Abstract
Herein we report an important role for the ferric uptake regulator (Fur) in the resistance of Salmonella enterica serovar Typhimurium to the reactive nitrogen species produced by inducible nitric oxide (NO) synthase in an NRAMP1r murine model of acute systemic infection. The expression of fur protected Salmonella grown under normoxic and hypoxic conditions against the bacteriostatic activity of NO. The hypersusceptibility of fur-deficient Salmonella to the cytotoxic actions of NO coincides with a marked repression of respiratory activity and the reduced ability of the bacteria to detoxify NO. A fur mutant Salmonella strain contained reduced levels of the terminal quinol oxidases of the electron transport chain. Addition of the heme precursor δ-aminolevulinic acid restored the cytochrome content, respiratory activity, NO consumption, and wild-type growth in bacteria undergoing nitrosative stress. The innate antinitrosative defenses regulated by Fur added to the adaptive response associated with the NO-detoxifying activity of the flavohemoprotein Hmp. Our investigations indicate that, in addition to playing a critical role in iron homeostasis, Fur is an important antinitrosative determinant of Salmonella pathogenesis.
INTRODUCTION
Salmonellosis encompasses a spectrum of clinical diseases ranging from typhoid fever, a serious condition that kills about 600,000 people annually (http://www.who.int/inf-pr-1998/en/pr98-18.html), to nontyphoidal zoonotic infections that afflict more than 1.4 million Americans a year (http://www.who.int/mediacentre/factsheets/fs139/en/). The parasitic relationship between Salmonella and host mononuclear phagocytes is one of the hallmarks of both typhoidal and nontyphoidal salmonellosis (1). Within macrophages, Salmonella is sequentially exposed to the antimicrobial activity of the NADPH oxidase and inducible nitric oxide (NO) synthase (iNOS) flavohemoproteins (2). Following the initial oxidative burst emanating from the NADPH oxidase (2), reactive nitrogen species derived from iNOS-expressing human (3–5) and murine (2, 6) macrophages exert sustained bacteriostatic activity at later stages of the intracellular Salmonella infection.
The coordinated actions of low-molecular-weight thiols and superoxide dismutases detoxify a variety of reactive nitrogen species produced from the reaction of NO with either oxygen (O2) or superoxide anion (7–9). These innate detoxification systems are strengthened upon the induction of Hmp, a flavohemoprotein that denitrosylates NO to nitrate (NO3−) (10), thereby protecting Salmonella against NO congeners produced by human and murine macrophages (3, 11, 12). Overall, the enzymatic activity of Hmp appears to be the most important antinitrosative defense in Salmonella pathogenesis (11). Salmonella can also diminish the extent of nitrosative stress endured in macrophages through the expression of the Salmonella pathogenicity island 2 type III secretion system that excludes iNOS-containing vesicles from phagosomes (13). Despite these protective mechanisms, reactive nitrogen species must damage Salmonella biomolecules, as demonstrated by the fact that DNA damage repair systems contribute to the antinitrosative arsenal of this enteric pathogen (14). Many of these antinitrosative defenses are carefully orchestrated at the transcriptional level. The proteins NsrR and NorR are NO-dedicated sensors that regulate genes encoding antinitrosative activity in aerobic and anaerobic bacteria (15, 16), whereas OxyR, SoxR, FNR, DksA, and Fur, which primarily serve as sensors of reactive oxygen species, O2, nutritional starvation, and iron, contribute important aspects of the antinitrosative arsenal of enteric bacteria (17–21).
Traditionally, the ferric uptake regulator (Fur) has been described to be a major determinant in the control of iron homeostasis. Binding of Fe2+ Fur to −35 and −10 promoter regions represses transcription by interfering with the RNA polymerase, whereas binding to upstream regions activates transcription (22). In addition to serving as a cofactor that modulates Fur activity in response to intracytoplasmic metal concentrations, the iron cofactor is a sensor of nitrosative stress, as indicated by the fact that iron-nitrosyl Fur complexes derepress gene transcription (18). The ability of Fur to sense nitrosative stress appears to be important for the antinitrosative defenses of Bacillus subtilis, Staphylococcus aureus, Helicobacter pylori, and Escherichia coli (23–26). Because Fur has recently been shown to play a critical role in Salmonella virulence (27), we tested whether Fur regulates antinitrosative defenses in Salmonella pathogenesis.
MATERIALS AND METHODS
Bacterial strains.
Salmonella enterica serovar Typhimurium strain ATCC 14028s was used as the wild type and as a background for the construction of mutants (Table 1). The hmp, hmp::km, and fur::bla mutants used in the course of our investigations have been described earlier (6, 28). The mutations were confirmed by PCR analysis. A Δhmp::km mutation was moved from strain AV13110 into S. Typhimurium strain KLM001 by P22-mediated transduction to generate strain AV10274. Pseudolysogens were eliminated by streaking on Evans blue uranine agar plates.
TABLE 1.
Bacterial strains
| S. enterica serovar Typhimurium strain | Relevant characteristic | Source or reference |
|---|---|---|
| 14028s | Wild type | ATCC |
| AV13110 | Δhmp::km | 6 |
| AV0468 | Δhmp::FRTa | 6 |
| KLM001 | Δfur::bla | 28 |
| AV10274 | Δfur::bla Δhmp::km | This study |
FRT, FLP recombination target.
Cytochrome spectroscopy.
Wild-type and fur mutant Salmonella strains grown overnight in LB broth were subcultured in fresh LB broth to an optical density at 600 nm (OD600) of 0.5. Where indicated, some of the cultures were supplemented with 100 μM δ-aminolevulinic acid (δ-ALA). Inner membranes were prepared as described by Husain et al. (29). Briefly, bacterial pellets were resuspended in 10 mM EDTA, 100 mM Tris HCl buffer, pH 8.5. The bacteria were lysed by passing the cell suspension through a French press cell disruptor (Thermo Electron Corporation, Milford, MA) 3 times at 18,000 lb/in2 at a flow rate of 5 ml/min. Cell debris was removed after centrifugation at 10,000 × g for 20 min. The supernatants were then centrifuged at 200,000 × g for 1 h, and the pellets were solubilized in 75 mM K2HPO4, 150 mM KCl, 5 mM EDTA, and 60 mM N-dodecyl-N,N dimethyl-3-ammonio-1-propane sulfonate buffer, pH 6.4. Supernatants containing inner membranes were collected, and the protein concentration was assayed using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Rockford, IL). The protein concentration was adjusted to 1.5 mg/ml in 75 mM K2HPO4, 150 mM KCl, 5 mM EDTA, 10 mM ascorbate, and 60 mM N-dodecyl-N,N-dimethyl-3-ammonio-1-propane sulfonate buffer, pH 6.4. Spectra were collected by absorbance spectroscopy with a Hewlett-Packard diode array spectrophotometer (model 8452A).
O2 and NO measurements.
Overnight Salmonella cultures diluted 1:100 in EG medium (1.66 mM MgSO4, 9.5 mM citric acid monohydrate, 57 mM K2HPO4, 16.7 mM NaNH3PO4, 0.4% [wt/vol] glucose) were grown to an OD600 of 0.5 in a shaker incubator at 37°C. The cultures were diluted to an OD600 of 0.2 with EG medium and equilibrated in a shaker incubator at 37°C for 3 min before transfer into an air-sealed, multiport measurement chamber equipped with ISO-OXY-2 O2 and ISO-NOP NO probes. The evolution of O2 and NO in the cultures was recorded with an Apollo 4000 free radical analyzer (World Precision Instruments, Inc., Sarasota, FL). Selected cultures were supplemented with 100 μM δ-ALA. The fast inhibition of respiratory activity in response to NO was studied by adding 5 μM proli NONOate [1-(hydroxy-NNO-azoxy)-l-proline] (half-life [t1/2] = 1.8 s at 37°C) to the bacterial cultures when the concentration of O2 in the chamber was ∼130 μM. To assess the ability of the bacteria to adapt to NO, O2 consumption was also studied in cultures treated for 10 min with 50 μM spermine NONOate (t1/2 = 39 min at 37°C). The rates of oxygen consumption supported by bacteria at a cell density of an OD600 of 1 are expressed as μM/s. The concentration of NO in the cultures is expressed as μM.
Bacterial growth kinetics.
The growth kinetics of wild-type and mutant Salmonella were studied using a Bioscreen C growth analyzer (Labsystems, Helsinki, Finland). Salmonella strains grown overnight in LB broth were diluted to 2 × 106 CFU/ml with fresh LB broth. The cultures were treated with 2.5 to 7.5 mM diethylentriamine (DETA) polyamine control or the NO donor DETA NONOate. Two hundred microliters of the bacterial suspension was dispensed into a Bioscreen 100-well microtiter plate. For analysis of growth under a normoxic environment, the microtiter plates were incubated at 37°C for 30 h with continuous shaking. The OD600 of the cultures was recorded every 15 min. To study the effect of δ-ALA on normoxic growth, some of the cultures diluted 1:100 in LB broth were supplemented with 100 μM δ-ALA until they reached an OD600 of 0.5. The cultures were then diluted to 2 × 106 CFU/ml with fresh LB broth, and the bacterial growth was recorded under normoxic conditions as described above. To measure growth under hypoxic conditions, the bacterial cultures in the microtiter plates were overlaid with 50 μl of mineral oil. The cultures were incubated at 37°C for 30 h without shaking, and the bacterial density at 600 nm was recorded every 15 min.
Bacterial virulence in mice.
Eight- to 10-week-old NRAMP1r C3H/HeN mice were bred at the animal facility of the University of Colorado School of Medicine according to the University of Colorado at Denver, Anschutz Medical Campus, Institutional Animal Care and Use Committee guidelines. NRAMP1r C3H/HeN mice were challenged intraperitoneally with 3 × 103 CFU/mouse of wild-type or isogenic fur mutant Salmonella prepared in phosphate-buffered saline from overnight cultures grown in LB broth. To evaluate the role of Fur in the resistance of Salmonella to iNOS-dependent cytotoxicity, the drinking water of selected groups of C3H/HeN mice was supplemented with 500 μg/ml of the specific iNOS inhibitor N6-(1-iminoethyl)-l-lysine, dihydrochloride (L-NIL). The survival of Salmonella-infected mice was recorded over time.
Statistical analysis.
Data are presented as the mean ± standard error of the mean or standard deviation. The statistical significance was calculated with a two-way analysis of variance, followed by a Bonferroni posttest. The statistical significance of the difference in O2 consumption between the control and spermine NONOate-treated cells was calculated using a paired Student's t test. Differences in mouse survival after Salmonella infection were determined by a log-rank Martel-Cox test. Data were considered statistically significant when the P value was <0.05.
RESULTS
Fur contributes to the antinitrosative defenses of Salmonella.
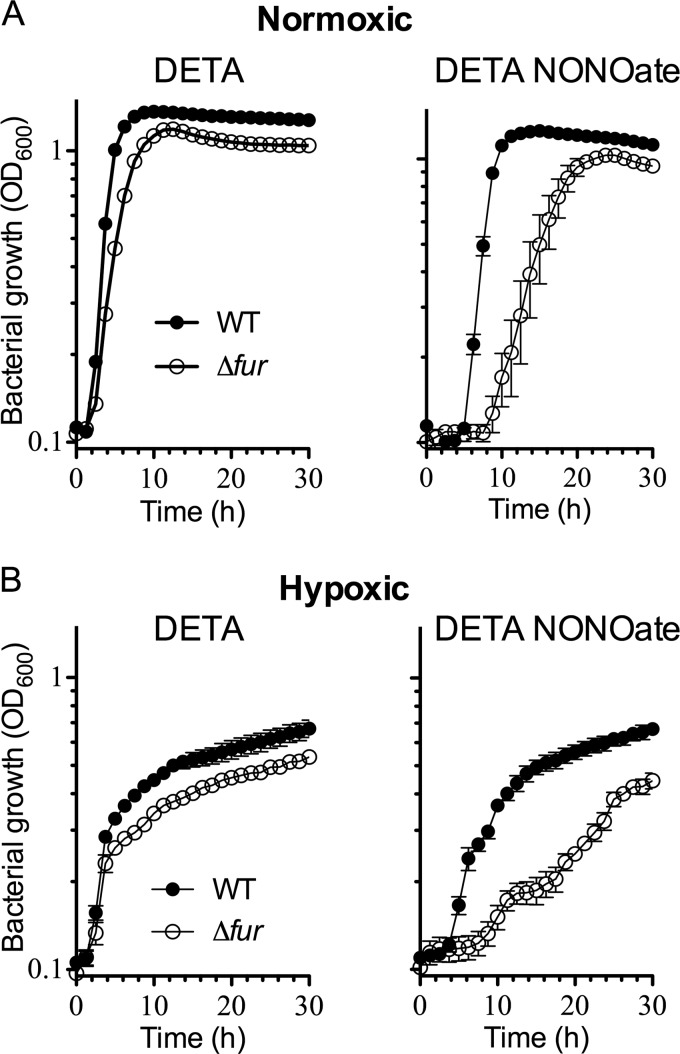
The cytoplasm of E. coli exposed to NO harbors nitrosyl-iron Fur complexes; and fur mutants of E. coli, H. pylori, S. aureus, and B. subtilis are hypersusceptible to the cytotoxicity of this diatomic radical. We therefore tested whether Fur contributes to the antinitrosative armamentarium of Salmonella. Wild-type and fur mutant Salmonella strains grown overnight to stationary phase in LB broth were diluted 1:100 in fresh LB broth. The cultures were treated with 7.5 mM either DETA NONOate or the DETA polyamine base. The fur mutant grew similarly to the isogenic wild-type controls during the first 6 h of culture in LB broth supplemented with DETA but exhibited slightly lower growth rates once the bacterial cells entered late log phase (Fig. 1A). The overall doubling times of wild-type and fur mutant bacteria growing in LB broth were 1.1 and 1.7 h, respectively. The addition of 7.5 mM DETA NONOate, which generates about 7.5 μM NO for the duration of the experiment (30), inhibited the growth of wild-type bacteria. The fur mutant was even more sensitive to the bacteriostatic activity of DETA NONOate, showing an extended lag period compared to wild-type controls. The doubling time of wild-type Salmonella after the initial bacteriostasis imposed upon NO treatment was similar to that of the untreated controls (1.2 versus 1.1 h, respectively). In comparison to the untreated controls, NO significantly (P < 0.001) slowed the doubling time (i.e., 3.9 h) of fur mutant Salmonella. Cumulatively, these data indicate that Salmonella fur mutant strains are hypersusceptible to NO during both the lag and log phases of growth. We also tested the anti-Salmonella activity of NO under O2-limiting conditions (Fig. 1B). To attain a hypoxic environment, 50 μl of sterile mineral oil was overlaid on top of the bacterial cultures. The addition of 7.5 mM DETA NONOate was highly inhibitory for both wild-type and fur mutant bacteria grown under hypoxic conditions. Therefore, we tried lower concentrations of DETA NONOate. It was found that fur mutant bacteria grown under hypoxic conditions were hypersusceptible to 2.5 mM DETA NONOate compared to wild-type Salmonella. Together, these data indicate that Fur contributes to the antinitrosative defenses of Salmonella.
FIG 1.
Effect of NO on bacterial growth. (A) Growth of wild-type (WT) Salmonella and its isogenic fur mutant in LB broth supplemented with either 7.5 mM DETA or DETA NONOate in a 100-well microtiter plate with shaking (normoxic). (B) Bacterial growth was also monitored in standing cultures overlaid with mineral oil after treatment with 2.5 mM DETA or DETA NONOate (hypoxic). The data are representative of those from experiments performed on three independent days with five replicates each day.
Fur protects the respiratory activity of Salmonella undergoing nitrosative stress.
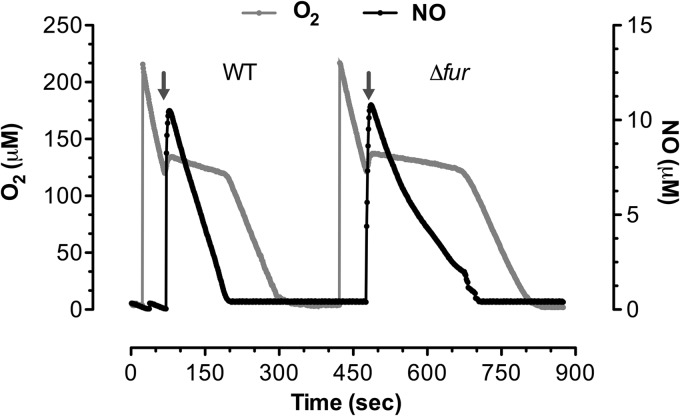
NO nitrosylates a variety of biomolecules, including [4Fe-4S] clusters, redox-active cysteines, organic radicals, and quinol oxidases of the electron transport chain. NO reacts with metal groups in the cytochromes of terminal oxidases of the electron transport chain with kon (association constant) rates of 1.25 × 107 to 2.3 × 108 M−1 s−1 (31), making these biomolecules one of the preferred targets of NO. We therefore tested the respiratory activity of fur mutant bacteria undergoing nitrosative stress (Fig. 2). Under the experimental conditions of our investigations, log-phase wild-type and fur mutant Salmonella bacteria respired at a rate of about 3.5 μM O2/s/OD600 unit. Proli NONOate, which at 37°C and pH 7.4 releases NO with a half-life of 1.8 s, allowed us to deliver a bolus of ∼10 μM NO. We then followed the evolution of NO in the cultures over time. The addition of 5 μM proli NONOate inhibited the respiration of wild-type Salmonella. After 2 min, wild-type Salmonella resumed respiration at rates similar to the ones sustained before exposure to NO. The reestablishment of respiratory activity coincided with the detoxification of NO from the bacterial cultures. In comparison, the respiratory activity of fur mutant Salmonella was inhibited for about 3 min after NO treatment. The longer period of inhibition of respiration in proli NONOate-treated, fur mutant bacteria coincided with a prolonged presence of NO in the cultures (P < 0.05).
FIG 2.
Consumption of O2 by fur mutant Salmonella undergoing nitrosative stress. The consumption of O2 was recorded in Salmonella grown to an OD600 of 0.5 in EG medium. The bacteria were diluted to an OD600 of 0.2 in an air-sealed chamber. Bacterial cultures were treated with 5 μM proli NONOate (arrows) at a time when the concentration of O2 in the medium was about 130 μM. The evolution of NO in the cultures was also recorded. The data are representative of those from three independent experiments performed on three separate days.
Hmp-independent, Fur-dependent antinitrosative defenses.
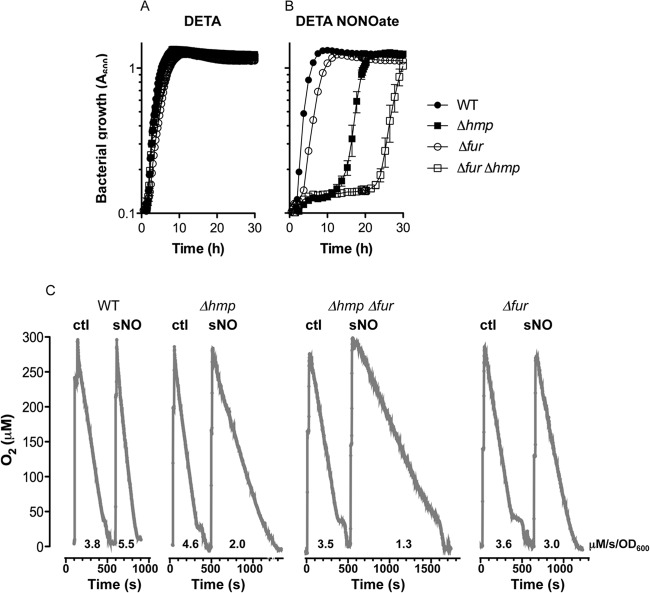
The flavohemoprotein Hmp catalyzes the denitrosylation of NO to NO3−, thereby serving as a primary defense mechanism against the nitrosative stress generated by iNOS (11). The transcription of hmp is derepressed in a fur mutant grown under anaerobic conditions (32), raising the possibility that the sustained inhibition of respiratory activity endured by fur-deficient Salmonella undergoing nitrosative stress is independent of Hmp function. To test this hypothesis, we constructed a double hmp fur mutant strain. Wild-type Salmonella grew in LB broth with kinetics similar to those for the hmp, fur, and fur hmp mutants (Fig. 3A). The addition of 7.5 mM DETA NONOate completely inhibited the growth of the hmp mutant for the 30 h tested. Therefore, 1 mM DETA NONOate was used instead. The hmp mutant was significantly more susceptible than the fur-deficient strain to the bacteriostasis of 1 mM DETA NONOate (Fig. 3B), indicating that the flavohemoprotein Hmp contributes to the antinitrosative defenses of Salmonella more than Fur. Interestingly, the fur hmp double mutant was even more susceptible than single fur or hmp mutant strains, supporting our hypothesis that Hmp and Fur independently contribute to the antinitrosative defenses of Salmonella.
FIG 3.
Contribution of Hmp and Fur to the antinitrosative defenses of Salmonella. Growth of stationary-phase Salmonella diluted to 2 × 106 CFU/ml in LB medium over time. The bacterial cultures were treated with either 1 mM DETA (A) and DETA NONOate (B). (C) O2 consumption in log-phase Salmonella grown in EG medium was monitored 10 min after the addition of 50 μM the spermine control (ctl) or 50 μM the NO donor spermine NONOate (sNO). The data are representative of those from three independent experiments performed on three separate days. The O2 consumption rates supported by bacterial cells at a cell density of OD600 of 1 are expressed as μM/s.
Hmp protects the respiratory activity of terminal cytochromes of the electron transport chain (33). Therefore, the contribution of Fur and Hmp to Salmonella's antinitrosative defenses was independently tested by monitoring the respiratory activity of log-phase bacteria after spermine NONOate treatment. The bacteria were exposed to 50 μM spermine or spermine NONOate. This donor was chosen because its 39-min half-life facilitates the long-term release of NO, thereby allowing the expression of inducible antinitrosative determinants. After 10 min of treatment, the bacterial cultures were transferred to an air-sealed, multiport measurement chamber and the evolution of O2 in the cultures was measured using an Apollo 4000 free radical analyzer. The respiratory activity of wild-type bacteria was not inhibited (P > 0.05) after treatment with 50 μM spermine NONOate (i.e., 3.8 versus 5.5 μM O2/s/OD600 unit for spermine and spermine NONOate, respectively). In contrast, the respiratory activity of log-phase hmp-deficient Salmonella was still inhibited 10 min after treatment with spermine NONOate (i.e., 4.6 versus 2.0 μM O2/s/OD600 unit for spermine and spermine NONOate, respectively). The respiration of the fur mutant was not affected (P > 0.05) 10 min after the addition of spermine NONOate (3.6 versus 3.0 μM O2/s/OD600 unit for spermine or spermine NONOate, respectively), suggesting that fur mutant Salmonella can adapt to prolonged exposure to NO. Remarkably, a lack of both fur and hmp further reduced the respiratory activity of Salmonella treated with 50 μM spermine NONOate (i.e., 1.3 μM O2/s/OD600 unit). Cumulatively, these findings indicate that Hmp- and Fur-regulated targets can independently protect respiration against the inhibitory actions of the diatomic radical NO.
Cytochrome content in Fur-deficient Salmonella and susceptibility to nitrosative stress.
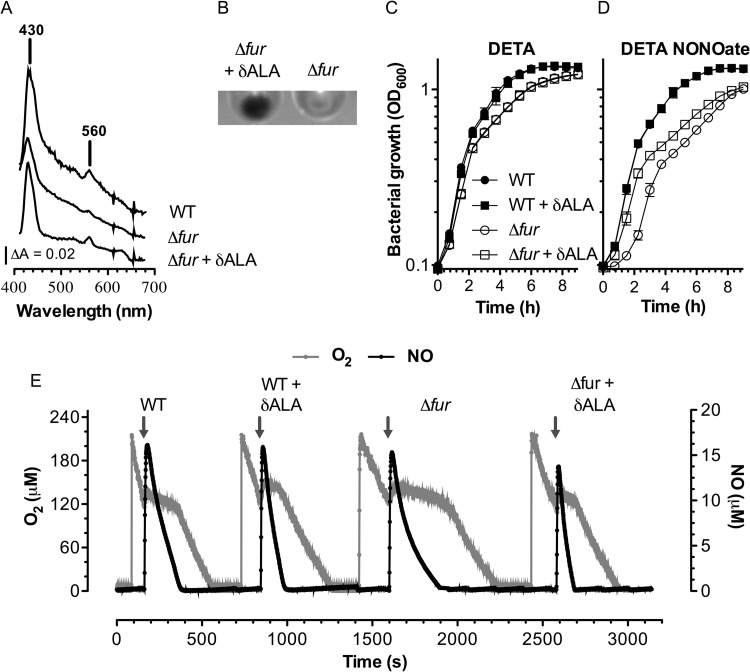
UV-visible spectroscopic analysis of cytoplasmic membranes revealed that Fur-deficient Salmonella strains have decreased absorbance peaks at the 430-nm Soret and 560-nm visible regions of the spectrum (Fig. 4A), indicating that Fur regulates the expression of cytochromes in Salmonella. The addition of δ-ALA, which is an intermediate of heme biosynthesis, restored the 430- and 560-nm absorption peaks in fur mutant Salmonella. In agreement with the spectroscopy data, membranes isolated from fur mutant bacteria grown in LB broth containing 100 μM δ-ALA yielded a pellet with a brown stain that is consistent with an increase in cytochrome content (Fig. 4B). We tested whether the addition of δ-ALA could enhance the resistance of fur mutant bacteria to the antimicrobial activity of 2 mM DETA NONOate. The bacteria grew in the presence of 100 μM δ-ALA; however, the addition of 2 mM DETA NONOate to LB broth containing δ-ALA completely inhibited the growth of both wild-type and fur mutant bacteria, which might reflect the toxicity associated with high levels of heme (34). Therefore, to test the effect of δ-ALA on the susceptibility of Salmonella to DETA NONOate, the bacteria were grown to an OD600 of 0.5 in LB broth supplemented with 100 μM δ-ALA. The cells were then washed and exposed to 2 mM DETA or DETA NONOate in fresh LB broth in the absence of δ-ALA. Log-phase wild-type Salmonella bacteria were highly resistant to the bacteriostatic activity of 2 mM DETA NONOate (Fig. 4C and D). In contrast, fur mutant bacteria exposed to 2 mM DETA NONOate were susceptible to NO. The fur mutant bacteria that had been grown in the presence of 100 μM δ-ALA were as resistant to NO as the wild-type controls during the first 2 h of challenge but became susceptible thereafter. The transient protective effects may be explained by the dwindling δ-ALA concentrations as fur mutant bacteria grew in fresh LB broth. The addition of 5 μM proli NONOate more profoundly inhibited the consumption of O2 in fur mutant Salmonella than in the wild-type controls (Fig. 4E). Wild-type Salmonella growth in EG medium supplemented with 100 μM δ-ALA detoxified NO more efficiently than control growth in EG medium, an activity that coincided with a shortened inhibition of respiration. Remarkably, the robust repression of respiratory activity noted in NO-treated fur-deficient Salmonella was prevented by growing the bacteria in EG medium containing 100 μM δ-ALA. Together, these findings suggest that the reduced cytochrome content of fur mutant bacteria decreases antinitrosative defenses.
FIG 4.
Effect of δ-ALA supplementation on the cytochrome content, growth, and O2 consumption. (A) UV/visible difference absorbance (ΔA) spectra were recorded in Salmonella grown in LB broth with or without 100 μM δ-ALA. The absorption band at 560 and the Soret peak at 430 nm correspond to the fully reduced cytochrome b. (B) Inner membrane preparations from fur mutant Salmonella grown with or without δ-ALA. (C, D) Effect of 100 μM δ-ALA on the growth of log-phase wild-type and fur mutant Salmonella in the presence of 2 mM DETA (C) or DETA NONOate (D). Where indicated, the Salmonella strains for which the results are presented in panels C and D were grown to log phase in LB broth supplemented with 100 μM δ-ALA. The cells were then washed and exposed to NO in fresh LB broth. (E) Effect of 100 μM δ-ALA on the respiratory activity of fur mutant Salmonella grown to log phase in EG medium. Selected cultures were treated with 5 μM proli NONOate (arrows) when the concentration of O2 in the cultures reached about 130 μM.
Fur contributes to Salmonella virulence by protecting against NO-dependent host defenses.
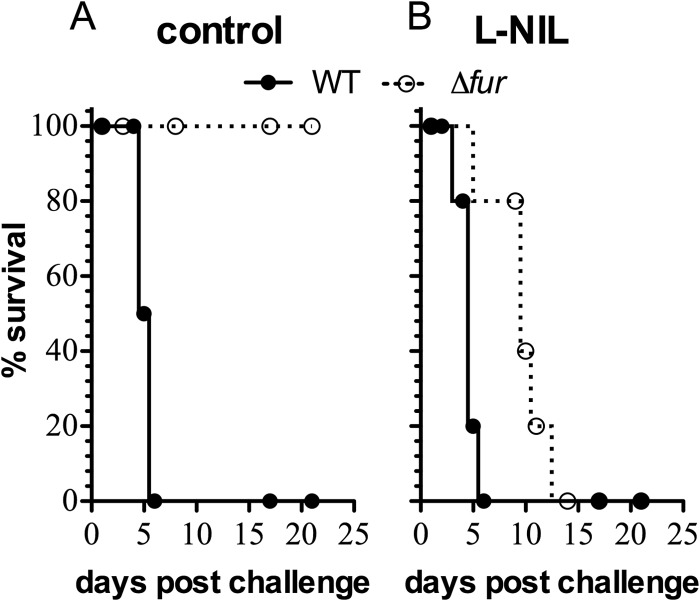
The results of the experiments described above demonstrate the importance of Fur in the antinitrosative arsenal of Salmonella. Next, we tested the possibility that Fur-dependent antinitrosative defenses may contribute to Salmonella virulence in a murine model of acute infection. Because the antinitrosative defenses of Salmonella have consistently been better appreciated in mice expressing the wild-type NRAMP1r allele (7, 11, 35), we used C3H/HeN mice in these investigations. C3H/HeN mice were inoculated intraperitoneally with about 3,000 CFU of either wild-type or fur mutant Salmonella. The fur mutant was found to be profoundly attenuated in this acute model of Salmonella infection (Fig. 5A), an observation that is consistent with our previously published data (27). Using this murine model of salmonellosis, we tested whether Fur antagonizes iNOS-dependent anti-Salmonella host defenses. This idea was examined by adding 500 μg/ml of the iNOS-specific inhibitor L-NIL to the drinking water of C3H/HeN mice. As previously noted (35), the addition of L-NIL to drinking water did not increase the virulence of wild-type Salmonella. In contrast, the addition of this iNOS competitive inhibitor to the drinking water of C3H/HeN mice significantly (P < 0.05) increased the virulence of fur-deficient Salmonella (Fig. 5B). These findings indicate that Fur-regulated antinitrosative defenses contribute to the virulence potential of Salmonella. It should be noted, however, that fur mutant Salmonella was slightly more attenuated in L-NIL-treated mice than wild-type isogenic bacteria, indicating that some aspects of the Fur-mediated Salmonella virulence cannot be accounted for by the contribution of this regulator to antinitrosative defenses.
FIG 5.
Effect of iNOS inhibition on the virulence of fur mutant Salmonella. (A) The survival of C3H/HeN mice infected intraperitoneally with wild-type or fur mutant Salmonella strains was studied over time. (B) Some of the animals were treated with 500 μg/ml of the iNOS-inhibitor L-NIL in drinking water. The data are from two independent experiments (n = 5 to 11 mice per group).
DISCUSSION
Fur has been shown to be important in bacterial pathogenesis. The Fur metalloprotein could coordinate virulence through the regulation of metabolic processes, and more specifically, in the case of enteropathogenic bacteria, it could enhance pathogenicity by regulating the acid tolerance response that increases bacterial fitness in the low pH of the stomach (36). Our investigations have identified regulation of antinitrosative defenses as a novel mechanism by which Fur contributes to Salmonella pathogenesis in an acute model of infection.
Our investigations indicate that Fur helps maintain normal cytochrome content in Salmonella. The regulation of cytochrome expression could mediate Salmonella virulence by at least two non-mutually exclusive mechanisms (Fig. 6). First, the proton-translocating activity of terminal cytochromes of the electron transport chain is essential for the production of ATP during aerobic metabolism. Low cytochrome levels in fur mutant bacteria could affect Salmonella virulence because production of ATP is correlated with bacterial cell growth. In this context, cytochromes were recently found to add to the pathogenicity of S. aureus (37). Copper and iron cofactors in quinol cytochrome oxidases are some of the preferred targets of the cytotoxicity of NO (38). Therefore, the extended inhibition of respiration experienced by fur mutant bacteria undergoing nitrosative stress could have a particularly devastating effect on ATP synthesis, cell growth, and Salmonella pathogenesis. Second, lower concentrations of the cytochrome bd oxidase, which is known to detoxify NO (31), might have contributed to the slow detoxification of this diatomic radical in fur mutant Salmonella. The lingering concentrations of NO noted in cultures of fur mutant Salmonella could exert lasting cytotoxicity to critical enzymes, such as ribonucleotide reductase and dihydroxy acid dehydratase (38). Low concentrations of cytochrome bd oxidase could also explain why the doubling time of fur mutant Salmonella undergoing nitrosative stress is less than the one recorded for wild-type controls.
FIG 6.
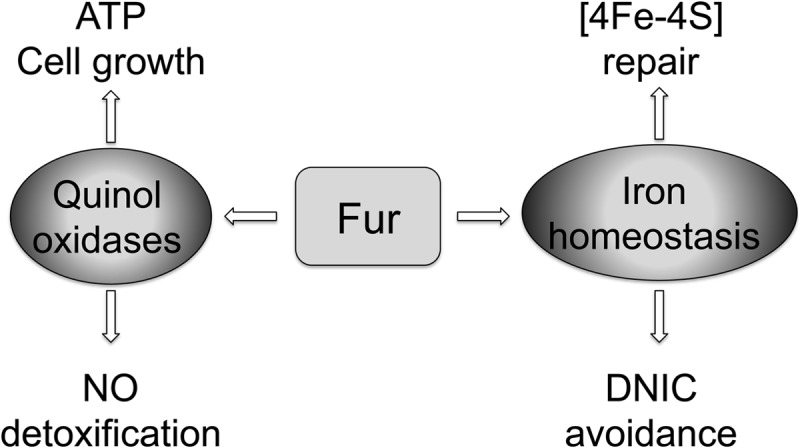
Model for Fur-mediated, innate, antinitrosative defenses. Fur-mediated regulation of terminal quinol oxidases of the electron transport chain can boost the innate antinitrosative defenses of Salmonella by (i) generating the ATP that is necessary for bacterial growth and (ii) directly detoxifying NO. The critical role played by Fur in iron homeostasis may also add to the defense mechanisms against nitrosative stress. For example, Fur-regulated expression of the sufABCDSE system could help repair [4Fe-4S] clusters damaged by NO and its oxidative congeners. Moreover, the Fur-dependent control of the labile iron pool could limit the formation of cytotoxic dinitrosyl-iron complexes (DNIC).
The reduced cytochrome content in fur mutant Salmonella is surprising since the cyoABCDE and cydAB genes encoding cytochrome bo and bd oxidases, respectively, are overexpressed in the absence of this regulatory metalloprotein (27). These observations suggest that the decreased cytochrome content of fur-deficient Salmonella must be explained by defects at steps of their biosynthesis other than the transcriptional regulation of the cyoABCDE and cydAB operons. The addition of δ-ALA to the culture medium not only increased the cytochrome content of fur mutant bacteria to wild-type levels but also enhanced NO detoxification. These findings indicate that Fur must directly or indirectly control a step in the biosynthesis of δ-ALA. This model is consistent with the fact that the supplementation of culture medium with δ-ALA reestablishes wild-type expression levels of the hemoproteins hydroperoxidase I and II in fur mutant bacteria (39). In Salmonella and E. coli, the hemA-encoded δ-aminolevulinic acid synthase generates δ-ALA from glutamate by the C5 pathway (40). Analysis of a lacZY transcriptional fusion revealed similar expression of hemA in wild-type and fur mutant controls (M. Song and A. Vázquez-Torres, unpublished data). Further investigations are needed in order to address how Fur regulates the biosynthesis of heme proteins.
Our investigations suggest that Fur regulates an innate antinitrosative detoxification system. However, fur mutant Salmonella strains respire and consume NO after prolonged exposure to NO, indicating that they are capable of mounting a delayed protective adaptive response against NO. The marked susceptibility of fur hmp mutant bacteria to nitrosative stress indicates that the flavohemoprotein Hmp mediates the adaptive antinitrosative defenses seen in fur mutant bacteria. Our investigations are consistent with a model in which Fur controls an innate mechanism of detoxification of NO (data herein), whereas the induction of the flavohemoprotein Hmp in response to the nitrosylation of the NsrR repressor provides adaptive, long-term, and amplified NO resistance (41). Terminal cytochromes of the electron transport chain are logical suspects for the Fur-regulated innate NO detoxification noted in our investigations, as suggested by previous studies in E. coli (31). Our observations also indicate that Fur- and Hmp-mediated antinitrosative defenses mostly operate independently. In support of this notion, a fur hmp double mutant not only was significantly more susceptible to the bacteriostatic actions of NO, but it also detoxified NO with apparently slower kinetics than fur and hmp single mutant isogenic Salmonella strains. Collectively, our investigations indicate that both innate and adaptive antinitrosative defenses are necessary for the growth of Salmonella in the presence of reactive nitrogen species generated by the inducible NO synthase.
In addition to the regulation of cytochrome expression, Fur could enhance the antinitrosative defenses of Salmonella by other mechanisms as well (Fig. 6). For example, Fur is a positive regulator of the sufABCDSE operon that encodes an enzymatic pathway for the repair of oxidized [4Fe-4S] clusters (42). As mentioned earlier, [4Fe-4S] clusters in dehydratases are some of the preferred targets of NO and some of its oxidative products, such as peroxynitrite (38, 43). Low levels of expression of SufABCDSE in fur mutant bacteria could have devastating effects on the repair of dehydratases, such as aconitase, the nuo-encoded NADH dehydrogenase, glutamate synthetase, and dihydroxy acid dehydratase, which are responsible for key aspects of central metabolism. In addition, deregulated iron homeostasis could play a key role in the hypersusceptibility of fur mutant Salmonella to nitrosative stress. Increases in the cytoplasmic pool of labile iron in fur mutant bacteria could lead to the formation of highly cytotoxic dinitrosyl-iron complexes that, according to work published by Lancaster and collaborators (44), are the leading nitrosating species in the cell.
ACKNOWLEDGMENTS
This research was supported by NIH grant AI54959, U.S. Department of Veterans Affairs grant 1I01BX002073, and the Burroughs Wellcome Fund.
We are grateful to Michael Vasil from the University of Colorado School of Medicine for helpful discussions during the preparation of the manuscript.
Footnotes
Published ahead of print 28 October 2013
REFERENCES
- 1.Fields PI, Swanson RV, Haidaris CG, Heffron F. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. U. S. A. 83:5189–5193. 10.1073/pnas.83.14.5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192:227–236. 10.1084/jem.192.2.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevanin TM, Poole RK, Demoncheaux EA, Read RC. 2002. Flavohemoglobin Hmp protects Salmonella enterica serovar Typhimurium from nitric oxide-related killing by human macrophages. Infect. Immun. 70:4399–4405. 10.1128/IAI.70.8.4399-4405.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paolillo R, Carratelli CR, Rizzo A. 2011. Effect of resveratrol and quercetin in experimental infection by Salmonella enterica serovar Typhimurium. Int. Immunopharmacol. 11:149–156. 10.1016/j.intimp.2010.10.019 [DOI] [PubMed] [Google Scholar]
- 5.Azenabor AA, Kennedy P, York J. 2009. Free intracellular Ca2+ regulates bacterial lipopolysaccharide induction of iNOS in human macrophages. Immunobiology 214:143–152. 10.1016/j.imbio.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 6.McCollister BD, Bourret TJ, Gill R, Jones-Carson J, Vazquez-Torres A. 2005. Repression of SPI2 transcription by nitric oxide-producing, IFNγ-activated macrophages promotes maturation of Salmonella phagosomes. J. Exp. Med. 202:625–635. 10.1084/jem.20050246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song M, Husain M, Jones-Carson J, Liu L, Henard CA, Vazquez-Torres A. 2013. Low-molecular-weight thiol-dependent antioxidant and antinitrosative defences in Salmonella pathogenesis. Mol. Microbiol. 87:609–622. 10.1111/mmi.12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Groote MA, Testerman T, Xu Y, Stauffer G, Fang FC. 1996. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium. Science 272:414–417. 10.1126/science.272.5260.414 [DOI] [PubMed] [Google Scholar]
- 9.Fang FC, DeGroote MA, Foster JW, Baumler AJ, Ochsner U, Testerman T, Bearson S, Giard JC, Xu Y, Campbell G, Laessig T. 1999. Virulent Salmonella typhimurium has two periplasmic Cu, Zn-superoxide dismutases. Proc. Natl. Acad. Sci. U. S. A. 96:7502–7507. 10.1073/pnas.96.13.7502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hausladen A, Gow A, Stamler JS. 2001. Flavohemoglobin denitrosylase catalyzes the reaction of a nitroxyl equivalent with molecular oxygen. Proc. Natl. Acad. Sci. U. S. A. 98:10108–10112. 10.1073/pnas.181199698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bang IS, Liu L, Vazquez-Torres A, Crouch ML, Stamler JS, Fang FC. 2006. Maintenance of nitric oxide and redox homeostasis by the Salmonella flavohemoglobin hmp. J. Biol. Chem. 281:28039–28047. 10.1074/jbc.M605174200 [DOI] [PubMed] [Google Scholar]
- 12.Crawford MJ, Goldberg DE. 1998. Role for the Salmonella flavohemoglobin in protection from nitric oxide. J. Biol. Chem. 273:12543–12547. 10.1074/jbc.273.20.12543 [DOI] [PubMed] [Google Scholar]
- 13.Chakravortty D, Hansen-Wester I, Hensel M. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J. Exp. Med. 195:1155–1166. 10.1084/jem.20011547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson AR, Soliven KC, Castor ME, Barnes PD, Libby SJ, Fang FC. 2009. The base excision repair system of Salmonella enterica serovar Typhimurium counteracts DNA damage by host nitric oxide. PLoS Pathog. 5:e1000451. 10.1371/journal.ppat.1000451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodenmiller DM, Spiro S. 2006. The yjeB (nsrR) gene of Escherichia coli encodes a nitric oxide-sensitive transcriptional regulator. J. Bacteriol. 188:874–881. 10.1128/JB.188.3.874-881.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Autreaux B, Tucker NP, Dixon R, Spiro S. 2005. A non-haem iron centre in the transcription factor NorR senses nitric oxide. Nature 437:769–772. 10.1038/nature03953 [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Ramos H, Crack J, Wu G, Hughes MN, Scott C, Thomson AJ, Green J, Poole RK. 2002. NO sensing by FNR: regulation of the Escherichia coli NO-detoxifying flavohaemoglobin, Hmp. EMBO J. 21:3235–3244. 10.1093/emboj/cdf339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Autreaux B, Touati D, Bersch B, Latour JM, Michaud-Soret I. 2002. Direct inhibition by nitric oxide of the transcriptional ferric uptake regulation protein via nitrosylation of the iron. Proc. Natl. Acad. Sci. U. S. A. 99:16619–16624. 10.1073/pnas.252591299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding H, Demple B. 2000. Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator. Proc. Natl. Acad. Sci. U. S. A. 97:5146–5150. 10.1073/pnas.97.10.5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hausladen A, Privalle CT, Keng T, DeAngelo J, Stamler JS. 1996. Nitrosative stress: activation of the transcription factor OxyR. Cell 86:719–729. 10.1016/S0092-8674(00)80147-6 [DOI] [PubMed] [Google Scholar]
- 21.Henard CA, Vazquez-Torres A. 2012. DksA-dependent resistance of Salmonella enterica serovar Typhimurium against the antimicrobial activity of inducible nitric oxide synthase. Infect. Immun. 80:1373–1380. 10.1128/IAI.06316-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiro S, D'Autreaux B. 2012. Non-heme iron sensors of reactive oxygen and nitrogen species. Antioxid. Redox Signal. 17:1264–1276. 10.1089/ars.2012.4533 [DOI] [PubMed] [Google Scholar]
- 23.Moore CM, Nakano MM, Wang T, Ye RW, Helmann JD. 2004. Response of Bacillus subtilis to nitric oxide and the nitrosating agent sodium nitroprusside. J. Bacteriol. 186:4655–4664. 10.1128/JB.186.14.4655-4664.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson AR, Dunman PM, Fang FC. 2006. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol. Microbiol. 61:927–939. 10.1111/j.1365-2958.2006.05290.x [DOI] [PubMed] [Google Scholar]
- 25.Qu W, Zhou Y, Shao C, Sun Y, Zhang Q, Chen C, Jia J. 2009. Helicobacter pylori proteins response to nitric oxide stress. J. Microbiol. 47:486–493. 10.1007/s12275-008-0266-0 [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhyay P, Zheng M, Bedzyk LA, LaRossa RA, Storz G. 2004. Prominent roles of the NorR and Fur regulators in the Escherichia coli transcriptional response to reactive nitrogen species. Proc. Natl. Acad. Sci. U. S. A. 101:745–750. 10.1073/pnas.0307741100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troxell B, Sikes ML, Fink RC, Vazquez-Torres A, Jones-Carson J, Hassan HM. 2011. Fur negatively regulates hns and is required for the expression of HilA and virulence in Salmonella enterica serovar Typhimurium. J. Bacteriol. 193:497–505. 10.1128/JB.00942-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velayudhan J, Castor M, Richardson A, Main-Hester KL, Fang FC. 2007. The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron-sulphur cluster repair and virulence. Mol. Microbiol. 63:1495–1507. 10.1111/j.1365-2958.2007.05600.x [DOI] [PubMed] [Google Scholar]
- 29.Husain M, Bourret TJ, McCollister BD, Jones-Carson J, Laughlin J, Vazquez-Torres A. 2008. Nitric oxide evokes an adaptive response to oxidative stress by arresting respiration. J. Biol. Chem. 283:7682–7689. 10.1074/jbc.M708845200 [DOI] [PubMed] [Google Scholar]
- 30.Henard CA, Bourret TJ, Song M, Vazquez-Torres A. 2010. Control of redox balance by the stringent response regulatory protein promotes antioxidant defenses of Salmonella. J. Biol. Chem. 285:36785–36793. 10.1074/jbc.M110.160960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason MG, Shepherd M, Nicholls P, Dobbin PS, Dodsworth KS, Poole RK, Cooper CE. 2009. Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nat. Chem. Biol. 5:94–96. 10.1038/nchembio.135 [DOI] [PubMed] [Google Scholar]
- 32.Troxell B, Fink RC, Porwollik S, McClelland M, Hassan HM. 2011. The Fur regulon in anaerobically grown Salmonella enterica sv. Typhimurium: identification of new Fur targets. BMC Microbiol. 11:236. 10.1186/1471-2180-11-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevanin TM, Ioannidis N, Mills CE, Kim SO, Hughes MN, Poole RK. 2000. Flavohemoglobin Hmp affords inducible protection for Escherichia coli respiration, catalyzed by cytochromes bo′ or bd, from nitric oxide. J. Biol. Chem. 275:35868–35875. 10.1074/jbc.M002471200 [DOI] [PubMed] [Google Scholar]
- 34.Anzaldi LL, Skaar EP. 2010. Overcoming the heme paradox: heme toxicity and tolerance in bacterial pathogens. Infect. Immun. 78:4977–4989. 10.1128/IAI.00613-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Husain M, Jones-Carson J, Song M, McCollister BD, Bourret TJ, Vazquez-Torres A. 2010. Redox sensor SsrB Cys203 enhances Salmonella fitness against nitric oxide generated in the host immune response to oral infection. Proc. Natl. Acad. Sci. U. S. A. 107:14396–14401. 10.1073/pnas.1005299107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster JW, Moreno M. 1999. Inducible acid tolerance mechanisms in enteric bacteria. Novartis Found. Symp. 221:55–69 [DOI] [PubMed] [Google Scholar]
- 37.Hammer ND, Reniere ML, Cassat JE, Zhang Y, Hirsch AO, Indriati Hood M, Skaar EP. 2013. Two heme-dependent terminal oxidases power Staphylococcus aureus organ-specific colonization of the vertebrate host. mBio 4(4):e00241–13. 10.1128/mBio.00241-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henard CA, Vazquez-Torres A. 2011. Nitric oxide and Salmonella pathogenesis. Front. Microbiol. 2:84. 10.3389/fmicb.2011.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troxell B. 2010. The role of ferric uptake regulator in regulation of metal homeostasis, metabolism, virulence, and protection against hydrogen peroxide in Salmonella enterica serovar Typhimurium. Ph.D. thesis North Carolina State University, Raleigh, NC [Google Scholar]
- 40.Avissar YJ, Beale SI. 1989. Identification of the enzymatic basis for delta-aminolevulinic acid auxotrophy in a hemA mutant of Escherichia coli. J. Bacteriol. 171:2919–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hausladen A, Gow AJ, Stamler JS. 1998. Nitrosative stress: metabolic pathway involving the flavohemoglobin. Proc. Natl. Acad. Sci. U. S. A. 95:14100–14105. 10.1073/pnas.95.24.14100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nachin L, El Hassouni M, Loiseau L, Expert D, Barras F. 2001. SoxR-dependent response to oxidative stress and virulence of Erwinia chrysanthemi: the key role of SufC, an orphan ABC ATPase. Mol. Microbiol. 39:960–972. 10.1046/j.1365-2958.2001.02288.x [DOI] [PubMed] [Google Scholar]
- 43.Henard CA, Vazquez-Torres A. 2013. Regulation of Salmonella resistance to oxidative and nitrosative stress, p 425–440 In Vasil ML, Darwin AJ. (ed), Regulation of bacterial virulence. ASM Press, Washington, DC [Google Scholar]
- 44.Bosworth CA, Toledo JC, Jr, Zmijewski JW, Li Q, Lancaster JR., Jr 2009. Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 106:4671–4676. 10.1073/pnas.0710416106 [DOI] [PMC free article] [PubMed] [Google Scholar]