Abstract
Staphylococcus aureus is an invasive bacterial pathogen, and antibiotic resistance has impeded adequate control of infections caused by this microbe. Moreover, efforts to prevent human infections with single-component S. aureus vaccines have failed. In this study, we evaluated the protective efficacy in rats of vaccines containing both S. aureus capsular polysaccharides (CPs) and proteins. The serotypes 5 CP (CP5) and 8 CP (CP8) were conjugated to tetanus toxoid and administered to rats alone or together with domain A of clumping factor A (ClfA) or genetically detoxified alpha-toxin (dHla). The vaccines were delivered according to a preventive or a therapeutic regimen, and their protective efficacy was evaluated in a rat model of osteomyelitis. Addition of dHla (but not ClfA) to the CP5 or CP8 vaccine induced reductions in bacterial load and bone morphological changes compared with immunization with either conjugate vaccine alone. Both the prophylactic and therapeutic regimens were protective. Immunization with dHla together with a pneumococcal conjugate vaccine used as a control did not reduce staphylococcal osteomyelitis. The emergence of unencapsulated or small-colony variants during infection was negligible and similar for all of the vaccine groups. In conclusion, addition of dHla to a CP5 or CP8 conjugate vaccine enhanced its efficacy against S. aureus osteomyelitis, indicating that the inclusion of multiple antigens will likely enhance the efficacy of vaccines against both chronic and acute forms of staphylococcal disease.
INTRODUCTION
Staphylococcus aureus is a medically important opportunistic pathogen that affects individuals in the hospital setting as well as in the community. S. aureus can provoke skin and soft tissue infections, and it can also disseminate to cause invasive life-threatening infections, including septic arthritis and osteomyelitis (1). Osteomyelitis is a progressive infection of the bone marrow and cortex and is frequently caused by S. aureus (2). It is usually preceded by trauma, other nosocomial infections, or orthopedic (3) or maxillofacial (4) surgery. The control of S. aureus infections in patients with either nosocomial or community-acquired infections has been hampered by the emergence of methicillin-resistant S. aureus (MRSA) (5–7). The high worldwide prevalence of nosocomial MRSA infections was responsible for the intensive use of glycopeptide therapy (8). Although glycopeptides have long been used to treat severe MRSA infections, the increasing prevalence of clinically relevant isolates with reduced susceptibility to vancomycin (9) and the appearance of MRSA resistant to vancomycin (10) have prompted a search for a suitable immunoprophylactic approach to prevent S. aureus infections.
Due to its vast array of virulence factors and the myriad of infection types that it causes, S. aureus presents a unique challenge for vaccine development. A number of S. aureus antigens have been explored as potential vaccine components. Among these, S. aureus capsular polysaccharides (CPs), which are antiphagocytic and crucial for immune evasion, have been utilized (11). In a phase III clinical trial, a conjugate vaccine including CP serotype 5 (CP5) and CP8 significantly (P = 0.02) reduced the incidence of S. aureus bacteremia in patients receiving hemodialysis between weeks 3 and 40 after immunization (12). However, at the study endpoint (week 54) the vaccine efficacy was only 26%, which was not statistically significant. A confirmatory phase III clinical trial failed to reduce bacteremia in hemodialysis patients (http://www.bizjournals.com/southflorida/stories/2005/10/31/daily27.html?page=all). Because of the complexity of S. aureus and its myriad of virulence factors, the inclusion of multiple staphylococcal antigens would likely result in a more effective vaccine.
Numerous studies suggest that cell wall-linked surface protein clumping factor A (ClfA) is a promising antigen for inclusion in an S. aureus multicomponent vaccine. Preclinically, ClfA was shown to be protective in rodent models of arthritis, sepsis, and endocarditis (13–15). Alpha-toxin (Hla) is a pore-forming exotoxin expressed by S. aureus that is cytolytic for a variety of cell types, including platelets, endothelial cells, and monocytes (16). Detoxified Hla induces protection in murine models of lethal pneumonia, subcutaneous abscess formation, and peritonitis (17–19). The next-generation S. aureus vaccine may benefit from the inclusion of both CPs and protein antigens. The selection of surface antigens for inclusion in an experimental vaccine is difficult because S. aureus produces a wide array of surface proteins that promote its virulence but are often redundant in function (20). Moreover, the immune correlates of protection against S. aureus infection have not yet been elucidated.
Efforts to prevent staphylococcal osteomyelitis by immunization are few (21). In this study, we evaluated the ability of active immunization to reduce the severity of experimental staphylococcal osteomyelitis, and we compared vaccines that were delivered in a preventive or a therapeutic fashion. CP conjugate vaccines were evaluated alone and in combination with S. aureus ClfA or detoxified Hla for their ability to reduce the bacterial burden associated with the disease, as well as to reduce the gross morphological changes that occur in the bone during chronic staphylococcal infection.
MATERIALS AND METHODS
Bacterial strains.
S. aureus clinical strains HU-1 and HU-92a were obtained in 2007 from patients with chronic osteomyelitis at the Hospital de Clínicas José de San Martín, Universidad de Buenos Aires. HU-1 is a CC97 strain that produces CP5, and HU-92a is a CC45 strain that produces CP8. Strains were kept frozen in Trypticase soy broth (TSB) at −20°C, and S. aureus was routinely cultured at 37°C for 24 h on Columbia agar supplemented with 2% NaCl to enhance CP production. Bacterial cells were harvested and suspended to the appropriate density in TSB. S. aureus HU-1 was used as the challenge strain for the CP5 experiments, and S. aureus HU-92a was used for the CP8 experiments.
Rat osteomyelitis model.
Outbred Wistar adult rats weighing 280 to 320 g were purchased from the vivarium of (i) Facultad de Odontología, Universidad de Buenos Aires, (ii) Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, or (iii) Comisión Nacional de Energía Atómica, Ezeiza, Buenos Aires. The rats were housed at the vivarium of the Instituto de Microbiología y Parasitología Médica (IMPaM), Universidad de Buenos Aires and CONICET. Animal care was in accordance with the guidelines set forth by the National Institutes of Health (22). The use of the rat osteomyelitis model was evaluated by the “Comisión Institucional para el Cuidado y Uso de Animales de Laboratorio” (CICUAL), Facultad de Medicina, Universidad de Buenos Aires, and approved through resolution CD 1938/07.
The rats were anesthetized with ketamine/xylazine, the left tibia was exposed, and a hole in the bone was made with a high-speed drill using a 0.4-mm diameter bit. Each tibia was injected with a 5-μl suspension containing ∼1 × 106 CFU S. aureus suspended in fibrin glue (Tissucol kit 1 ml; Baxter Argentina-AG, Vienna, Austria) (23). Fourteen weeks after bacterial challenge, the rats were euthanized by CO2 overdose. Left and right tibias were excised, and adjacent tissue, especially at the distal epiphysis, was removed. The following measurements were made using calipers: (i) the distance between the inoculation point and the distal end of the left tibia (DT); (ii) the left tibia section diameter at the inoculation site (Di) and the perpendicular diameter at the same site (Dp); (iii) Di and Dp were also measured in the uninfected right tibia of the same rat at the DT determined in the diseased left tibia (control). The osteomyelitic index (OI) was determined as follows: OI = (Dp + Di)infected − (Dp + Di)control. In pilot experiments, we determined that the OI at 7 or 14 weeks after injection of sterile Tissucol did not differ significantly from that in uninjected tibias. Therefore, in order to avoid unnecessary discomfort to rats, only the left tibia was injected, and the OI was determined by subtraction of values from the healthy right tibia (control). One-centimeter segments involving the infected bone were crushed and homogenized in sterile mortars. Homogenates were quantitatively cultured overnight on Trypticase soy agar, and the CFU were determined. Plates were incubated for 48 h to evaluate the CFU of S. aureus small-colony variants (SCVs), which were characterized by catalase and coagulase tests and confirmed by a species-specific PCR (24). The OI, the wild-type S. aureus CFU, and the S. aureus SCV CFU from each experimental group (infected and control) were compared. CP5 and CP8 production by S. aureus recovered from the rat tibias was assessed by colony immunoblotting as described previously (25).
Vaccination schemes.
Rats were vaccinated according to two different schemes. In the preventive scheme, rats were immunized on days 0 and 7. The rats were challenged by the intratibial route with S. aureus on day 28. In the therapeutic scheme, rats were vaccinated 21 days after bacterial challenge and boosted 7 days later (day 28). For both regimens, the rats were euthanized by CO2 exposure 14 weeks (ca. 98 days) after challenge with S. aureus.
Immunogens and controls.
Rats were immunized as follows: group 1, 10 μg of CP5 or CP8 conjugated to tetanus toxoid [CP5(8)-TT]; group 2, 10 μg of CP5(8)-TT plus 20 μg of recombinant ClfA region A, domain N123 (ClfA); group 3, 10 μg of CP5(8)-TT plus 20 μg of purified, genetically detoxified S. aureus alpha-toxin (dHla); group 4, 10 μg of Streptococcus pneumoniae CP18C conjugated to TT [Spn-TT] (the 18C-TT conjugate is a pre-GMP lot of one of the conjugates included in a commercial vaccine, and the 18C polysaccharide was fully characterized and devoid of cell wall polysaccharide contamination); group 5, 10 μg of Spn-TT plus 20 μg ClfA; group 6, 10 μg of Spn-TT plus 20 μg dHla; group 7, phosphate-buffered saline (PBS) (control group). The CP/TT ratios were 1/2.6 for the CP5-TT conjugate and 1/2.2 for the CP8-TT conjugate. The vaccine antigens (except PBS) were adsorbed on 200 μg aluminum [AlPO4 or Al(OH)3] and delivered subcutaneously to the rats in a 0.1-ml volume. CP5-TT, CP8-TT, dHla, and Spn-TT (CP18C) were adsorbed on AlPO4, whereas ClfA was adsorbed on Al(OH)3. The selection of AlPO4 or Al(OH)3 was based upon intrinsic characteristic of the antigen (such as the isoelectric point) and the pH of the antigen buffer. Preliminary experiments in other settings have shown that the use of AlPO4 or Al(OH)3 did not change significantly the adjuvant effect or impact on protection (S. Germain and P. Denoël, unpublished data).
Antibody determinations.
Blood was collected from all rats by cardiac puncture under anesthesia prior to euthanasia, and the serum was stored at −20°C. Preliminary experiments showed that the titer of antibodies to CP increased after vaccination, whereas the titer of antibodies in nonvaccinated rats was undetectable and not modified after 14 weeks. Therefore, rats were bled only once at the end of the experiment, and the serum from nonimmunized rats served as the control. A similar criterion was adopted for other antibody determinations in order to reduce discomfort and further handling of the animals, as recommended by the CICUAL. Titers of serum antibodies to CP5, CP8, Spn CP18C, ClfA, and dHla were determined by enzyme-linked immunosorbent assay (ELISA) using Nunc Maxisorp microtiter plates. Optimal coating conditions were defined for each antigen to ensure optimal adsorption on the plates and to allow reproducible detection of specific rat serum antibodies. Plates were coated overnight at 4°C with solutions of purified CP5 (1 μg/ml), CP8 (30 μg/ml), Spn CP18C (40 μg/ml), ClfA (1 μg/ml), or dHla (20 μg/ml) in PBS. The plates were blocked with PBS + 1% bovine serum albumin (BSA) for 30 min at room temperature with agitation. Twofold dilutions of rat sera (initial dilution, 1:10) were added to the plate and incubated with agitation at room temperature for 30 min. After washing, peroxidase-AffiniPure goat anti-rat IgG, Fcγ fragment specific (product 112-035-008; Jackson ImmunoResearch Laboratories Inc.), diluted 1:5,000 in PBS, 0.2% BSA, and 0.05% Tween 20, was added, and the plate was incubated with agitation for 30 min at room temperature. The color was developed using 4 mg o-phenylenediamine + 0.05% H2O2 in 0.1 M citrate buffer, pH 4.5, for 15 min in the dark at room temperature. The reaction was stopped with 50 μl 3 M HCl, and the optical density was recorded at 490 nm relative to 620 nm. The level of antibodies present in the serum was expressed in midpoint titers. Midpoint ELISA titers were calculated by 4-parameter regression analysis and were defined as the reciprocal of the serum dilution that produced an absorbance (at 490 nm) equal to 50% of the maximum value.
Statistical analysis.
Bone bacterial burdens and OI values were compared by one-way analysis of variance (ANOVA), and multiple comparisons were performed with the Bonferroni posttest. Prism 5.0 software (GraphPad) was used for all calculations, and P values of <0.05 were considered significant.
RESULTS
CP5 vaccine experiments: preventive scheme.
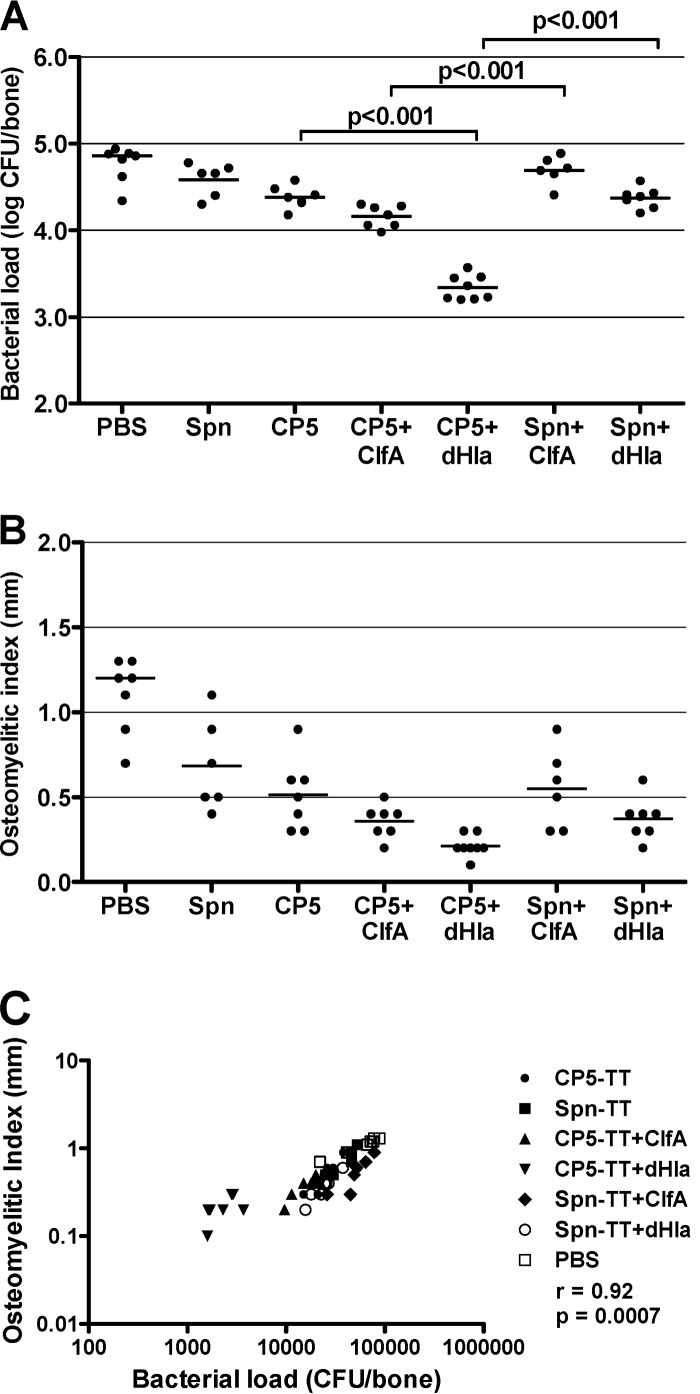
Two experiments of identical design using rats from the same source and vaccinated with preparations from the same lot of CP5 vaccines were performed. Since the data from the individual experiments were similar, the results from identical groups were pooled and are presented in Fig. 1. The validity of the experiment was confirmed by the significant correlation of the bacterial load and the OI (r = 0.92; P < 0.0007) (Fig. 1C).
FIG 1.
Protective efficacy of S. aureus CP5-TT conjugate vaccines administered alone or in combination with ClfA or dHla. Groups of 6 to 8 rats were immunized according to a preventive scheme (vaccination before bacterial challenge) and challenged by the intratibial route with S. aureus HU-1. Effects of immunization on bacterial load (CFU/bone) (A) and OI (mm) (B). Each dot represents determinations made on an individual rat, and the bars represent the geometric means (bacterial load) or the arithmetic means (OI) of each group. Comparisons were performed by one-way ANOVA followed by the Bonferroni posttest, and the P values for relevant comparisons are shown. (C) Correlation of the bacterial load (CFU/bone) and the OI (mm) (Spearman's rank correlation test).
The addition of ClfA to CP5-TT slightly reduced the bone bacterial load and the OI compared to those of rats given CP5-TT alone. The differences, however, did not reach significance (Fig. 1A and B). The addition of ClfA to the Spn-TT conjugate did not improve the protective efficacy of the CP conjugate (Fig. 1A and B), indicating the limitations of ClfA as a protective immunogen in the osteomyelitis infection model. Nonetheless, the rats given CP5-TT + ClfA show a reduced bacterial burden compared to those animals given Spn-TT + ClfA, suggesting a role for capsular antibodies in reducing infection.
Of note, the addition of dHla to the CP5-TT preparation produced a significant reduction in bacterial load compared to that of CP5-TT alone (CP5 versus CP5 + dHla, P < 0.001; Fig. 1A). The specificity of this synergistic response is demonstrated by the fact that dHla did not improve the protective efficacy of Spn-TT (Spn versus Spn + dHla; Fig. 1A). Rats given the CP5-TT + dHla vaccine showed significantly fewer CFU/bone than the rats given Spn-TT + dHla (P < 0.001). The relevance of dHla as vaccine antigen was underscored by the fact that rats given the CP5-TT + dHla showed significantly fewer CFU/bone than the rats given CP5-TT + ClfA (P < 0.001). The greatest reduction in OI occurred in the rats given the CP5-TT + dHla vaccine (Fig. 1B), but the reduction in OI did not reach significance.
CP5 vaccine experiments: therapeutic scheme.
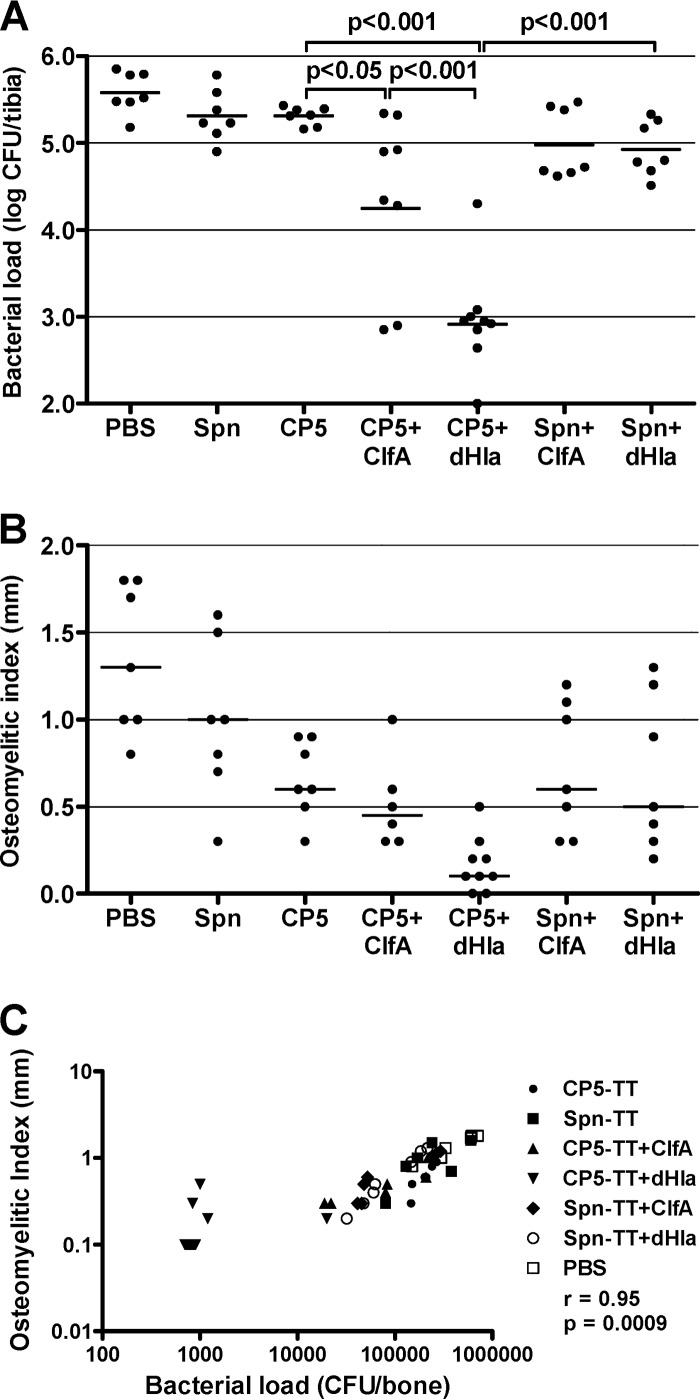
Two experiments of identical design were performed, and the results were pooled as described above. The overall results of the therapeutic vaccine approach correlated with those obtained with the preventive scheme. The CP-TT conjugates alone showed little effect therapeutically (Fig. 2A). In contrast to the results obtained in the prophylactic regimen, however, the addition of ClfA to the CP5-TT preparation resulted in a modest but significant reduction in the bone bacterial burden compared to that of CP5-TT alone (P < 0.05) (Fig. 2A). Similar to the results of the preventive vaccine approach, the therapeutic addition of dHla to the CP5-TT vaccine provided the greatest and most consistent protection against infection compared to CP5-TT alone or Spn-TT + dHla (P < 0.001). The greatest reductions in OI (Fig. 2B) occurred in the rats immunized with CP5-TT + dHla, but the differences did not achieve statistical significance. The validity of the experiment was confirmed by the significant correlation of the bacterial load and the OI (r = 0.95; P < 0.0009) (Fig. 2C).
FIG 2.
Therapeutic efficacy of S. aureus CP5-TT conjugate vaccines administered alone or in combination with ClfA or dHla. Groups of 7 to 9 rats were immunized according to a therapeutic scheme (vaccination after bacterial challenge) and challenged by the intratibial route with S. aureus HU-1. Effects of immunization on bacterial load (CFU/bone) (A) and OI (mm) (B). Each dot represents determinations made on an individual rat, and the bars represent the geometric means (bacterial load) or the arithmetic means (OI) of each group. Comparisons were performed by one-way ANOVA followed by the Bonferroni posttest, and P values for relevant comparisons are shown. (C) Correlation of the bacterial load (CFU/bone) and the OI (mm) (Spearman's rank correlation test).
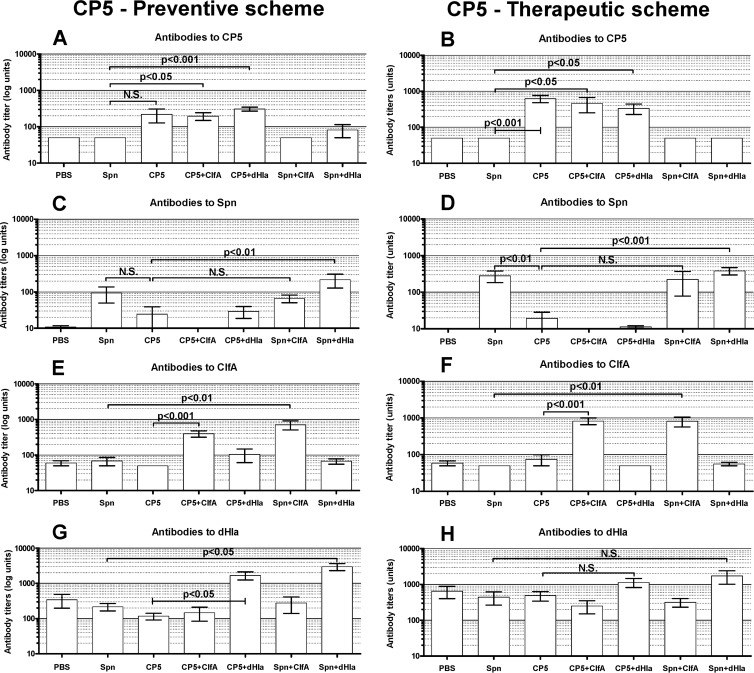
Vaccine antibody levels: CP5 studies.
Serum was obtained from experimental and control groups on the day the rats were euthanized. Serum antibody titers in the rats immunized with the antigens used for the CP5 experiments are shown in Fig. 3. Overall, the antibody responses of the immunized rats were specific for the individual immunogens. The CP5-TT antibody response was generally higher than that elicited by the Spn-TT vaccine (Fig. 3A, B, C, and D). Antibody titers resulting from vaccination with either CP conjugate tended to be higher in the rats immunized therapeutically (Fig. 3B and D). The overall antibody response to dHla was greater than that of the other antigens (Fig. 3G and H). However, dHla antibody titers in rats immunized with antigens other than dHla were also somewhat higher than those of the heterologous antigens, particularly in the rats immunized therapeutically (Fig. 3H). These data suggest that dHla is an immunodominant protein elicited during staphylococcal infection. Whether protection against infection mediated by the CP5-TT vaccine administered with dHla is mediated by antibodies or by immune T cells remains to be determined.
FIG 3.
Levels of serum antibodies to CP5, Spn, ClfA, and dHla in groups of immunized rats and controls. Animals were immunized according to a preventive (A, C, E, and G) or a therapeutic (B, D, F, and H) scheme. Blood was collected 14 weeks after bacterial challenge. Each bar represents the arithmetic mean ± standard error of the mean (SEM). P values for selected comparisons were determined by ANOVA for nonparametric data (Kruskal-Wallis test) followed by Dunn's multiple-comparison posttest.
CP8 vaccine experiments.
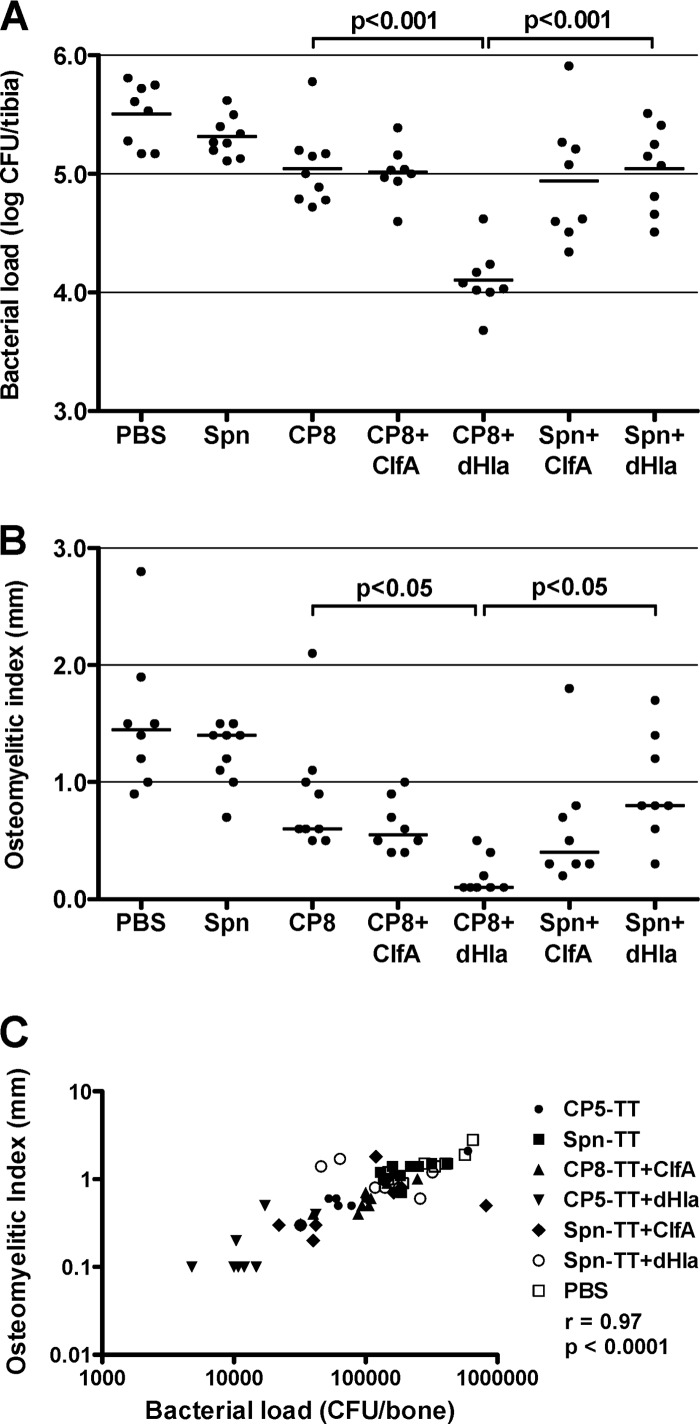
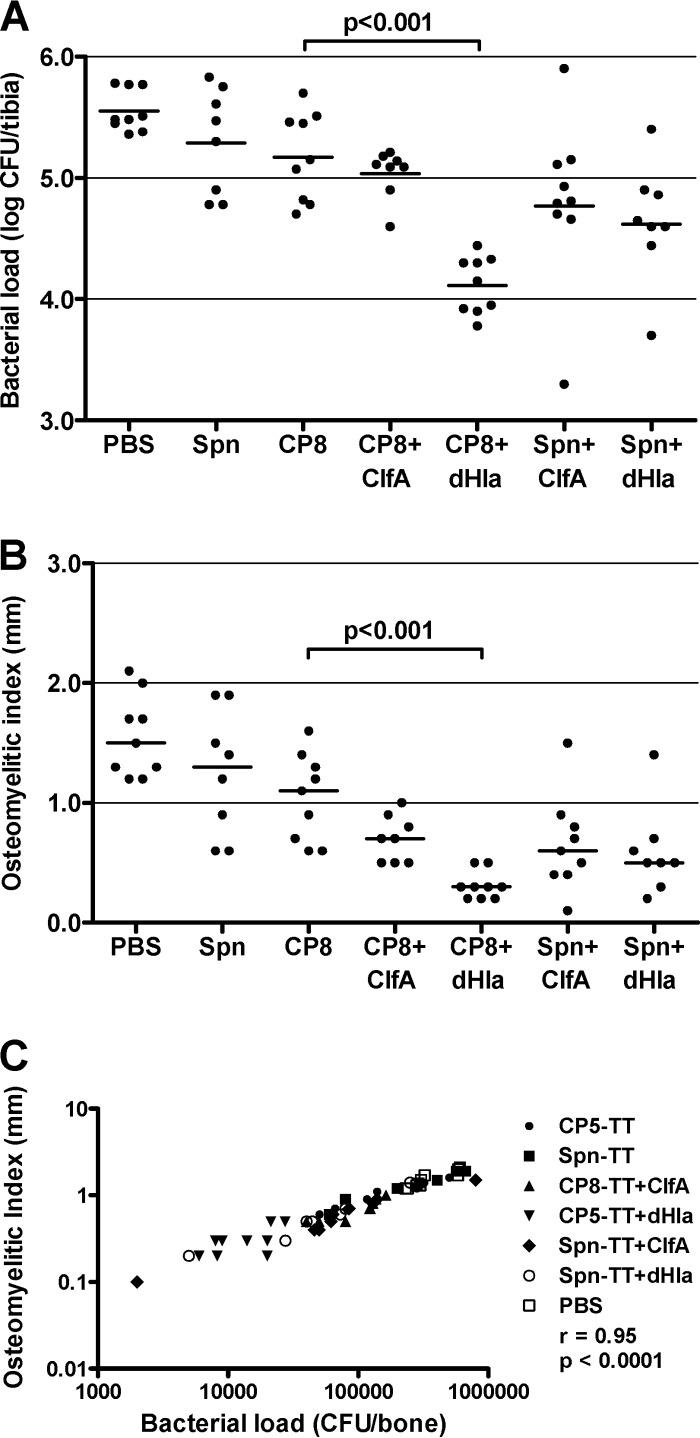
Two experiments each were performed using the preventive and therapeutic vaccination schemes, and the results were pooled as described above. The validity of the CP8 experiments was confirmed by the significant correlation of the bacterial load and the OI: preventive scheme, r = 0.97 and P < 0.0001 (Fig. 4C); therapeutic scheme, r = 0.95 and P < 0.0001 (Fig. 5C).
FIG 4.
Protective efficacy of S. aureus CP8-TT conjugate vaccines administered alone or in combination with ClfA or dHla. Groups of 8 or 9 rats were immunized according to a preventive scheme (vaccination before bacterial challenge) and challenged by the intratibial route with S. aureus HU-92a. Effects of immunization on bacterial load (CFU/bone) (A) and OI (mm) (B). Each dot represents determinations made on an individual rat, and the bars represent the geometric means (bacterial load) or the arithmetic means (OI) of each group. Comparisons were performed by one-way ANOVA followed by the Bonferroni posttest, and P values for relevant comparisons are shown. (C) Correlation of the bacterial load (CFU/bone) and the OI (mm) (Spearman's rank correlation test).
FIG 5.
Therapeutic efficacy of S. aureus CP8-TT conjugate vaccines administered alone or in combination with ClfA or dHla. Groups of 8 or 9 rats were immunized according to a therapeutic scheme (vaccination after bacterial challenge) and challenged by the intratibial route with S. aureus HU-92a. Effects of immunization on bacterial load (CFU/bone) (A) and OI (mm) (B). Each dot represents determinations made on individual rats, and the bars represent the geometric means (bacterial load) or the arithmetic means (OI) of each group. Comparisons were performed by one-way ANOVA followed by the Bonferroni posttest, and the P values for relevant comparisons are shown. (C) Correlation of the bacterial load (CFU/bone) and the OI (mm) (Spearman's rank correlation test).
The results obtained with the CP8 vaccine groups were similar to those obtained with the CP5 vaccines. In the preventive scheme, vaccination with CP8-TT + dHla significantly reduced the bacterial load (P < 0.001) and the OI (P < 0.05) compared to vaccination with CP8-TT alone (Fig. 4A and B). Similar findings can be seen for rats immunized therapeutically with either CP8-TT + dHla or CP8-TT alone (Fig. 5A and B). In contrast, the addition of ClfA to the CP8-TT vaccine did not improve CP8-TT vaccine efficacy. Of note are the results showing that when dHla was combined with Spn-TT, no protection against osteomyelitis was observed. Thus, the combination of S. aureus CP antigens and dHla provided the best protection against experimental infection with encapsulated staphylococci.
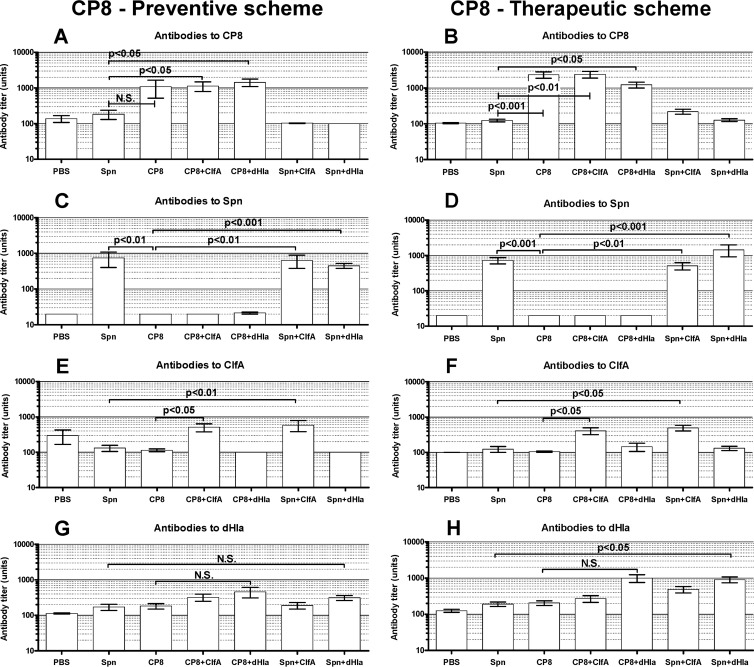
Vaccine antibody levels: CP8 studies.
Specific serum antibody responses to the various vaccine antigens used for the CP8 experiments are shown in Fig. 6. Overall, the antibody responses of the immunized rats were specific for the individual immunogens (Fig. 6A, B, C, and D). Notable differences in antibody titers were not observed in rats immunized with CP-TT conjugates before or after bacterial challenge (Fig. 6A, B, C, and D). The antibody responses to dHla in the rats immunized prophylactically (Fig. 6G) were not as striking as those observed in the CP5 experiments (Fig. 3G). Within the CP8 experiment, the most consistent antibody response to Hla was seen in rats immunized therapeutically with dHla given with CP8-TT or Spn-TT (Fig. 6H). Whether protection against infection afforded by the CP8-TT vaccine administered with dHla is mediated by antibodies or by immune T cells is not yet known.
FIG 6.
Levels of serum antibodies to CP8, Spn, ClfA, and dHla in groups of immunized rats and controls. Animals were immunized according to a preventive (A, C, E, and G) or a therapeutic (B, D, F, and H) scheme. Blood was collected 14 weeks after bacterial challenge. Each bar represents the arithmetic mean ± SEM. P values for selected comparisons were determined by ANOVA for nonparametric data (Kruskal-Wallis test) followed by Dunn's multiple-comparison posttest.
Emergence of SCVs.
The percentages of SCV colonies recovered from the bone cultures of each rat were determined. Despite the chronic nature of the experimental infection (14 weeks), the emergence of SCVs was <1.6% for all of the experimental groups, with a mean recovery of only 0.86%. Statistical analysis by ANOVA revealed no significant differences among any of the rat groups within each of the 4 experiments (CP5-preventive, CP5-therapeutic, CP8-preventive, or CP8-therapeutic) (data not shown). Similarly, cultures from only three rats yielded colonies of S. aureus that no longer produced CP5 or CP8 (measured by colony immunoblotting), and there was no obvious association between experimental group and loss of CP production.
DISCUSSION
A number of S. aureus target antigens have been evaluated as vaccine components in preclinical active or passive immunization studies (20, 26–30). Because capsular antigens comprise effective vaccines against other encapsulated bacterial pathogens, early efforts in S. aureus vaccine development focused on these components. Covalent coupling of CP5 and CP8 to protein carrier molecules significantly increases their immunogenicity and T-cell-dependent properties (31–33). CP5/CP8-based conjugate vaccines alone failed in clinical trials aimed at reducing bacteremia in hemodialysis patients (34, 35). Similarly, a vaccine targeting a single protein (IsdB) failed to reduce surgical wound infections in patients undergoing a median sternotomy (26, 28, 36). Because the nature of S. aureus pathogenicity is multifactorial, it is likely that the protective responses required to prevent infection should necessarily target a number of virulence factors (37, 38). The fact that CP-based vaccines induce functional antibodies that mediate opsonophagocytic killing of encapsulated staphylococci supports the inclusion of CP5/CP8 in a multicomponent vaccine. Indeed, CP5 and CP8 conjugates have been included in a recently developed tetravalent vaccine that has entered phase I/II clinical trials (36). In the present study, however, vaccination of rats with the CP5 or CP8 conjugates alone did not significantly diminish the bone bacterial load or the severity of bone infection. The addition of staphylococcal proteins (dHla and to a lesser extent ClfA) significantly reduced the bacterial burden in rats with osteomyelitis.
ClfA is a cell wall-anchored adhesin that mediates S. aureus binding to fibrinogen and promotes the attachment of S. aureus to biomaterial surfaces, fibrin clots, platelets, and damaged endothelial surfaces (39). This surface protein is conserved among S. aureus isolates and has shown protective efficacy in diverse animal models (13–15, 40, 41). A pooled human immunoglobulin G preparation (INH-A21; Veronate) from healthy donors selected for high antibody titers to S. aureus ClfA and S. epidermidis SdrG failed to protect neonates from staphylococcal sepsis in a phase III clinical trial (42). Despite the failure of this passive immunotherapy, there is sustained interest in ClfA as a vaccine component for active immunization against S. aureus (43). We demonstrated the benefit of targeting both CP5 and ClfA antigens in a mouse mastitis model, in which passive immunization with antibodies to the two antigens had an additive effect on reducing bacterial burden (44). In the present study, the addition of ClfA to the CP5-TT vaccine only reduced the bacterial load in the CP5 therapeutic regimen.
Alpha toxoid was first suggested as a vaccine component in 1977, when it was shown to prevent the lethal gangrenous form of S. aureus mastitis in rabbits (45). Subsequently, a number of S. aureus vaccines composed of inactivated toxins or their subunits have been evaluated preclinically, and the results of these studies were reviewed recently (20, 26). In addition, S. aureus Hla activates the immune system via a toll-like receptor 2 (TLR2)-independent mechanism whereby NOD2 signaling results in protection against a murine staphylococcal infection (46). The fact that a Th17 response is relevant to host defense against S. aureus (47, 48) and that Hla is able to induce interleukin 17A (IL-17A) in blood (49) has renewed the interest in Hla as vaccine component. Our results in the osteomyelitis model support the use of dHla in a multicomponent S. aureus vaccine. The addition of dHla to either the CP5-TT or CP8-TT conjugate vaccine provided the most striking and consistent protection against the severity of experimental osteomyelitis, as well as the bacterial burden in the infected bone. Whereas vaccination with dHla plus an irrelevant polysaccharide vaccine was not protective, the addition of dHla to CP5(8)-TT induced a significant reduction of the S. aureus bone load and the severity of experimental osteomyelitis, supporting a synergistic effect of the two antigens resulting from immunization. However, only 37% of 76 highly cited animal research studies were replicated in human randomized trials (50). Similarly, a recent study aimed at evaluating how well research from murine clinical models mimics human disease indicates that the transcriptional response to inflammatory stresses in mice is a poor reflection of the human response to similar stresses (51). Thus, data from animal models, although informative, are not necessarily predictive of efficacy in humans (47, 48). Further clinical trials of vaccines that include CP5/8, ClfA, and/or dHla are required to ascertain whether the findings of the present investigation translate into success in humans.
Immunization with CP5(8) may result in production of opsonic antibodies that enhance phagocytic clearance of S. aureus, whereas immunization with dHla may induce neutralizing antibodies that reduce the tissue damage associated with infection. S. aureus is a ubiquitous microbe, and thus it is feasible that the rats were exposed to S. aureus prior to experimental infection. This fact may explain the observed elevated baseline antibody levels to Hla in animals not administered dHla. Because all of the animals were subjected to experimental infection, the measurable titers of antibodies to Hla might have resulted from an enhanced response to this antigen by the rats, compared with the other, presumably weaker antigens. The immunization schedule utilized was based upon preliminary experiments. An extended immunization schedule might have resulted in a better immune response in the immunized rats. Even though the vaccination schedule might be considered suboptimal, protective efficacy was observed in our studies, supporting the use of multiple antigens in an S. aureus vaccine.
SCVs, which have been isolated from patients with chronic, recurrent, or antibiotic-resistant diseases (52), have phenotypes that hold low virulence and are particularly adapted to the intracellular environment for long-term persistence (53). Once SCVs have emerged, they are very difficult to eradicate (54). When S. aureus was passaged in vivo in the mouse model of mastitis under the selective pressure of antibodies to CP5 or CP8, SCVs were recovered in high numbers from the infected glands. However, SCVs did not emerge when the mice were treated with both CP antibodies and monoclonal antibodies to ClfA (Aurexis). In the present study, only a small percentage of SCVs were recovered from the rats with osteomyelitis, irrespective of the vaccine antigen(s) administered. It is likely that the levels of serum antibodies induced by active immunization with the CP5(8) conjugates were insufficient to generate SCVs. In conclusion, the addition of S. aureus protein antigens to a capsular conjugate vaccine enhanced its efficacy in the prevention and therapeutic treatment of experimental S. aureus osteomyelitis in rats. ClfA induced only a modest improvement to the efficacy of CP5(8) conjugate, but dHla induced a significant enhancement of conjugate vaccine efficacy. The inclusion of vaccine components designed to neutralize staphylococcal toxins and block adhesins into a multicomponent vaccine preparation may be an effective strategy for the prevention of S. aureus infections.
ACKNOWLEDGMENTS
This study was partially supported by GlaxoSmithKline Biologicals S.A. (Rixensart, Belgium), by the Agencia Nacional de Promoción de la Ciencia y la Tecnología, Argentina (ANPCyT PICT 2010-01039), and by the Secretaría de Ciencia y Técnica, Universidad de Buenos Aires, Argentina (UBACyT 200 201 001 003 47). F.R.B. is supported by ANPCyT (PICT 2010-0733).
We thank Lorena Medina for her dedicated technical assistance.
Footnotes
Published ahead of print 14 October 2013
REFERENCES
- 1.Que YA, Moreillon P. 2010. Staphylococcus aureus (including staphylococcal toxic shock), p 2543–2578 In Mandell GL, Bennet JE, Dolin R. (ed), Principles and practice of infectious diseases, 7 ed, vol 2 Churchill-Livingstone-Elsevier, Philadelphia, PA [Google Scholar]
- 2.Montanaro L, Speziale P, Campoccia D, Ravaioli S, Cangini I, Pietrocola G, Giannini S, Arciola CR. 2011. Scenery of Staphylococcus implant infections in orthopedics. Future Microbiol. 6:1329–1349. 10.2217/fmb.11.117 [DOI] [PubMed] [Google Scholar]
- 3.Mader JT, Cripps MW, Calhoun JH. 1999. Adult posttraumatic osteomyelitis of the tibia. Clin. Orthop. Relat. Res. 1999(360):14–21 [DOI] [PubMed] [Google Scholar]
- 4.Ogawa A, Miyate H, Nakamura Y, Shimada M, Seki S, Kudo K. 2001. Treating chronic diffuse sclerosing osteomyelitis of the mandible with saucerization and autogenous bone grafting. Oral Surg. Oral Med. Oral Pathol. Oral Radiol Endod. 91:390–394. 10.1067/moe.2001.113347 [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- 6.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. 10.1016/S0140-6736(09)61999-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kluytmans-Vandenbergh MF, Kluytmans JA. 2006. Community-acquired methicillin-resistant Staphylococcus aureus: current perspectives. Clin. Microbiol. Infect. 12(Suppl 1):9–15. 10.1111/j.1469-0691.2006.01341.x [DOI] [PubMed] [Google Scholar]
- 8.Uckay I, Bernard L, Buzzi M, Harbarth S, Francois P, Huggler E, Ferry T, Schrenzel J, Renzoni A, Vaudaux P, Lew DP. 2012. High prevalence of isolates with reduced glycopeptide susceptibility in persistent or recurrent bloodstream infections due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 56:1258–1264. 10.1128/AAC.05808-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosowska-Shick K, Ednie LM, McGhee P, Smith K, Todd CD, Wehler A, Appelbaum PC. 2008. Incidence and characteristics of vancomycin nonsusceptible strains of methicillin-resistant Staphylococcus aureus at Hershey Medical Center. Antimicrob. Agents Chemother. 52:4510–4513. 10.1128/AAC.01073-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda S, Hanaki H, Yanagisawa C, Ikeda-Dantsuji Y, Matsui H, Iwatsuki M, Shiomi K, Nakae T, Sunakawa K, Omura S. 2010. Identification of the active component that induces vancomycin resistance in MRSA. J. Antibiot. (Tokyo) 63:533–538. 10.1038/ja.2010.75 [DOI] [PubMed] [Google Scholar]
- 11.Jones T. 2002. StaphVAX (Nabi). Curr. Opin. Investig. Drugs 3:48–50 [PubMed] [Google Scholar]
- 12.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, Muenz L, Fuller S, Johnson J, Fireman B, Alcorn H, Naso R. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346:491–496. 10.1056/NEJMoa011297 [DOI] [PubMed] [Google Scholar]
- 13.Josefsson E, Hartford O, O'Brien L, Patti JM, Foster T. 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 184:1572–1580. 10.1086/324430 [DOI] [PubMed] [Google Scholar]
- 14.Josefsson E, Higgins J, Foster TJ, Tarkowski A. 2008. Fibrinogen binding sites P336 and Y338 of clumping factor A are crucial for Staphylococcus aureus virulence. PLoS One 3:e2206. 10.1371/journal.pone.0002206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vernachio J, Bayer AS, Le T, Chai YL, Prater B, Schneider A, Ames B, Syribeys P, Robbins J, Patti JM, Vernachio J, Bayer AS, Le T, Chai Y-L, Prater B, Schneider A, Ames B, Syribeys P, Robbins J, Patti JM. 2003. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob. Agents Chemother. 47:3400–3406. 10.1128/AAC.47.11.3400-3406.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menzies BE, Kernodle DS. 1994. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect. Immun. 62:1843–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J. Infect. Dis. 202:1050–1058. 10.1086/656043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauch S, DeDent AC, Kim HK, Bubeck Wardenburg J, Missiakas DM, Schneewind O. 2012. Abscess formation and alpha-hemolysin induced toxicity in a mouse model of Staphylococcus aureus peritoneal infection. Infect. Immun. 80:3721–3732. 10.1128/IAI.00442-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bubeck Wardenburg J, Schneewind O. 2008. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 205:287–294. 10.1084/jem.20072208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otto M. 2010. Novel targeted immunotherapy approaches for staphylococcal infection. Expert Opin. Biol. Ther. 10:1049–1059. 10.1517/14712598.2010.495115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brady RA, O'May GA, Leid JG, Prior ML, Costerton JW, Shirtliff ME. 2011. Resolution of Staphylococcus aureus biofilm infection using vaccination and antibiotic treatment. Infect. Immun. 79:1797–1803. 10.1128/IAI.00451-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins P, Morton DB, Burman O, Dennison N, Honess P, Jennings M, Lane S, Middleton V, Roughan JV, Wells S, Westwood K. 2011. A guide to defining and implementing protocols for the welfare assessment of laboratory animals: eleventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Anim. 45:1–13. 10.1258/la.2010.010031 [DOI] [PubMed] [Google Scholar]
- 23.Spagnolo N, Greco F, Rossi A, Ciolli L, Teti A, Posteraro P. 1993. Chronic staphylococcal osteomyelitis: a new experimental rat model. Infect. Immun. 61:5225–5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martineau F, Picard FJ, Roy PH, Ouellette M, Bergeron MG. 1998. Species-specific and ubiquitous DNA-based assays for rapid identification of Staphylococcus aureus. J. Clin. Microbiol. 36:618–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JC, Liu MJ, Parsonnet J, Arbeit RD. 1990. Expression of type-8 capsular polysaccharide and production of toxic shock syndrome toxin-1 are associated among vaginal isolates of Staphylococcus aureus. J. Clin. Microbiol. 28:2612–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaffer AC, Lee JC. 2008. Vaccination and passive immunisation against Staphylococcus aureus. Int. J. Antimicrob. Agents 32(Suppl 1):S71–S78. 10.1016/j.ijantimicag.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 27.Daum RS, Spellberg B. 2012. Progress toward a Staphylococcus aureus vaccine. Clin. Infect. Dis. 54:560–567. 10.1093/cid/cir828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, Chan IS, McNeely TB, Kartsonis NA, Guris D, Onorato MT, Smugar SS, DiNubile MJ, Sobanjo-ter Meulen A. 2013. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309:1368–1378. 10.1001/jama.2013.3010 [DOI] [PubMed] [Google Scholar]
- 29.Tuchscherr L, Loffler B, Buzzola FR, Sordelli DO. 2010. Staphylococcus aureus adaptation to the host and persistence: role of loss of capsular polysaccharide expression. Future Microbiol. 5:1823–1832. 10.2217/fmb.10.147 [DOI] [PubMed] [Google Scholar]
- 30.Verkaik NJ, van Wamel WJ, van Belkum A. 2011. Immunotherapeutic approaches against Staphylococcus aureus. Immunotherapy 3:1063–1073. 10.2217/imt.11.84 [DOI] [PubMed] [Google Scholar]
- 31.Fattom A, Schneerson R, Watson DC, Karakawa WW, Fitzgerald D, Pastan I, Li X, Shiloach J, Bryla DA, Robbins JB. 1993. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect. Immun. 61:1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert FB, Poutrel B, Sutra L. 1994. Immunogenicity in cows of Staphylococcus aureus type 5 capsular polysaccharide-ovalbumin conjugate. Vaccine 12:369–374. 10.1016/0264-410X(94)90103-1 [DOI] [PubMed] [Google Scholar]
- 33.Reynaud-Rondier L, Voiland A, Michel G. 1991. Conjugation of capsular polysaccharide to alpha-haemolysin from Staphylococcus aureus as a glycoprotein antigen. FEMS Microbiol. Immunol. 3:193–199 [DOI] [PubMed] [Google Scholar]
- 34.Fattom A, Fuller S, Propst M, Winston S, Muenz L, He D, Naso R, Horwith G. 2004. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine 23:656–663. 10.1016/j.vaccine.2004.06.043 [DOI] [PubMed] [Google Scholar]
- 35.Shinefield HR. 2006. Use of a conjugate polysaccharide vaccine in the prevention of invasive staphylococcal disease: is an additional vaccine needed or possible? Vaccine 24(Suppl 2):65–69. 10.1016/j.vaccine.2005.01.126 [DOI] [PubMed] [Google Scholar]
- 36.Anderson AS, Miller AA, Donald RG, Scully IL, Nanra JS, Cooper D, Jansen KU. 2012. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum. Vaccin. Immunother. 8:1585–1594. 10.4161/hv.21872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HK, Kim HY, Schneewind O, Missiakas D. 2011. Identifying protective antigens of Staphylococcus aureus, a pathogen that suppresses host immune responses. FASEB J. 25:3605–3612. 10.1096/fj.11-187963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patti JM. 2011. Will we ever see the approval of a Staphylococcus aureus vaccine? Expert Rev. Anti Infect. Ther. 9:845–846. 10.1586/eri.11.99 [DOI] [PubMed] [Google Scholar]
- 39.McDevitt D, Francois P, Vaudaux P, Foster TJ. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237–248. 10.1111/j.1365-2958.1994.tb00304.x [DOI] [PubMed] [Google Scholar]
- 40.Murphy E, Lin SL, Nunez L, Andrew L, Fink PS, Dilts DA, Hoiseth SK, Jansen KU, Anderson AS. 2011. Challenges for the evaluation of Staphylococcus aureus protein based vaccines: monitoring antigenic diversity. Hum. Vaccin. 7(Suppl):51–59. 10.4161/hv.7.0.14562 [DOI] [PubMed] [Google Scholar]
- 41.Narita K, Hu DL, Mori F, Wakabayashi K, Iwakura Y, Nakane A. 2010. Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect. Immun. 78:4234–4242. 10.1128/IAI.00447-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeJonge M, Burchfield D, Bloom B, Duenas M, Walker W, Polak M, Jung E, Millard D, Schelonka R, Eyal F, Morris A, Kapik B, Roberson D, Kesler K, Patti J, Hetherington S. 2007. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J. Pediatr. 151:260–265. 10.1016/j.jpeds.2007.04.060 [DOI] [PubMed] [Google Scholar]
- 43.Hawkins J, Kodali S, Matsuka YV, McNeil LK, Mininni T, Scully IL, Vernachio JH, Severina E, Girgenti D, Jansen KU, Anderson AS, Donald RG. 2012. A recombinant clumping factor A-containing vaccine induces functional antibodies to Staphylococcus aureus that are not observed after natural exposure. Clin. Vaccine Immunol. 19:1641–1650. 10.1128/CVI.00354-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuchscherr LP, Buzzola FR, Alvarez LP, Lee JC, Sordelli DO. 2008. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice. Infect. Immun. 76:5738–5744. 10.1128/IAI.00874-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adlam C, Ward PD, McCartney AC, Arbuthnott JP, Thorley CM. 1977. Effect of immunization with highly purified alpha- and beta-toxins on staphylococcal mastitis in rabbits. Infect. Immun. 17:250–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hruz P, Zinkernagel AS, Jenikova G, Botwin GJ, Hugot JP, Karin M, Nizet V, Eckmann L. 2009. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc. Natl. Acad. Sci. U. S. A. 106:12873–12878. 10.1073/pnas.0904958106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proctor RA. 2012. Challenges for a universal Staphylococcus aureus vaccine. Clin. Infect. Dis. 54:1179–1186. 10.1093/cid/cis033 [DOI] [PubMed] [Google Scholar]
- 48.Frank KM, Zhou T, Moreno-Vinasco L, Hollett B, Garcia JG, Bubeck Wardenburg J. 2012. Host response signature to Staphylococcus aureus alpha-hemolysin implicates pulmonary Th17 response. Infect. Immun. 80:3161–3169. 10.1128/IAI.00191-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niebuhr M, Gathmann M, Scharonow H, Mamerow D, Mommert S, Balaji H, Werfel T. 2011. Staphylococcal alpha-toxin is a strong inducer of interleukin-17 in humans. Infect. Immun. 79:1615–1622. 10.1128/IAI.00958-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hackam DG, Redelmeier DA. 2006. Translation of research evidence from animals to humans. JAMA 296:1731–1732. 10.1001/jama.296.14.1731 [DOI] [PubMed] [Google Scholar]
- 51.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U. S. A. 110:3507–3512. 10.1073/pnas.1222878110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295–305. 10.1038/nrmicro1384 [DOI] [PubMed] [Google Scholar]
- 53.Tuchscherr L, Heitmann V, Hussain M, Viemann D, Roth J, von Eiff C, Peters G, Becker K, Loffler B. 2010. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J. Infect. Dis. 202:1031–1040. 10.1086/656047 [DOI] [PubMed] [Google Scholar]
- 54.Kahl BC, Mellmann A, Deiwick S, Peters G, Harmsen D. 2005. Variation of the polymorphic region X of the protein A gene during persistent airway infection of cystic fibrosis patients reflects two independent mechanisms of genetic change in Staphylococcus aureus. J. Clin. Microbiol. 43:502–505. 10.1128/JCM.43.1.502-505.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]