Abstract
T cell-independent antibody responses develop rapidly, within 3 to 4 days, and are critical for preventing blood-borne pathogens from evolving into life-threatening infections. The interaction of BAFF, also known as BLyS, with its receptors BAFFR and TACI on B cells is critical for B cell homeostasis and function. Using a synthetic polysaccharide antigen, it has previously been shown that TACI is critical for T cell-independent antibody responses. To examine the role of BAFFR and TACI in T cell-independent antibody responses to an active infection, we utilized the Borrelia hermsii infection system. In this infection system, T cell-independent responses mediated by the B1b cell subset are critical for controlling bacteremia. We found that B1b cells express BAFFR and TACI and that the surface expression of both receptors is upregulated on B1b cells following exposure to whole B. hermsii cells. Surprisingly, we found that TACI−/− mice are not impaired either in specific antibody responses to B. hermsii or in controlling B. hermsii bacteremia. In contrast, TACI-deficient mice immunized with heat-killed type 3 serotype pneumococcus cells are impaired in generating pneumococcal polysaccharide-specific responses and succumb to challenge with live type 3 serotype pneumococcus, indicating that TACI is required for T cell-independent antibody responses to bacterial-associated polysaccharides. Although we have found that TACI is dispensable for controlling B. hermsii infection, mice deficient in BAFFR or BAFF exhibit impairment in B. hermsii-specific IgM responses and clearance of bacteremia. Collectively, these data indicate a disparity in the roles for TACI and BAFFR in primary T cell-independent antibody responses to bacterial pathogens.
INTRODUCTION
Humoral responses are central to protection against a variety of microbial pathogens. Mature B cells that generate protective immunity can be classified as follicular (FOB), marginal zone (MZB), B1a, and B1b cell subsets (1). These subsets are developmentally, phenotypically, and functionally distinct. FOB cells are critical for responding to T cell-dependent antigens, whereas MZB, B1a, and B1b cell subsets play a dominant role in T cell-independent (TI) antibody responses. Previously, it was thought that TI responses were short lived; however, recent studies using several infection systems have revealed that the B1b cell subset is capable of generating antigen-specific, long-lasting protection in a TI fashion (2–5).
TI antibody responses develop significantly more rapidly than T cell-dependent responses, as early as 3 to 4 days following antigen encounter, and are highly protective and, therefore, a critical factor in controlling bacteremia. Borrelia hermsii infection is a well-defined system in which to study bacteremia. B. hermsii is a causative agent of tick-borne relapsing fever, which is endemic to the western United States (6). Experimental infections via needle inoculation in mice have been shown to recapitulate the key pathophysiological characteristics of the human disease (7–14). B. hermsii infection is characterized by recurrent episodes of high-level bacteremia (∼108 bacteria/ml blood), with each wave of bacteremia accompanied by a febrile episode. IgM, a dominant isotype in TI responses, is both necessary and sufficient for the rapid clearance of B. hermsii bacteremia (3, 15–17). In order to evade the adaptive immune response of the mammalian host, B. hermsii utilizes a complex genetic expression system, which switches the surface expression of antigenically distinct variable major proteins (Vmp) in 10−4 to 10−3 bacteria per generation (18, 19). This system results in waves of bacteremia, with each wave associated with an antigenically distinct bacterial population. Initially, the protective IgM responses recognize the Vmp of B. hermsii, which facilitates the clearance of each individual wave of bacteremia (3, 15–17). Although immunocompetent mice suffer multiple episodes of bacteremia, the severity of bacteremia typically decreases in each subsequent episode, and by 4 weeks, the bacteremia is undetectable (3, 15–17). In mice, the decrease in the severity of bacteremic episodes correlates to the gradual generation of TI IgM responses specific to factor H-binding protein (FhbA) (20, 21), an outer membrane protein and putative virulence factor that is constitutively expressed by B. hermsii (22, 23), suggesting that the gradual generation of IgM specific for this conserved antigenic target may be responsible for the eventual resolution of infection. Using the B. hermsii infection system, we have defined a major role for B1b cells in TI immune responses (2, 3, 16, 20). Specifically, we have found that B1b cells are the dominant B cell subset that generates anti-B. hermsii- and anti-FhbA IgM responses. Additionally, we have shown that antigen-experienced B1b cells are responsible for long-lasting protection against reinfection (3). Although the importance of humoral immunity in the B. hermsii system has been well defined, the mechanisms responsible for the generation and maintenance of the B cells required for these functional responses are not known.
B cell activating factor of the tumor necrosis factor (TNF) family (BAFF: also known as BLyS) and a proliferation inducing ligand (APRIL) are members of the TNF superfamily that play important roles in B cell function and normal B cell homeostasis (24). Mature B cells express receptors for BAFF and APRIL, namely, BAFF receptor (BAFFR; also known as BR3), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), and B cell maturation antigen (BCMA) (24). BAFF binds to BAFFR, TACI, and BCMA, whereas APRIL binds to TACI and BCMA (24). BCMA is expressed on plasma cells but not on mature B cells and is critical for plasma cell maintenance (25). By engaging these receptors, BAFF and/or APRIL induces the activation of the NF-κB pathway and the expression of prosurvival molecules, such as Mcl-1 and Bcl-xL (24, 26). Mice deficient in BAFF have significantly smaller FOB and MZB cell compartments but have a normal frequency of B1 cells (27). These findings suggest that BAFF is not required for the generation of B1 cell subsets. However, the impairment in TI responses in these mice indicates that the B1 cells in BAFF-deficient mice may be functionally compromised (28, 29).
NP-Ficoll (4-hydroxy-3-nitrophenyl-acetyl conjugated to Ficoll) and bacterial polysaccharides are referred to as TI type 2 (TI-2) antigens. TI-2 antigens are defined by the fact that antibody responses to these antigens are primarily mediated by B cell antigen receptor (BCR) cross-linking, and mice defective in BCR signaling (e.g., x-linked immunodeficient; xid mice) are severely impaired in mounting a response to this type of antigens (2, 30). Although the antibody responses required to control B. hermsii infection are also independent of T cell help (3, 15, 31), xid mice can mount a protective anti-B. hermsii response (31), albeit with a delay, suggesting that in the context of an infection, TI responses involve more complex signaling pathways in addition to BCR signaling. Previous studies revealed that mice deficient in TACI have impaired responses to purified TI-2 antigens (32–34). To examine whether TACI is also required for TI responses in the context of pathogen encounters, we have utilized Staphylococcus pneumoniae and the B. hermsii infection system.
MATERIALS AND METHODS
Mice.
The Institutional Animal Care and Use Committee at Thomas Jefferson University approved these studies. Mice were housed in microisolator cages with free access to food and water and were maintained in a specific-pathogen-free facility. C57BL6/J (wild-type), B6.129S2-Tnfsf13btm1Msc/J (BAFF−/−), and B6(Cg)-Tnfrsf13ctm1Mass/J (BAFFR−/−) mice were purchased from Jackson Laboratories (Bar Harbor, ME). TACI-deficient (TACI−/−) mice on a C57BL/6 background were described previously (33).
Cell culture.
Peritoneal exudate cells were harvested by peritoneal lavage, and 1 × 106 cells were plated in each well of a 12-well plate in 2 ml of R-15 medium (RPMI 1640 [with 25 mM HEPES and l-glutamine] plus 15% heat-inactivated fetal bovine serum and penicillin-streptomycin). Cells were stimulated with the synthetic Toll-like receptor 2 (TLR2) agonist Pam3Cys-kkkk (100 ng/ml; EMC Microcollections, Tübingen, Germany) and/or the BCR-specific agonist AffiniPure F(ab′)2 fragment goat anti-mouse IgM, μ-chain specific (100 ng/ml; Jackson ImmunoResearch laboratories, West Grove, PA). Preparation of heat-killed B. hermsii for in vitro cell culture was performed as described previously (35).
Infections.
Although B. hermsii is naturally transmitted by tick bites, needle inoculations can deliver a defined dose of spirochetes, and such inoculation has been shown to recapitulate the pathophysiology of relapsing fever in humans. Therefore, in this study, mice were infected intravenously via the tail vein with 5 × 104 bacteria of B. hermsii strain DAH-p1 (from the blood of an infected mouse), and the bacteremia was monitored by dark-field microscopy as described previously (3). For S. pneumoniae challenge, 5,000 CFU of fully virulent WU-2 strain S. pneumoniae bacteria were injected intraperitoneally. Mice were monitored daily for survival.
Immunization.
For NP-Ficoll immunization, 50 μg of 77-NP-loaded-Ficoll (Biosearch Technologies, Petaluma, CA) dissolved in 100 μl Dulbecco's phosphate-buffered saline was used to immunize mice intraperitoneally (36). For pneumococcal immunizations, mice were injected intraperitoneally with 1 × 108 encapsulated, heat-killed cells of the WU-2 strain of S. pneumoniae (36).
ELISA.
The level of B. hermsii-, FhbA-, NP-, or type 3 pneumococcal polysaccharide (PPS3)-specific IgM or IgG was determined as described previously (3, 20, 21, 36) Specific antibody levels were interpreted as ng/μl equivalents using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (Bethyl Laboratories, Montgomery, TX).
Flow cytometry.
To determine the frequency of B cell subsets, peritoneal cavity cells were harvested from individual mice and the cell concentration was adjusted to 2.5 × 107/ml in staining medium (deficient RPMI medium 1640 [Irvine Scientific, Santa Ana, CA] with 3% new calf serum, 1 mM EDTA). After blocking Fc receptors with 2.4G2 antibody (1 μg per 106 cells), an aliquot of 25 μl of peritoneal cavity cells was incubated in a microtiter plate with appropriately diluted antibodies. Anti-IgM-fluorescein isothiocyanate (FITC) (clone 1B4B1), anti-Mac1-AF700 (clone M1/70), anti-CD5 Pacific blue (clone 53-7.3) anti-CD19-phycoerythrin (PE)-Cy7 (clone eBio1D3), anti-CD267 (TACI)-PE (clone eBio8F10-3), anti-BAFFR-allophycocyanin (APC) (clone eBio7H22E16), rat IgG2a-PE isotype control (clone eBR2a), and rat IgG1-APC isotype control (clone eBRG1) antibodies were purchased from eBioscience (San Diego, CA); anti-B220-peridinin chlorophyll protein (PerCP)-Cy5.5 (clone RA3-6B2) was purchased from Caltag (Burlingame, CA). After staining, cells were washed twice with staining medium and the preparations were analyzed on an LSRII (Becton, Dickinson, Mountain View, CA), using the FACS Diva software (Becton, Dickinson). Data were analyzed using the FlowJo software program (TreeStar, San Carlos, CA).
Statistical analysis.
Statistical analyses were performed using the Prism 5 software program (GraphPad Software, Inc., La Jolla, CA). To analyze statistical significance, the unpaired Student's t test (two-tailed), one-way or two-way ANOVA, and Bonferroni's posttest or log-rank (Mantel-Cox) test were used as necessary.
RESULTS
B1b cells express both TACI and BAFFR, which are upregulated by TLR2 agonist and B. hermsii.
B cell responses to TI-2 antigens are dependent on TACI, which has been shown to be required for efficient differentiation of B cells into plasma cells (32–34). The expression of TACI, as well as that of BAFFR, has been well characterized on FOB, MZB, and B1a but not B1b subsets (37–39). Since B1b cells are the major cell subset that mounts responses to TI-2 antigens, such as NP-Ficoll and type 3 pneumococcal polysaccharide (PPS3), as well as B. hermsii (2–4, 36, 40), and since TACI and BAFFR are the primary receptors for BAFF and APRIL on mature B cells, we evaluated the expression of both TACI and BAFFR on B1b cells. As shown previously, we found that the B1a cell subset expressed both TACI and BAFFR (Fig. 1A). We also found that B1b cells expressed TACI (Fig. 1A). Interestingly, the relative level of BAFFR on B1b cells appeared to be much higher than that of TACI.
FIG 1.
Expression of TACI and BAFFR on B1 cell subsets. (A) Peritoneal cavity cells of C57BL/6 mice (n = 3) were stained with antibodies specific for surface CD19, B220, IgM, Mac1, and CD5, as well as TACI and BAFFR or appropriate isotype controls, and analyzed by flow cytometry. Gating strategies in each plot are indicated (arrows), and the expression of TACI and BAFFR (bold lines) and appropriate isotype controls (gray lines) is shown. Geometric mean fluorescence intensity (MFI) values of isotype control (regular font) and TACI or BAFFR antibodies (boldface font) are shown within the plots. Data are representative of two independent experiments. (B and C) A million peritoneal exudate cells from C57BL/6 mice were stimulated as described for 18 h, at which time cells were stained as described above to determine TACI and BAFFR expression. (B) Representative histograms indicating TACI and BAFFR expression on B1b cells stimulated with no agonist (gray fill), anti-BCR (gray line), TLR2 agonist (thin black line), and anti-BCR and TLR2 agonist (bold black line) are shown. Three experimental replicates were used, and cells for each replicate were harvested from different wild-type mice. Data are representative of two independent experiments. The data are presented as the means and standard deviations. The differences in MFI were analyzed by one-way ANOVA and Bonferroni's multiple comparison test or Student's t test as appropriate (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant).
Classical TI-2 antigens require BCR-mediated signaling, whereas anti-Borrelia responses are dependent on both BCR- and TLR-mediated signaling. Specifically, we have shown that protective antibody responses to B. hermsii require TLR2 (31, 35). It has been previously shown that the surface expression of both TACI and BAFFR on FOB, MZB, and B1a cells increases in response to CpG DNA, which is a TLR9 agonist. A separate study examined TACI expression on total B cells in response to a variety of TLR ligands (41), but no studies to date have examined TACI and BAFF surface expression on B1b cells in response to in vitro stimulation. Considering the importance of TLR2 in protective, TI anti-B. hermsii antibody responses and since B1b cells are the primary subset of B cells that confer protective immunity to B. hermsii, we examined whether a TLR2 agonist could influence the expression of TACI and BAFFR on B1b cells. We found that stimulation of B cells with anti-BCR stimulation alone, a mimic of TI-2 antigen, did not significantly alter the levels of TACI or BAFFR on B1b cells in vitro (Fig. 1B). We also found that exposure to TLR2 agonist resulted in a 2-fold upregulation of TACI and BAFFR on B1b cells compared to their levels on unstimulated cells (Fig. 1B). Furthermore, we found that BCR stimulation significantly enhanced the upregulation of TACI and BAFFR when coadministered with TLR ligands. This led us to examine whether B. hermsii alone would be capable of influencing TACI or BAFFR expression on B1b cells in vitro. In fact, we found that exposure to intact, heat-killed bacteria was capable of significantly increasing both TACI and BAFFR surface expression on B1b cells (Fig. 1C). Collectively, these findings showed that the B1b cell subset expressed both TACI and BAFFR and that the surface expression of both receptors could be influenced by exposure to TLR2 ligands and B. hermsii cells.
Analysis of B cell subsets and anti-NP-Ficoll responses in TACI-, BAFF- and BAFFR-deficient mice.
Since B1b cells are the primary responders to the prototypical TI-2 antigen NP-Ficoll, as well as to B. hermsii (2, 4, 36, 40), we analyzed the B1b cell compartments of TACI−/−, BAFFR−/−, and BAFF−/− mice. We found that TACI-, BAFFR-, and BAFF-deficient mice all possessed a comparable frequency of B1b cells, though BAFFR- and BAFF-deficient mice possessed significantly fewer B1 cells than did wild-type or TACI-deficient mice (Fig. 2A and B). Although we found that the B1 cell compartments in TACI-deficient mice were comparable to those in wild-type mice (Fig. 2A and B), they were severely impaired in responding to NP-Ficoll (Fig. 2C), as shown previously (33, 42). Similarly, while BAFF−/− mice possessed a B1b compartment, they were severely impaired in responding to NP-Ficoll (Fig. 2C). These data are consistent with the notion that responses to classical TI-2 antigens depend primarily upon the BAFF-TACI axis. There is currently no consensus regarding responses to NP-Ficoll in BAFFR-deficient mice (28, 29). We found that BAFFR−/− mice generated NP-specific IgM responses that were significantly more robust than those of either TACI- or BAFF-deficient mice but were still slightly impaired compared to the responses of wild-type mice (Fig. 2C). The paucity of MZB and FOB cells in BAFFR-deficient mice (28, 29) suggests that these cells are not required for responses to TI-2 antigens, though the impairment seen in BAFFR-deficient mice, particularly at the earliest time points, could indicate a role for MZB cells in early responses immediately following TI antigen encounter (43).
FIG 2.
Analysis of B1a and B1b cell subsets and NP-specific IgM responses in mice deficient in TACI, BAFFR, or BAFF. (A and B) Peritoneal cavity cells of mice were stained with antibodies specific for surface CD19, B220, IgM, CD23, Mac1, and CD5 and analyzed by flow cytometry. All cells were first identified as B1 cells (IgM+, CD19+ CD23−, and Mac1+ cells [plots not shown]). (A) The frequencies of B1a (CD5+) and B1b (CD5−) subsets among those B cells are shown. Five-percent contour plots are shown. (B) The numbers of B1a and B1b cells in the indicated mice are shown as means + standard deviations. These data were generated by analyzing a minimum of 20,000 cells and are representative of at least 4 mice from each indicated genotype. Differences between wild-type and mutant mice were determined using one-way ANOVA followed by Bonferroni's multiple comparison test. Data are representative of two independent experiments. (C) Wild-type (n = 5) and BAFFR−/− (n = 7), TACI−/− (n = 6), and BAFF−/− (n = 7) mice were immunized intraperitoneally with 50 μg of NP-77 Ficoll. Blood samples were obtained on days 0, 7, and 14 postimmunization, and NP-specific IgM was determined by ELISA. The differences in antibody responses were analyzed by two-way ANOVA with Bonferroni's posttest (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Data are representative of two independent experiments.
TACI is dispensable for control of B. hermsii infection.
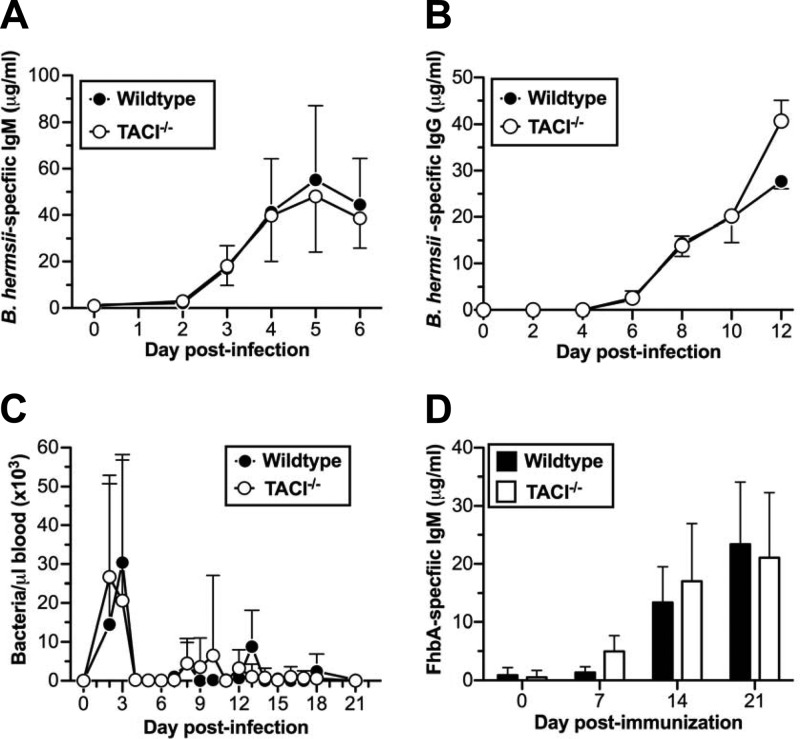
Since TACI is required for efficient responses to TI-2 antigens (32–34) and B1b cells play a significant role in generating TI antibody responses against both TI-2 antigens and B. hermsii (2–4, 36, 40), we expected that TACI would play a critical role in generating humoral responses against B. hermsii. Surprisingly, we found that, following infection, the kinetics of B. hermsii-specific IgM responses in TACI−/− mice were indistinguishable from those of wild-type mice (Fig. 3A). In response to TI-2 antigens, TACI−/− mice generate significantly reduced IgG responses (33). Although IgG responses are not essential for controlling B. hermsii, we also looked at specific IgG responses to B. hermsii. We found that, like the B. hermsii-specific IgM responses, the B. hermsii-specific IgG responses were indistinguishable from those of wild-type mice (Fig. 3B). Consistent with these observations, we found that the severity of the recurrent episodes of bacteremia in TACI−/− mice was indistinguishable from the severity of the episodes of bacteremia in wild-type mice (Fig. 3C). We also found that the FhbA-specific antibody responses in TACI−/− mice were comparable to the responses found in wild-type mice (Fig. 3D). These data indicated that protective TI responses to B. hermsii did not require TACI.
FIG 3.
Comparable B. hermsii-specific antibody responses in wild-type and TACI−/− mice. (A, C, and D) Wild-type (n = 9) or TACI−/− (n = 11) mice were infected intravenously with 5 × 104 B. hermsii bacteria (strain DAH-p1); bacteremia was monitored by dark-field microscopy as described previously (3) (C), and B. hermsii-specific IgM (A) and FhbA-specific IgM (D) were measured by ELISA. These data represent the pooled data from two independent experiments. (B) B. hermsii-specific IgG from B. hermsii-infected wild-type (n = 3) or TACI−/− (n = 3) mice was measured by ELISA. The data shown are representative of two independent experiments. Means ± standard deviations are shown for all panels. Differences in the antibody responses and bacteremia were not statistically significant when analyzed by two-way ANOVA with significance reached at a P value of <0.05.
BAFF and BAFFR are required for efficient anti-B. hermsii responses.
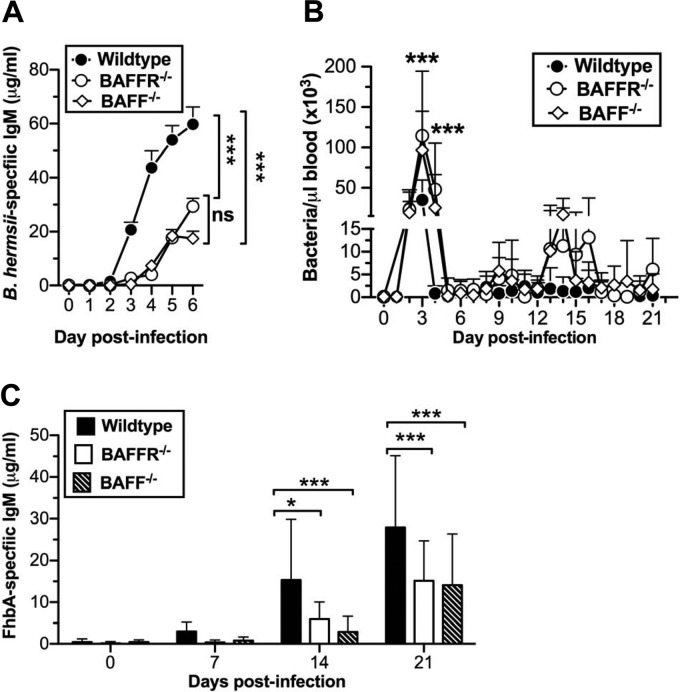
Since we found no impairment in anti-B. hermsii responses in TACI-deficient mice, we examined a role for BAFFR and BAFF. Following B. hermsii infection, we found that both BAFFR−/− and BAFF−/− mice were significantly impaired in generating B. hermsii-specific IgM compared to wild-type mice (Fig. 4A). Consistent with this impairment, both BAFFR−/− and BAFF−/− mice exhibited more-severe bacteremia than wild-type mice at days 3 and 4 postinfection, during the primary episode (Fig. 4B). Specifically, the peak bacteremia in BAFFR−/− and BAFF−/− mice was more than 2-fold higher than that in wild-type mice. Additionally, the primary bacteremic episode in both BAFFR−/− and BAFF−/− mice persisted for 1 day longer than was seen in wild-type mice (Fig. 4B).
FIG 4.
Efficient control of B. hermsii requires BAFF and BAFFR. Wild-type (n = 18) or BAFFR−/− (n = 12) and BAFF−/− (n = 13) mice were infected intravenously with 5 × 104 B. hermsii bacteria (strain DAH-p1). (A and C) B. hermsii-specific IgM (A) and FhbA-specific IgM (C) were measured by ELISA. Means + standard deviations are shown. Differences in the anti-B. hermsii IgM responses of wild-type and BAFFR−/− or BAFF−/− mice were significantly different from days 3 to 6 postimmunization when analyzed by two-way ANOVA followed by Bonferroni's posttest (A), and anti-FhbA responses of wild-type and BAFFR−/− or BAFF−/− mice were significantly different at the indicated days when analyzed by two-way ANOVA followed by Bonferroni's posttest (C) (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant). (B) Bacteremia was monitored by dark-field microscopy as described previously (4), and mean bacterial densities + standard deviations at indicated days postinfection are plotted. Differences in bacteremia between wild-type and BAFF- or BAFFR-deficient mice were determined by two-way ANOVA followed by Bonferroni's posttest (***, P < 0.001). These data represent the pooled data from two independent experiments.
In wild-type mice, the magnitude of the recurrent episodes of bacteremia diminished by 3 to 4 weeks postinfection. In contrast, both BAFFR−/− and BAFF−/− mice exhibited persistently higher bacteremia at these time points, though these differences were not statistically significant and the mice were able to eventually resolve the infection (Fig. 4B). Since specific antibody responses to FhbA inversely correlate with the magnitude of bacteremia, we expected that anti-FhbA responses in BAFF- and BAFFR-deficient mice would be compromised. In fact, we found that these mice had significantly impaired anti-FhbA responses compared to the responses in wild-type mice (Fig. 4C).
TACI is critical for protective humoral responses to heat-killed S. pneumoniae.
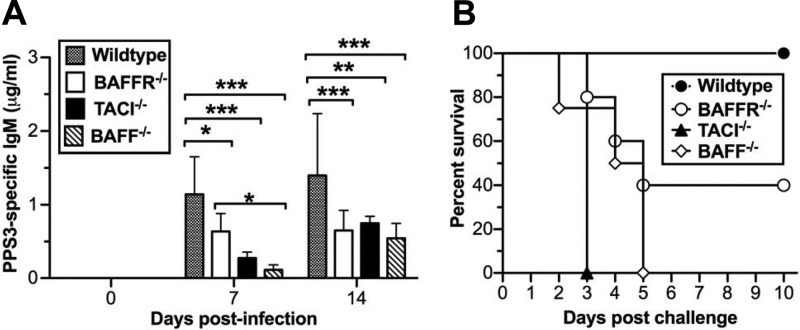
Having found that efficient TI antibody responses to B. hermsii did not require TACI, we set out to test whether this difference in terms of TACI dependence in TI humoral responses is related to the difference between using analytically pure antigens, such as NP-Ficoll or purified bacterial polysaccharide, and whole pathogens, such as B. hermsii. To test this, we utilized an S. pneumoniae strain possessing PPS3. The polysaccharide capsule of this pathogen typically elicits a PPS3-specific TI humoral response in less than 1 week and is generated by B1b cells (4). Infection in naive wild-type mice is lethal; therefore, we immunized wild-type and BAFFR-, TACI-, and BAFF-deficient mice with heat-killed, morphologically intact S. pneumoniae (strain WU-2). We found that the PPS3-specific IgM levels in all mutant strains at both 7 and 14 days postinfection were significantly lower than the levels in wild-type mice (Fig. 5A). However, at 7 days postinfection, BAFFR-deficient mice exhibited less impairment than either TACI- or BAFF-deficient mice. To determine the functionality of the antibody responses, we challenged the mice with the same strain of live S. pneumoniae 28 days postimmunization and monitored survival. We found that all TACI- and BAFF-deficient mice succumbed to the infection, while all wild-type mice survived (Fig. 5B). In comparison, roughly half of the BAFFR-deficient mice survived, seeming to mirror the slight impairment observed in the PPS3-specific IgM response. Collectively, these data suggest that TACI played the dominant role in anti-PPS responses to intact heat-killed S. pneumoniae cells and that BAFFR played a minor role in efficient responses to PPS, similar to the role observed following immunization with NP-Ficoll (Fig. 2B).
FIG 5.
Efficient responses to heat-killed PPS3-bearing S. pneumoniae cells require BAFF, BAFFR, and TACI. Wild-type (n = 4) and BAFFR−/− (n = 5), TACI−/− (n = 6), and BAFF−/− (n = 4) mice were immunized with 1 × 108 heat-killed, PPS3-bearing S. pneumoniae cells (strain WU-2). (A) PPS3-specific IgM was measured by ELISA. Data are represented as means + standard deviations. The differences in antibody responses were analyzed by two-way ANOVA followed by Bonferroni's posttest (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (B) At 28 days postimmunization, mice were infected with 5,000 CFU of the WU-2 strain of PPS3-bearing S. pneumoniae. Survival was monitored, and data were analyzed by log-rank (Mentel-Cox) test; only TACI−/− and BAFF−/− mice exhibited significantly reduced survival (P < 0.05). Data are representative of two independent experiments.
DISCUSSION
Antibody responses to analytically pure polysaccharide antigens are rapidly generated independently of T cell help and require BCR cross-linking. Mice impaired in BCR-mediated signaling, such as those deficient in Bruton's tyrosine kinase (Btk), are severely impaired in responding to TI-2 antigens (2). While protective immune responses to B. hermsii develop independently of T cell help, the ability of mice lacking Btk to control B. hermsii infection illustrates that the response to B. hermsii is distinct from classical TI-2 responses. In fact, we have previously shown that, in addition to Btk-dependent BCR signaling, Toll-like receptor (TLR) signaling contributes to the development of efficient antibody responses to B. hermsii, illustrating that the characteristics of TI B cell responses depend upon the immunological context in which they are encountered (2, 31). Considering that TLR ligands are possessed by all bacterial pathogens, including many clinically important bacterial species, the influence of TLRs on B cell responses to TI antigens has broad implications in the field of vaccine design.
In addition to the above-described differences, humoral responses to B. hermsii also differ from those of classical TI-2 antigens in terms of TACI dependency. While studies have demonstrated that TACI is required for efficient TI responses to polysaccharide antigens (32, 33, 42), we find that TACI is dispensable for TI humoral responses to B. hermsii (Fig. 3). Rather, we have found that BAFFR plays a discernible role in responses to B. hermsii. Both BAFF- and BAFFR-deficient mice have significantly reduced MZB cell compartments (28, 29). While MZB cells are not required for resolution of B. hermsii infection, they have been shown to contribute to the initial IgM response following infection (16, 44). Therefore, it is possible that the paucity of MZB cells in these mice is partially responsible for the phenotype observed in both BAFF- and BAFFR-deficient mice. However, in addition to impaired initial anti-B. hermsii IgM responses, we also observed impaired FhbA-specific IgM production and delayed resolution of later episodes of bacteremia. Considering that the role for MZB cells in B. hermsii infection appears limited to the first wave of bacteremia (16), reduced MZB cell numbers do not fully explain the observed phenotypes. Previous studies have focused on the fact that the frequencies of B1 cells are similar in BAFFR- or BAFF-deficient and wild-type mice (27–29). We report here that while the frequency is unchanged, the absolute number of B1b cells is significantly lower in BAFFR-deficient than in wild-type mice (Fig. 2B). Considering that B1b cells have been directly implicated in B. hermsii- and FhbA-specific IgM responses, the lower numbers of B1b cells in BAFFR- and BAFF-deficient mice may explain the observed impairments. Alternatively, it is possible that in the absence of BAFFR signaling, B1b cells are functionally compromised. Further study will be needed to determine the precise role of BAFFR in the B. hermsii infection system.
Humoral responses to classical TI-2 antigens, such as isolated PPS, have been previously shown to require TACI, but considering that efficient and protective TI responses to B. hermsii occur in the absence of TACI, we sought to determine whether TI responses generated to whole bacteria differed from responses to isolated antigens in terms of TACI dependency. After immunizing mice with heat-killed S. pneumoniae, we found that TACI is required for anti-PPS responses to whole heat-killed bacteria, similar to results obtained following immunization with purified PPS. This result leads to a puzzling question: why is TACI required for TI humoral responses to some pathogens but not others? It should be noted that B. hermsii infection results in extremely high levels of bacteremia, which could contribute to the ability of this infection to activate other costimulatory pathways, such as the TLR pathway. Thus, it is possible that TLR ligand availability and persistence may vary between an active infection and immunization with killed bacteria, resulting in a different requirement for TACI-mediated B cell differentiation. In fact, TACI signaling results primarily in activation of the canonical NF-κB pathway (24). Therefore, it is possible that persistent TLR signaling during active infection may bypass the need for TACI signaling through TLR-mediated canonical NF-κB pathway activation. Polysaccharide vaccines have been shown to have limited immunogenicity in children and also offer a relatively short period of protection and suboptimal boosting; understanding the ability of TLR signaling to augment or bypass the need for TACI-mediated B cell differentiation signals could provide insights for the development of novel immunization strategies, including improved polysaccharide vaccines.
In contrast to TI responses to polysaccharides, protective responses to B. hermsii infection have been shown to be long lived (2, 3). This has been attributed to the long-term maintenance of expanded populations of antigen-specific B1b cells (3, 5). BAFFR-mediated signaling has been shown to play an important role in promoting B cell survival through the production of prosurvival molecules, such as Bcl-2 and Mcl-1, as well as kinases that are important for metabolic fitness (24, 26). Therefore, understanding TI humoral responses that occur in the context of active infections, such as B. hermsii infection, and the importance of BAFF/BAFFR-mediated signaling in this process could help to identify novel strategies for improving vaccines.
ACKNOWLEDGMENTS
We thank Michael Cancro and Vishal Sindhava for stimulating discussions and David Briles for providing the S. pneumoniae strain WU-2.
This work was supported by the U.S. National Institutes of Health (grant R56AI097421 to K.R.A.).
Footnotes
Published ahead of print 11 November 2013
REFERENCES
- 1.Martin F, Kearney JF. 2001. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 13:195–201. 10.1016/S0952-7915(00)00204-1 [DOI] [PubMed] [Google Scholar]
- 2.Alugupalli KR. 2008. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr. Top. Microbiol. Immunol. 319:105–130. 10.1007/978-3-540-73900-5_5 [DOI] [PubMed] [Google Scholar]
- 3.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21:379–390. 10.1016/j.immuni.2004.06.019 [DOI] [PubMed] [Google Scholar]
- 4.Haas KM, Poe JC, Steeber DA, Tedder TF. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23:7–18. 10.1016/j.immuni.2005.04.011 [DOI] [PubMed] [Google Scholar]
- 5.Foote JB, Kearney JF. 2009. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J. Immunol. 183:6359–6368. 10.4049/jimmunol.0902473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dworkin MS, Anderson DE, Jr, Schwan TG, Shoemaker PC, Banerjee SN, Kassen BO, Burgdorfer W. 1998. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin. Infect. Dis. 26:122–131. 10.1086/516273 [DOI] [PubMed] [Google Scholar]
- 7.Cadavid D, Barbour AG. 1998. Neuroborreliosis during relapsing fever: review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clin. Infect. Dis. 26:151–164. 10.1086/516276 [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Monco JC, Miller NS, Backenson PB, Anda P, Benach JL. 1997. A mouse model of Borrelia meningitis after intradermal injection. J. Infect. Dis. 175:1243–1245. 10.1086/593681 [DOI] [PubMed] [Google Scholar]
- 9.Gebbia JA, Monco JC, Degen JL, Bugge TH, Benach JL. 1999. The plasminogen activation system enhances brain and heart invasion in murine relapsing fever borreliosis. J. Clin. Invest. 103:81–87. 10.1172/JCI5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alugupalli KR, Michelson AD, Joris I, Schwan TG, Hodivala-Dilke K, Hynes RO, Leong JM. 2003. Spirochete-platelet attachment and thrombocytopenia in murine relapsing fever borreliosis. Blood 102:2843–2850. 10.1182/blood-2003-02-0426 [DOI] [PubMed] [Google Scholar]
- 11.Alugupalli KR, Michelson AD, Barnard MR, Leong JM. 2001. Serial determinations of platelet counts in mice by flow cytometry. Thromb. Haemost. 86:668–671 [PubMed] [Google Scholar]
- 12.Liu H, Fitzgerald D, Gran B, Leong JM, Alugupalli KR. 2010. Induction of distinct neurologic disease manifestations during relapsing fever requires T lymphocytes. J. Immunol. 184:5859–5864. 10.4049/jimmunol.0902737 [DOI] [PubMed] [Google Scholar]
- 13.Vuyyuru R, Liu H, Manser T, Alugupalli KR. 2011. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc. Natl. Acad. Sci. U. S. A. 108:20707–20712. 10.1073/pnas.1108776109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamaei-Tousi A, Martin P, Bergh A, Burman N, Brannstrom T, Bergstrom S. 1999. Erythrocyte-aggregating relapsing fever spirochete Borrelia crocidurae induces formation of microemboli. J. Infect. Dis. 180:1929–1938. 10.1086/315118 [DOI] [PubMed] [Google Scholar]
- 15.Barbour AG, Bundoc V. 2001. In vitro and in vivo neutralization of the relapsing fever agent Borrelia hermsii with serotype-specific immunoglobulin M antibodies. Infect. Immun. 69:1009–1015. 10.1128/IAI.69.2.1009-1015.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. 2003. The resolution of relapsing fever Borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 170:3819–3827 [DOI] [PubMed] [Google Scholar]
- 17.Connolly SE, Benach JL. 2005. The versatile roles of antibodies in Borrelia infections. Nat. Rev. Microbiol. 3:411–420. 10.1038/nrmicro1149 [DOI] [PubMed] [Google Scholar]
- 18.Barbour AG. 1993. Linear DNA of Borrelia species and antigenic variation. Trends Microbiol. 1:236–239. 10.1016/0966-842X(93)90139-I [DOI] [PubMed] [Google Scholar]
- 19.Barbour AG. 1990. Antigenic variation of a relapsing fever Borrelia species. Annu. Rev. Microbiol. 44:155–171. 10.1146/annurev.mi.44.100190.001103 [DOI] [PubMed] [Google Scholar]
- 20.Colombo MJ, Alugupalli KR. 2008. Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J. Immunol. 180:4858–4864 [DOI] [PubMed] [Google Scholar]
- 21.Colombo MJ, Sun G, Alugupalli KR. 2010. T-cell-independent immune responses do not require CXC ligand 13-mediated B1 cell migration. Infect. Immun. 78:3950–3956. 10.1128/IAI.00371-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovis KM, Schriefer ME, Bahlani S, Marconi RT. 2006. Immunological and molecular analyses of the Borrelia hermsii factor H and factor H-like protein 1 binding protein, FhbA: demonstration of its utility as a diagnostic marker and epidemiological tool for tick-borne relapsing fever. Infect. Immun. 74:4519–4529. 10.1128/IAI.00377-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hovis KM, McDowell JV, Griffin L, Marconi RT. 2004. Identification and characterization of a linear-plasmid-encoded factor H-binding protein (FhbA) of the relapsing fever spirochete Borrelia hermsii. J. Bacteriol. 186:2612–2618. 10.1128/JB.186.9.2612-2618.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackay F, Schneider P. 2009. Cracking the BAFF code. Nat. Rev. Immunol. 9:491–502. 10.1038/nri2572 [DOI] [PubMed] [Google Scholar]
- 25.O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. 2004. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199:91–98. 10.1084/jem.20031330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elgueta R, de Vries VC, Noelle RJ. 2010. The immortality of humoral immunity. Immunol. Rev. 236:139–150. 10.1111/j.1600-065X.2010.00924.x [DOI] [PubMed] [Google Scholar]
- 27.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, McMillen S, Waggie K, Schreckhise RW, Shoemaker K, Vu T, Moore M, Grossman A, Clegg CH. 2001. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. Immunity 15:289–302. 10.1016/S1074-7613(01)00183-2 [DOI] [PubMed] [Google Scholar]
- 28.Shulga-Morskaya S, Dobles M, Walsh ME, Ng LG, MacKay F, Rao SP, Kalled SL, Scott ML. 2004. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J. Immunol. 173:2331–2341 [DOI] [PubMed] [Google Scholar]
- 29.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. 2004. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J. Immunol. 173:2245–2252 [DOI] [PubMed] [Google Scholar]
- 30.Vos Q, Lees A, Wu Z, Snapper CM, Mond JJ. 2000. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 176:154–170. 10.1034/j.1600-065X.2000.00607.x [DOI] [PubMed] [Google Scholar]
- 31.Alugupalli KR, Akira S, Lien E, Leong JM. 2007. MyD88- and Bruton's tyrosine kinase-mediated signals are essential for T cell-independent pathogen-specific IgM responses. J. Immunol. 178:3740–3749 [DOI] [PubMed] [Google Scholar]
- 32.Kanswal S, Katsenelson N, Selvapandiyan A, Bram RJ, Akkoyunlu M. 2008. Deficient TACI expression on B lymphocytes of newborn mice leads to defective Ig secretion in response to BAFF or APRIL. J. Immunol. 181:976–990 [DOI] [PubMed] [Google Scholar]
- 33.von Bulow GU, van Deursen JM, Bram RJ. 2001. Regulation of the T-independent humoral response by TACI. Immunity 14:573–582. 10.1016/S1074-7613(01)00130-3 [DOI] [PubMed] [Google Scholar]
- 34.Mantchev GT, Cortesao CS, Rebrovich M, Cascalho M, Bram RJ. 2007. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J. Immunol. 179:2282–2288 [DOI] [PubMed] [Google Scholar]
- 35.Dickinson GS, Piccone H, Sun G, Lien E, Gatto L, Alugupalli KR. 2010. Toll-like receptor 2 deficiency results in impaired antibody responses and septic shock during Borrelia hermsii infection. Infect. Immun. 78:4579–4588. 10.1128/IAI.00438-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shriner AK, Liu H, Sun G, Guimond M, Alugupalli KR. 2010. IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J. Immunol. 185:525–531. 10.4049/jimmunol.0902841 [DOI] [PubMed] [Google Scholar]
- 37.Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, Cancro MP, Khan WN. 2007. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J. Immunol. 178:7531–7539 [DOI] [PubMed] [Google Scholar]
- 38.Ng LG, Ng CH, Woehl B, Sutherland AP, Huo J, Xu S, Mackay F, Lam KP. 2006. BAFF costimulation of Toll-like receptor-activated B-1 cells. Eur. J. Immunol. 36:1837–1846. 10.1002/eji.200635956 [DOI] [PubMed] [Google Scholar]
- 39.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. 2007. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J. Exp. Med. 204:1959–1971. 10.1084/jem.20062567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu MC, Toellner KM, Vinuesa CG, Maclennan IC. 2006. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc. Natl. Acad. Sci. U. S. A. 103:5905–5910. 10.1073/pnas.0601502103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsenelson N, Kanswal S, Puig M, Mostowski H, Verthelyi D, Akkoyunlu M. 2007. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur. J. Immunol. 37:1785–1795. 10.1002/eji.200636800 [DOI] [PubMed] [Google Scholar]
- 42.Yan M, Wang H, Chan B, Roose-Girma M, Erickson S, Baker T, Tumas D, Grewal IS, Dixit VM. 2001. Activation and accumulation of B cells in TACI-deficient mice. Nat. Immunol. 2:638–643. 10.1038/89790 [DOI] [PubMed] [Google Scholar]
- 43.Martin F, Oliver AM, Kearney JF. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14:617–629. 10.1016/S1074-7613(01)00129-7 [DOI] [PubMed] [Google Scholar]
- 44.Belperron AA, Dailey CM, Bockenstedt LK. 2005. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174:5681–5686 [DOI] [PubMed] [Google Scholar]