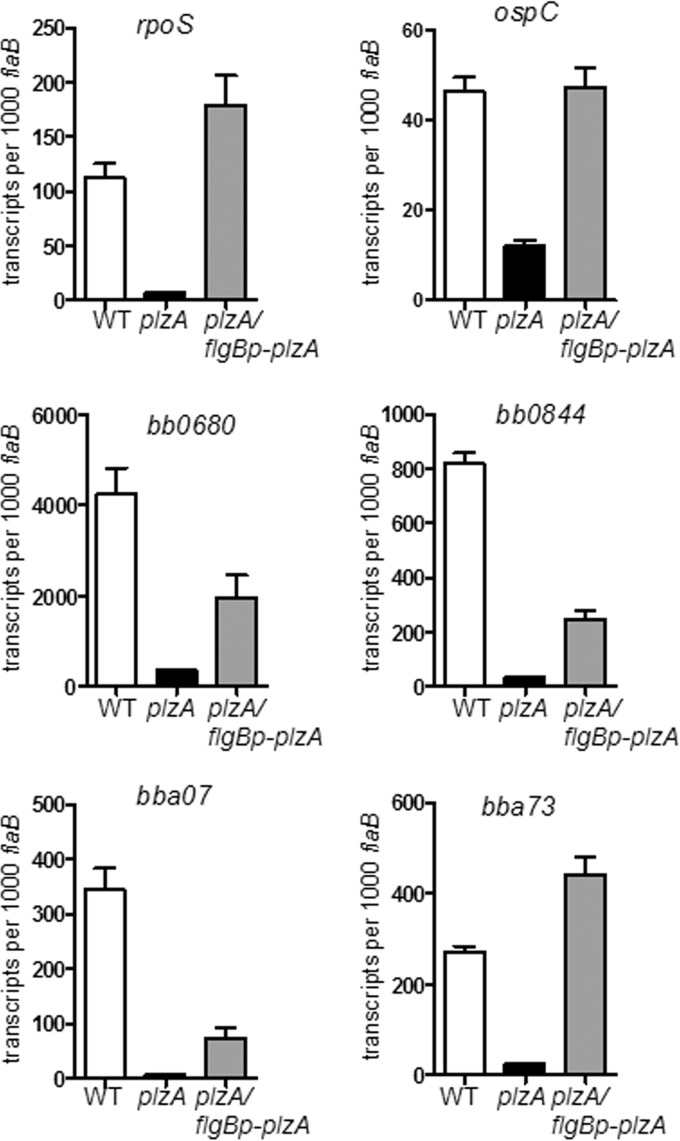
RpoS is known to be regulated at various levels in other bacteria, including transcription, translation, proteolysis, and protein activity (52). First, we tested mRNA levels of rpoS. qRT-PCR analyses demonstrated that PlzA influences rpoS expression at the mRNA level (Fig. 3). As expected, the transcript level of ospC, as well as the transcript levels of several other RpoS-dependent genes, including bb0680, bb0844, bba07, and bba73, were greatly reduced in the plzA mutant in comparison to the wild-type and the complemented strains (7, 45, 53, 54) (Fig. 3). Taken together, these results indicate that PlzA positively regulates the activation of the RpoS regulon in B. burgdorferi.
FIG 3.
PlzA controls expression of rpoS and RpoS-dependent genes. B. burgdorferi strains were harvested at late logarithmic phase and subjected to RNA extraction and qRT-PCR analyses for expression of the RpoS-dependent genes indicated at the tops of the graphs. qRT-PCR data were from three independent samples.
Inactivation of rrp1 reduces the production of RpoS and OspC, which can be overcome by overexpression of plzA.
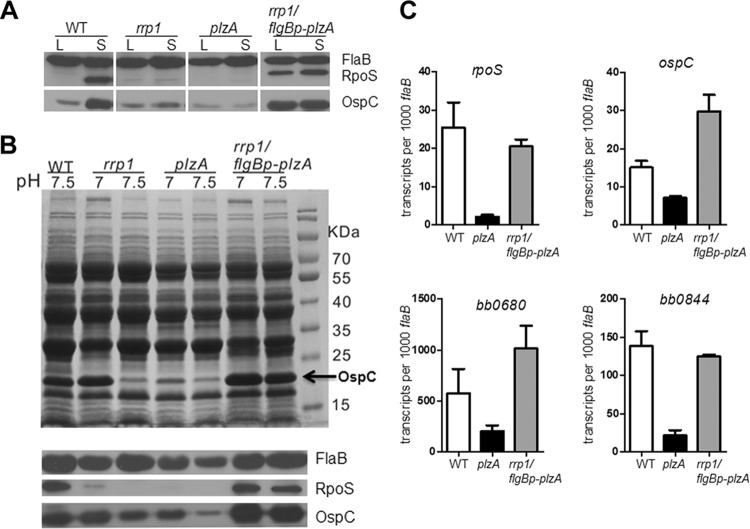
Previous reports indicated that Rrp1 influences rpoS expression (26, 32). Given that PlzA is a c-di-GMP-binding protein, we further investigated whether the effect of Rrp1 on rpoS is mediated by PlzA. We first confirmed the effect of Rrp1 on rpoS and ospC expression using our previously reported rrp1 mutant (23). Deletion of rrp1 reduced OspC and RpoS protein levels (Fig. 4A). Interestingly, lowering the culture pH (pH 7.0) could partially overcome the defect of rrp1 (Fig. 4B). Deletion of plzA also resulted in impaired RpoS and OspC levels (Fig. 4A). plzA appeared to have a greater impact on OspC levels than rrp1 at both pH 7.5 and pH 7.0 (Fig. 4B).
FIG 4.
The defect of the rrp1 mutant in rpoS expression can be overcome by overexpression of plzA or by low pH. (A) Wild-type B. burgdorferi B31-5A4NP1 (WT), the rrp1 mutant (rrp1), the plzA mutant (plzA), and the rrp1 mutant carrying a constitutive copy of plzA (rrp1/flgBp-plzA) were harvested at late logarithmic (L) or stationary (S) phase, and the whole-cell lysates were subjected to immunoblot analyses by chemiluminescence using a mix of monoclonal antibodies against RpoS and FlaB or antibody against OspC. FlaB serves as a loading control. (B) Cells were grown in BSK-II at pH 7.5 or 7.0 and harvested at stationary phase. Whole-cell lysates were subjected to SDS-PAGE (top) or immunoblotting (bottom) by chemiluminescence using monoclonal antibodies against RpoS, OspC, or FlaB. (C) qRT-PCR analysis of rpoS and RpoS-regulated genes in B. burgdorferi strains grown in standard BSK-II medium and harvested at late logarithmic phase.
The above observation suggests that the defects in the plzA and rrp1 mutants are similar but not identical. To further examine the relationship of PlzA and Rrp1 on influencing rpoS expression, we overexpressed PlzA in the rrp1 mutant. The rationale is that if the function of PlzA is solely dependent on c-di-GMP, then overproduction of PlzA in the rrp1 mutant should not rescue rpoS and ospC expression, since Rrp1 is the sole diguanylate cyclase in B. burgdorferi. Surprisingly, the result showed that overexpression of plzA could fully restore expression of rpoS and rpoS-controlled genes in the rrp1 mutant (Fig. 4). This result suggests that PlzA can influence rpoS expression independently of Rrp1.
PlzA controls RpoS through BosR.
Sze et al. reported that Rrp1 influences rpoS expression through BosR, a Fur/PerR homolog transcription regulator that positively regulates rpoS expression (32). Therefore, we examined whether PlzA also affects rpoS expression through BosR. As shown in Fig. 5A, deletion of rrp1 greatly reduced the BosR level. However, PlzA had a more dramatic effect than Rrp1: BosR was virtually undetectable in the plzA mutant. Furthermore, overexpression of plzA was able to restore BosR production in the rrp1 mutant (Fig. 5A).
FIG 5.
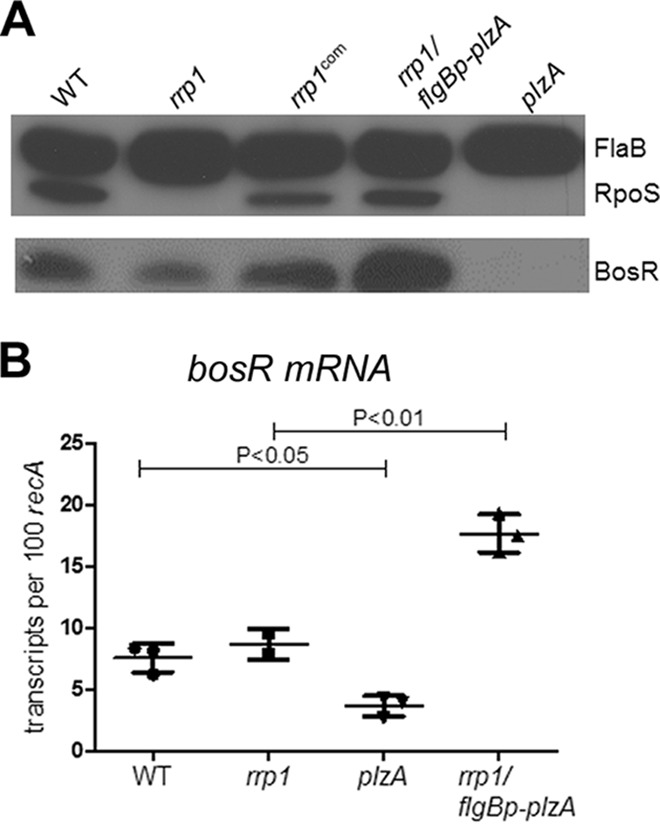
Transcriptional and posttranscriptional control of bosR expression by PlzA. (A) Wild-type B. burgdorferi B31-5A4NP1 (WT), the rrp1 mutant (rrp1), the rrp1 mutant with a wild-type copy of rrp1 (rrp1com), the rrp1 mutant carrying a constitutive copy of plzA (rrp1/flgBp-plzA), and the plzA mutant (plzA) were harvested at stationary phase, and the whole-cell lysates were subjected to immunoblot analyses using the chemiluminescent method. (B) RNAs were extracted from the cells described above and subjected to qRT-PCR analysis for bosR transcript levels.
It was previously reported that bosR expression is often regulated at the protein level, rather than the transcript level, by environmental factors such as CO2 and Mn2+ concentrations (43, 55). Thus, levels of bosR mRNA were determined in the plzA mutant as well as the rrp1 mutant. As shown in Fig. 5B, despite the obvious difference in BosR protein levels between wild-type and the rrp1 mutant, there was no significant difference in bosR mRNA levels between the two strains. This result indicates that Rrp1 influences bosR at a posttranscriptional level. On the other hand, the effect of PlzA on bosR expression appeared to differ from that of Rrp1. Although there was about a 2- to 3-fold reduction of bosR mRNA level in the plzA mutant compared to wild-type B. burgdorferi, this moderate reduction of bosR mRNA could not fully account for the lack of detectable BosR in the plzA mutant (Fig. 5B). These data suggest that PlzA influenced bosR both transcriptionally and posttranscriptionally. Overexpression of plzA in the rrp1 mutant resulted in 3-fold-higher levels of bosR mRNA than in the wild-type strain, leading to restoration of the RpoS level in the rrp1 mutant (Fig. 5A and B).
Overexpression of PlzA partially rescues the rrp1 mutant's defect in ticks.
The rrp1 mutant failed to survive in ticks, at least in part due to the inability to utilize glycerol and chitobiose (23–25, 32). Since Rrp1 influences the chitobiose transporter ChbC via RpoS (32), we sought to determine whether overexpression of plzA would affect the rrp1 phenotype in ticks. The rationale was that although overexpression of plzA in the rrp1 mutant background could not restore the function of PlzA that is dependent on c-di-GMP, it was able to rescue RpoS production in the rrp1 mutant. Pathogen-free larval ticks fed on mice that were infected with either wild-type B. burgdorferi, the rrp1 mutant, the wild-type strain overexpressing plzA, or the rrp1 mutant overexpressing plzA. After ticks were replete (4 to 5 days of feeding), engorged larvae were collected for qPCR analyses to determine B. burgdorferi burdens. As shown in Fig. 6, both the rrp1 mutant and the rrp1 mutant overexpressing plzA had dramatically lower spirochete loads than the wild-type strain. However, overexpression of plzA in the rrp1 mutant resulted in 5-fold-higher spirochetal loads than in the rrp1 mutant, suggesting that overexpression of plzA can partially rescue the survival defect of the rrp1 mutant in ticks.
FIG 6.
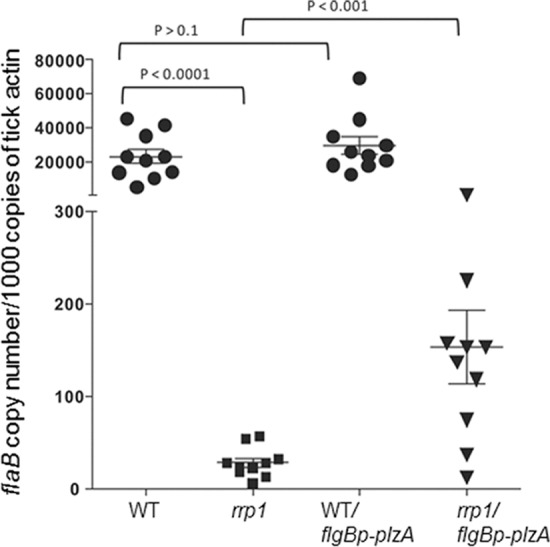
Overexpression of PlzA partially rescues the rrp1 mutant's defect in ticks. Pathogen-free unfed I. scapularis larvae fed on mice infected with various B. burgdorferi strains, and fed larvae were subjected to DNA extraction and qPCR analyses for spirochete burden. Each dot represents the B. burgdorferi flaB copy number in one fed larval tick (a total of 10 ticks examined for each group). The horizontal bar represents the mean value for each group.
DISCUSSION
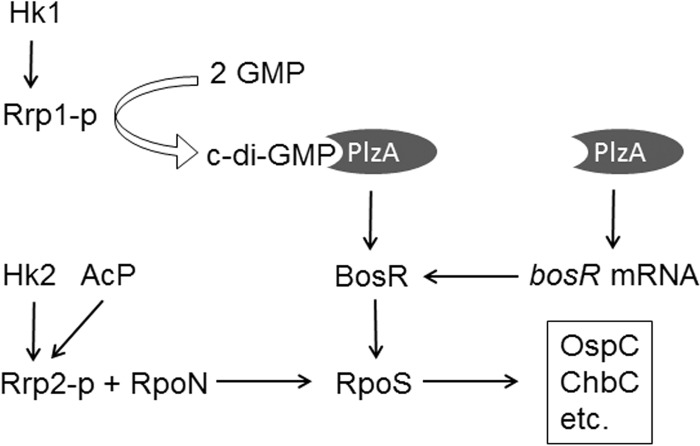
Accumulated evidence indicates that the two two-component systems in B. burgdorferi, Hk1-Rrp1 and Hk2-Rrp2, control spirochetal adaptation to each of its two hosts. While Hk2-Rrp2 is essential for mammalian infection (6, 10, 47), Hk1-Rrp1 is required for tick colonization (23–25). In the feeding gut, when spirochetes migrate from ticks to mammals, both Hk1-Rrp1 and Hk2-Rrp2 are activated (6, 24, 56). It is therefore not surprising that there is cross talk between these two systems. In this study, we provide evidence that the sole known c-di-GMP receptor protein PlzA links the two systems together and helps B. burgdorferi coordinate responses to multiple signals to modulate virulence gene expression (Fig. 7).
FIG 7.
Working model of PlzA connecting the two signaling systems in B. burgdorferi, Hk1-Rrp1 and Hk2-Rrp2. The product of the Hk1-Rrp1 signaling system, c-di-GMP, binds to the effector PlzA and positively regulates the BosR protein level. PlzA also regulates the bosR mRNA level independently of c-di-GMP. An elevated BosR level increases the transcription of rpoS from an RpoN (σ54)-type promoter whose activation is governed by RpoN and phosphorylated Rrp2 by Hk2 or acetyl phosphate (AcP). RpoS, in turn, controls expression of many genes, such as ospC (essential for mammalian infection) and chbC (important to tick colonization). For simplicity, other functions of Rrp1 and PlzA, such as motility and glycerol utilization, as well as other factors involved in RpoS regulation, are not included in the model.
We first confirmed the previous observation that Rrp1 affects the RpoS regulon, the downstream targets of Rrp2 (23, 26, 32). It is noteworthy that although Rrp1 influences expression of rpoS and RpoS-regulated genes, the degree of its impact on RpoS is much less than that reported previously for Rrp2, RpoN, or BosR: mutation of rrp2 or deletion of rpoN or bosR virtually abolishes the expression of rpoS and ospC, whereas deletion of rrp1 only partially reduces ospC expression (Fig. 4). In addition, certain growth conditions, such as lowered pH (pH 7.0), significantly restored OspC production in the rrp1 mutant (Fig. 4B).
Our observation that both the rrp1 and plzA mutants showed defects in rpoS expression suggests that Rrp1 may regulate rpoS through PlzA (Fig. 2 and 4). In addition, our data reveal that PlzA can influence rpoS expression even in the absence of c-di-GMP. Several lines of evidence support this notion. First, the plzA mutant had a more severe defect in OspC production than the rrp1 mutant (Fig. 4). Second, deletion of rrp1 affected only the level of BosR protein, not the level of bosR mRNA, suggesting that Rrp1 influences BosR at the posttranscriptional level, such as with protein stability. In contrast to Rrp1, PlzA appears to regulate BosR levels at both mRNA and protein levels, because a 2-fold reduction in the bosR mRNA levels could not fully account for the lack of detectable BosR protein in the plzA mutant (Fig. 5). Lastly, overexpression of plzA overcomes the defect of rrp1 on RpoS production (Fig. 4). It is worth noting that Sze et al. previously showed that their rrp1 mutant has a defect in bosR transcription (32). The reason for the discrepancy remains unclear. However, it has been reported that regulation of bosR often occurs at the posttranscriptional level (43, 55). Based on our findings, we postulate that PlzA influences rpoS expression via BosR through two mechanisms: (i) upon PlzA binding to c-di-GMP, PlzA–c-di-GMP positively regulates the BosR level by affecting translation efficiency or protein stability; (ii) in the absence of c-di-GMP, PlzA functions as a positive regulator for bosR transcription (Fig. 7). The c-di-GMP-independent functions of PlzA may explain why the plzA mutant had a more severe defect than the rrp1 mutant in mammalian infection (23, 25, 35).
The inability of the rrp1 mutant to survive in ticks is likely due to defects in several cellular functions. First, additional c-di-GMP-binding effectors, besides PlzA, may exist in B. burgdorferi. Second, as shown in this study and by Pitzer et al. (35), PlzA modulates multiple cellular functions, including cellular motility and transcriptional and posttranscriptional regulation of virulence determinants. Some of the functions of PlzA depend on c-di-GMP, while other functions may be c-di-GMP independent. Since overexpression of plzA in the rrp1 mutant could restore only c-di-GMP-independent functions of PlzA, it is not surprising that overexpression of plzA could not fully restore the rrp1 mutant's survival in ticks (Fig. 6). The result of partial restoration of the rrp1 mutant's survival in ticks further emphasized the important role of c-di-GMP in this process. Nevertheless, our finding that PlzA is a multifunctional c-di-GMP receptor appears to be a unique feature of B. burgdorferi signal transduction. Bacteria with numerous c-di-GMP signaling pathways often dedicate specific c-di-GMP receptors to control individual targets, in contrast to B. burgdorferi, which has a very limited number of signaling pathways (34).
ACKNOWLEDGMENTS
We thank M. Bauer and M. Gomelsky for helpful discussion, H. Kawabata, S. Norris, P. Rosa, M. Motaleb, D. S. Samuels, and G. Chaconas for strains and plasmids.
Funding for this work was partially provided by NIH grants AI083640 and AI085242, Indiana INGEN and METACyt grants of Indiana University, funded by the Lilly Endowment, Inc. (to X.F.Y.), and by the National Science Foundation of China (81171611) (to Y.L.). This investigation was partially conducted in a facility with support from research facilities improvement program grant number C06 RR015481-01 from the National Center for Research Resources, NIH.
Footnotes
Published ahead of print 11 November 2013
REFERENCES
- 1.Steere AC, Coburn J, Glickstein L. 2004. The emergence of Lyme disease. J. Clin. Invest. 113:1093–1101. 10.1172/JCI21681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuels DS. 2011. Gene regulation in Borrelia burgdorferi. Annu. Rev. Microbiol. 65:479–499. 10.1146/annurev.micro.112408.134040 [DOI] [PubMed] [Google Scholar]
- 3.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10:87–99. 10.1038/nrmicro2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586. 10.1038/37551 [DOI] [PubMed] [Google Scholar]
- 5.Das R, Hegyi H, Gerstein M. 2000. Genome analyses of spirochetes: a study of the protein structures, functions and metabolic pathways in Treponema pallidum and Borrelia burgdorferi. J. Mol. Microbiol. Biotechnol. 2:387–392 [PubMed] [Google Scholar]
- 6.Boardman BK, He M, Ouyang Z, Xu H, Pang X, Yang XF. 2008. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect. Immun. 76:3844–3853. 10.1128/IAI.00467-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, Radolf JD. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65:1193–1217. 10.1111/j.1365-2958.2007.05860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang XF, Alani SM, Norgard MV. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 100:11001–11006. 10.1073/pnas.1834315100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouyang Z, Blevins JS, Norgard MV. 2008. Transcriptional interplay among the regulators Rrp2, RpoN, and RpoS in Borrelia burgdorferi. Microbiology 154:2641–2658. 10.1099/mic.0.2008/019992-0 [DOI] [PubMed] [Google Scholar]
- 10.Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, Gherardini FC. 2005. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. U. S. A. 102:5162–5167. 10.1073/pnas.0408536102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyde JA, Shaw DK, Smith Iii R, Trzeciakowski JP, Skare JT. 2009. The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol. Microbiol. 74:1344–1355. 10.1111/j.1365-2958.2009.06951.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouyang Z, Kumar M, Kariu T, Haq S, Goldberg M, Pal U, Norgard MV. 2009. BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol. Microbiol. 74:1331–1343. 10.1111/j.1365-2958.2009.06945.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouyang Z, Deka RK, Norgard MV. 2011. BosR (BB0647) controls the RpoN-RpoS regulatory pathway and virulence expression in Borrelia burgdorferi by a novel DNA-binding mechanism. PLoS Pathog. 7:e1001272. 10.1371/journal.ppat.1001272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katona LI, Tokarz R, Kuhlow CJ, Benach J, Benach JL. 2004. The fur homologue in Borrelia burgdorferi. J. Bacteriol. 186:6443–6456. 10.1128/JB.186.19.6443-6456.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boylan JA, Posey JE, Gherardini FC. 2003. Borrelia oxidative stress response regulator, BosR: a distinctive Zn-dependent transcriptional activator. Proc. Natl. Acad. Sci. U. S. A. 100:11684–11689. 10.1073/pnas.2032956100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seshu J, Boylan JA, Hyde JA, Swingle KL, Gherardini FC, Skare JT. 2004. A conservative amino acid change alters the function of BosR, the redox regulator of Borrelia burgdorferi. Mol. Microbiol. 54:1352–1363. 10.1111/j.1365-2958.2004.04352.x [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Dadhwal P, Cheng Z, Zianni MR, Rikihisa Y, Liang FT, Li X. 2013. Borrelia burgdorferi oxidative stress regulator BosR directly represses lipoproteins primarily expressed in the tick during mammalian infection. Mol. Microbiol. 89:1140–1153. 10.1111/mmi.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller CL, Karna SLR, Seshu J. 2013. Borrelia host adaptation regulator (BadR) regulates rpoS to modulate host adaptation and virulence factors in Borrelia burgdorferi. Mol. Microbiol. 88:105–124. 10.1111/mmi.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lybecker MC, Samuels DS. 2007. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol. Microbiol. 64:1075–1089. 10.1111/j.1365-2958.2007.05716.x [DOI] [PubMed] [Google Scholar]
- 20.Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. 2005. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 187:1792–1798. 10.1128/JB.187.5.1792-1798.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sultan SZ, Pitzer JE, Boquoi T, Hobbs G, Miller MR, Motaleb MA. 2011. Analysis of the HD-GYP domain cyclic dimeric GMP phosphodiesterase reveals a role in motility and the enzootic life cycle of Borrelia burgdorferi. Infect. Immun. 79:3273–3283. 10.1128/IAI.05153-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sultan SZ, Pitzer JE, Miller MR, Motaleb MA. 2010. Analysis of a Borrelia burgdorferi phosphodiesterase demonstrates a role for cyclic-di-guanosine monophosphate in motility and virulence. Mol. Microbiol. 77:128–142. 10.1111/j.1365-2958.2010.07191.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, Norgard MV, Gomelsky M, Yang XF. 2011. Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathog. 7:e1002133. 10.1371/journal.ppat.1002133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S, Akins DR, Pal U, Radolf JD. 2011. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect. Immun. 79:3117–3130. 10.1128/IAI.05136-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N, Marconi RT. 2011. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol. Microbiol. 81:219–231. 10.1111/j.1365-2958.2011.07687.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers EA, Terekhova D, Zhang H, Hovis KM, Schwartz I, Marconi RT. 2009. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol. Microbiol. 71:1551–1573. 10.1111/j.1365-2958.2009.06621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pappas CJ, Iyer R, Petzke MM, Caimano MJ, Radolf JD, Schwartz I. 2011. Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathog. 7:e1002102. 10.1371/journal.ppat.1002102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee RE, Chen CP, Denlinger DL. 1987. A rapid cold-hardening process in insects. Science 238:1415–1417. 10.1126/science.238.4832.1415 [DOI] [PubMed] [Google Scholar]
- 29.Rhodes R, Coy W, Nelson D. 2009. Chitobiose utilization in Borrelia burgdorferi is dually regulated by RpoD and RpoS. BMC Microbiol. 9:108. 10.1186/1471-2180-9-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhodes R, Atoyan J, Nelson D. 2010. The chitobiose transporter, chbC, is required for chitin utilization in Borrelia burgdorferi. BMC Microbiol. 10:21. 10.1186/1471-2180-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilly K, Elias A, Errett J, Fischer E, Iyer R, Schwartz I, Bono J, Rosa P. 2001. Genetics and regulation of chitobiose utilization in Borrelia burgdorferi. J. Bacteriol. 183:5544–5553. 10.1128/JB.183.19.5544-5553.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sze CW, Smith A, Choi YH, Yang X, Pal U, Yu A, Li C. 2013. Study of the response regulator Rrp1 reveals its regulatory role in chitobiose utilization and virulence of Borrelia burgdorferi. Infect. Immun. 81:1775–1787. 10.1128/IAI.00050-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Römling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 71:1–52. 10.1128/MMBR.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryjenkov DA, Simm R, Romling U, Gomelsky M. 2006. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J. Biol. Chem. 281:30310–30314. 10.1074/jbc.C600179200 [DOI] [PubMed] [Google Scholar]
- 35.Pitzer JE, Sultan SZ, Hayakawa Y, Hobbs G, Miller MR, Motaleb MA. 2011. Analysis of the Borrelia burgdorferi cyclic-di-GMP-binding protein PlzA reveals a role in motility and virulence. Infect. Immun. 79:1815–1825. 10.1128/IAI.00075-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freedman JC, Rogers EA, Kostick JL, Zhang H, Iyer R, Schwartz I, Marconi RT. 2010. Identification and molecular characterization of a cyclic-di-GMP effector protein, PlzA (BB0733): additional evidence for the existence of a functional cyclic-di-GMP regulatory network in the Lyme disease spirochete, Borrelia burgdorferi. FEMS Immunol. Med. Microbiol. 58:285–294. 10.1111/j.1574-695X.2009.00635.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawabata H, Norris SJ, Watanabe H. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 72:7147–7154. 10.1128/IAI.72.12.7147-7154.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuels DS. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 45:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641–648. 10.1084/jem.20031960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purser JE, Norris SJ. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi Proc. Natl. Acad. Sci. U. S. A. 97:13865–13870. 10.1073/pnas.97.25.13865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Caimano MJ, Lin T, He M, Radolf JD, Norris SJ, Gheradini F, Wolfe AJ, Yang XF. 2010. Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog. 6:e1001104. 10.1371/journal.ppat.1001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moriarty TJ, Norman MU, Colarusso P, Bankhead T, Kubes P, Chaconas G. 2008. Real-time high resolution 3D imaging of the Lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog. 4:e1000090. 10.1371/journal.ppat.1000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Troxell B, Ye M, Yang Y, Carrasco SE, Lou Y, Yang XF. 2013. Manganese and zinc regulate virulence determinants in Borrelia burgdorferi. Infect. Immun. 81:2743–2752. 10.1128/IAI.00507-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akins DR, Bourell KW, Caimano MJ, Norgard MV, Radolf JD. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Invest. 101:2240–2250. 10.1172/JCI2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu H, He M, He JJ, Yang XF. 2010. Role of the surface lipoprotein BBA07 in the enzootic cycle of Borrelia burgdorferi. Infect. Immun. 78:2910–2918. 10.1128/IAI.00372-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. U. S. A. 98:12724–12729. 10.1073/pnas.231442498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caimano MJ, Eggers CH, Hazlett KR, Radolf JD. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433–6445. 10.1128/IAI.72.11.6433-6445.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbert MA, Morton EA, Bundle SF, Samuels DS. 2007. Artificial regulation of ospC expression in Borrelia burgdorferi. Mol. Microbiol. 63:1259–1273. 10.1111/j.1365-2958.2007.05593.x [DOI] [PubMed] [Google Scholar]
- 49.Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U. S. A. 101:3142–3147. 10.1073/pnas.0306845101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, Flavell RA, Norgard MV, Fikrig E. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 113:220–230. 10.1172/JCI19894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuchs R, Jauris S, Lottspeich F, Preac-Mursic V, Wilske B, Soutschek E. 1992. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22 kDa protein (pC) in Escherichia coli. Mol. Microbiol. 6:503–509. 10.1111/j.1365-2958.1992.tb01495.x [DOI] [PubMed] [Google Scholar]
- 52.Hengge R. 2008. The two-component network and the general stress sigma factor RpoS (sigma S) in Escherichia coli. Adv. Exp. Med. Biol. 631:40–53. 10.1007/978-0-387-78885-2_4 [DOI] [PubMed] [Google Scholar]
- 53.Banik S, Terekhova D, Iyer R, Pappas CJ, Caimano MJ, Radolf JD, Schwartz I. 2011. BB0844, an RpoS-regulated protein, is dispensable for Borrelia burgdorferi infectivity and maintenance in the mouse-tick infectious cycle. Infect. Immun. 79:1208–1217. 10.1128/IAI.01156-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilmore RD, Jr, Howison RR, Schmit VL, Carroll JA. 2008. Borrelia burgdorferi expression of the bba64, bba65, bba66, and bba73 genes in tissues during persistent infection in mice. Microb. Pathog. 45:355–368. 10.1016/j.micpath.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 55.Hyde JA, Trzeciakowski JP, Skare JT. 2007. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J. Bacteriol. 189:437–445. 10.1128/JB.01109-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ouyang Z, Narasimhan S, Neelakanta G, Kumar M, Pal U, Fikrig E, Norgard M. 2012. Activation of the RpoN-RpoS regulatory pathway during the enzootic life cycle of Borrelia burgdorferi. BMC Microbiol. 12:44. 10.1186/1471-2180-12-44 [DOI] [PMC free article] [PubMed] [Google Scholar]