Abstract
This communication describes ultrastructural variations among synaptosomal fractions isolated from the corpus striatum of the rat by incomplete equilibrium sedimentation in sucrose density gradients, and attempts to relate the variations to neurotransmitter-specific synaptosomes. The concentration of synaptosomes in each fraction of the density gradient was found to be correlated with the concentration of potassium, a marker for cytoplasm occluded within synaptosomes. Monoamine oxidase activity was found to be correlated with the incidence of free mitochondria in the gradients.
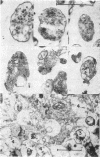
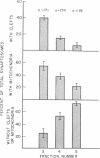
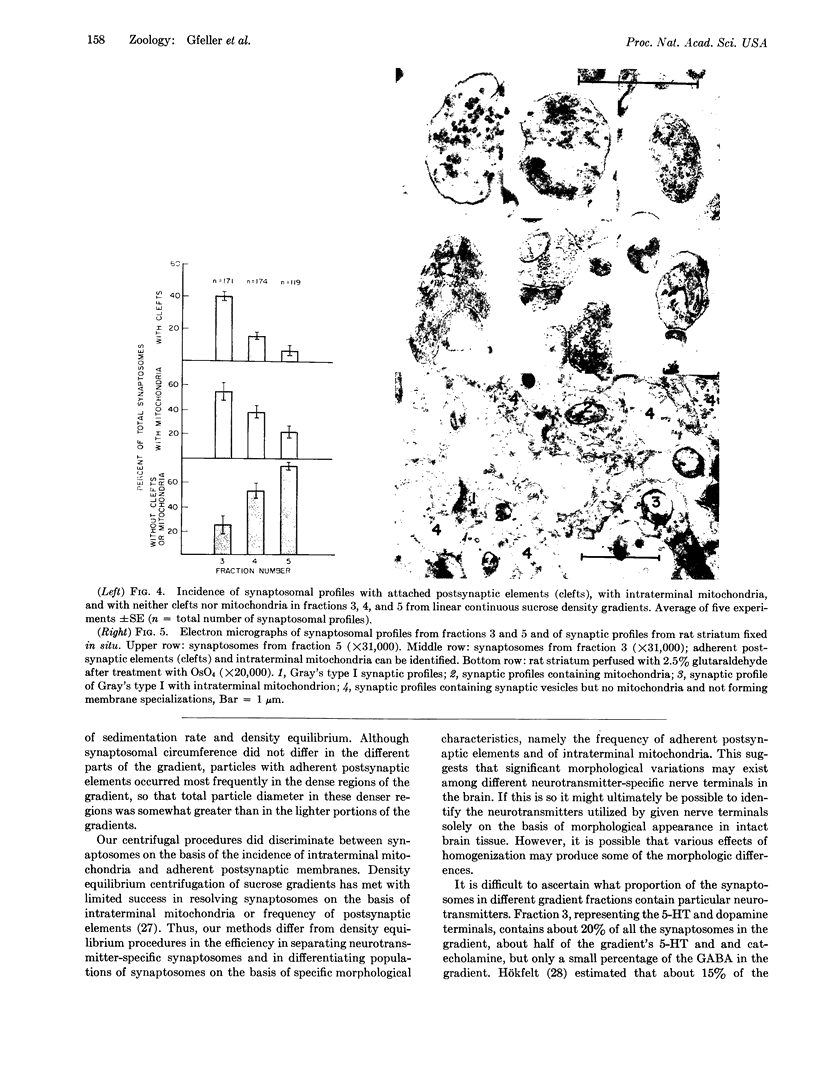
Synaptosomes from denser gradient fractions showed a markedly increased frequency of adherent postsynaptic elements and intraterminal mitochondria. These denser gradient fractions were rich in synaptosomes containing norepinephrine, dopamine, serotonin, and acetylcholine, while synaptosomes in lighter portions of the gradients were rich in γ-aminobutyric acid and other amino acids. These data suggest that significant morphological variations may exist among different neurotransmitter-specific nerve terminals in the brain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn K. J., French P. C., Merrills R. J. 5-hydroxytryptamine uptake by rat brain in vitro. Life Sci. 1967 Aug 1;6(15):1653–1663. doi: 10.1016/0024-3205(67)90176-2. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. The chemical excitation of spinal neurones by certain acidic amino acids. J Physiol. 1960 Mar;150:656–682. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C., Brown D. H., Harrell B. W., Anderson N. G. Analytical differential centrifugation: an analysis of the sedimentation properties of synaptosomes, mitochondria and lysosomes from rat brain homogenates. Arch Biochem Biophys. 1970 Feb;136(2):436–447. doi: 10.1016/0003-9861(70)90215-8. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Snyder S. H. Catecholamine uptake by synaptosomes in homogenates of rat brain: stereospecificity in different areas. J Pharmacol Exp Ther. 1969 Dec;170(2):221–231. [PubMed] [Google Scholar]
- De Robertis E. Ultrastructure and cytochemistry of the synaptic region. The macromolecular components involved in nerve transmission are being studied. Science. 1967 May 19;156(3777):907–914. doi: 10.1126/science.156.3777.907. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Green A. I., Snyder S. H., Iversen L. L. Separation of catecholamine-storing synaptosomes in different regions of rat brain. J Pharmacol Exp Ther. 1969 Aug;168(2):264–271. [PubMed] [Google Scholar]
- Hökfelt T. In vitro studies on central and peripheral monoamine neurons at the ultrastructural level. Z Zellforsch Mikrosk Anat. 1968;91(1):1–74. doi: 10.1007/BF00336984. [DOI] [PubMed] [Google Scholar]
- Israël M., Whittaker V. P. The isolation of mossy fibre endings from the granular layer of the cerebellar cortex. Experientia. 1965 Jun 15;21(6):325–326. doi: 10.1007/BF02144693. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Neal M. J. The uptake of [3H]GABA by slices of rat cerebral cortex. J Neurochem. 1968 Oct;15(10):1141–1149. doi: 10.1111/j.1471-4159.1968.tb06831.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Snyder S. H. Synaptosomes: different populations storing catecholamines and gamma-aminobutyric acid in homogenates of rat brain. Nature. 1968 Nov 23;220(5169):796–798. doi: 10.1038/220796a0. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., De Robertis E. Histamine in isolated small nerve endings and synaptic vesicles of rat brain cortex. J Pharmacol Exp Ther. 1967 Apr;156(1):114–125. [PubMed] [Google Scholar]
- Kuhar M. J., Green A. I., Snyder S. H., Gfeller E. Separation of synaptosomes storing catecholamines and gamma-aminobutyric acid in rat corpus striatum. Brain Res. 1970 Jul 29;21(3):405–417. doi: 10.1016/0006-8993(70)90420-8. [DOI] [PubMed] [Google Scholar]
- Kuriyama K., Roberts E., Vos J. Some characteristics of binding of gamma-aminobutyric acid and acetylcholine to a synaptic vesicle fraction form mouse brain. Brain Res. 1968 Jul;9(2):231–252. doi: 10.1016/0006-8993(68)90232-1. [DOI] [PubMed] [Google Scholar]
- MICHAELSON I. A., WHITTAKER V. P. The subcellular localization of 5-hydroxytryptamine in guinea pig brain. Biochem Pharmacol. 1963 Feb;12:203–211. doi: 10.1016/0006-2952(63)90185-0. [DOI] [PubMed] [Google Scholar]
- Neal M. J., Iversen L. L. Subcellular distribution of endogenous and (3H) gamma-aminobutyric acid in rat cerebral cortex. J Neurochem. 1969 Aug;16(8):1245–1252. doi: 10.1111/j.1471-4159.1969.tb05972.x. [DOI] [PubMed] [Google Scholar]
- Roberts E., Kuriyama K. Biochemical-physiological correlations in studies of the gamma-aminobutyric acid system. Brain Res. 1968 Apr;8(1):1–35. doi: 10.1016/0006-8993(68)90170-4. [DOI] [PubMed] [Google Scholar]
- Shaskan E. G., Snyder S. H. Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther. 1970 Nov;175(2):404–418. [PubMed] [Google Scholar]
- Snyder S. H., Green A. I., Hendley E. D. Kinetics of H3-norepinephrine accumulation into slices from different regions of the rat brain. J Pharmacol Exp Ther. 1968 Nov;164(1):90–102. [PubMed] [Google Scholar]
- Snyder S. H., Green A., Hendley E. D., Gfeller E. Noradrenaline: kinetics of accumulation into slices from different regions of rat brain. Nature. 1968 Apr 13;218(5137):174–176. doi: 10.1038/218174a0. [DOI] [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P. The morphology of fractions of rat forebrain synaptosomes separated on continuous sucrose density gradients. Biochem J. 1968 Jan;106(2):412–417. doi: 10.1042/bj1060412. [DOI] [PMC free article] [PubMed] [Google Scholar]