Abstract
Xenopus laevis embryos are particularly well suited to address questions requiring either knockdown or overexpression of genes in a tissue-specific fashion during vertebrate embryonic development. These manipulations are achieved by targeted injection of either antisense morpholino oligonucleotides, or synthetic mRNAs, respectively, into the early embryo. Herein we offer detailed protocols describing how to design and perform these experiments successfully, as well as a brief discussion of considerations for performing a microarray analysis in this organism.
Keywords: Xenopus laevis, embryogenesis, microinjection, morpholinos, gene knockdown, microarray
1. Introduction
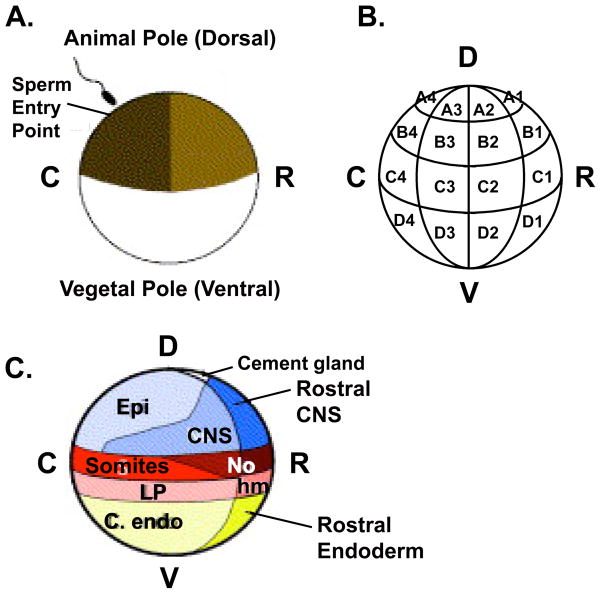
Among the many advantages of working with Xenopus laevis embryos as compared with other vertebrate embryos are that they are very large (approximately 1mm in diameter at the one-cell stage), and they develop rapidly and externally. Their size and accessibility allow for microdissection and manipulation of specific tissues at even the earliest stages of development. In addition, their characteristic pigmentation and cleavage patterns together with extensive lineage tracing studies (1–3), facilitate targeted injection of constructs in order to manipulate early gene expression in a tissue specific fashion (see Figure 3.1). Individual blastomeres may be targeted reasonably well up to the 32-cell stage. For later stage targeting, it is preferable to use an alternate strategy, such as transgenesis employing the appropriate DNA promoter, to achieve overexpression in a regulated fashion. This technique is described in an accompanying chapter, as is the use of chemicals in order to induce competency in specific tissue types (e.g. mesoderm induction by Animal Cap exposure to Activin (4)), to perturb specific signaling pathways (e.g. inhibition of FGF signaling by incubation in SU5402 (5)) or to alter cell fate (e.g. UV or LiCl treatment to ventralize or dorsalize embryos, respectively (6, 7)) all of which are common approaches used to study changes in gene expression in X. laevis.
Figure 3.1. Fate map of a 32-cell Xenopus laevis embryo.
(A) Following fertilization and cortical rotation, cells derived from the lightly pigmented side of the embryo will form rostral (R) structures and the darkly pigmented side will give rise to more caudal (C) structures. This schematic represents a 90° rotation from the traditional dorsal/ventral (D/V) axis. It should be noted that neither the original fate map, nor the updated version is strictly accurate in assigning the D/V axis since dorsal and rostral fates are closely aligned, as are ventral and caudal fates. (B) Blastomere nomenclature of a 32-cell (stage 6) embryo (only cells in one of two sides with respect to the left-right axis are shown). (C) Prospective adult tissues derived from regions of a 32-cell embryo, as seen from a side view. C). Adapted with permission from (21). D = Dorsal, V = Ventral, R = Rostral, C= Caudal, Epi = Epidermis, CNS = Central Nervous System, No = Notochord, LP = Lateral Plate Mesoderm, hm = head mesoderm, endo = endoderm.
In this chapter we describe methods to generate and culture Xenopus embryos, and to perform targeted injection of antisense morpholino oligonucleotides (MOs) and capped mRNAs in order to manipulate gene expression in various tissues. It is worth noting that the microinjection techniques described herein are not limited to straightforward overexpression and knockdown of gene function, and are best applied in the context of established Xenopus resources for optimum utility. For example, many signaling pathways responsible for early vertebrate axis formation and tissue patterning (such as the bone morphogenetic protein (BMP) or the Wnt pathway) have been very well studied in Xenopus. The defects specific to alterations in these pathways may therefore be used as readouts for perturbations at particular steps of the given pathway. This phenomenon is well illustrated by studies that take advantage of the BMP signaling pathway. During Xenopus development, BMP is expressed in a caudal to rostral gradient across the early embryo. High BMP expression on the caudal side of the embryo is required to specify ventral and caudal fates and misexpression of BMPs or molecules that are BMP downstream effectors on the rostral side of the early embryo causes characteristic ventralization of dorsal structures and caudalization of anterior structures (8, 9). Conversely, injection of BMP inhibitors, or proteins that have a dominant negative effect on BMP signaling, on the caudal side of the embryo results in inappropriate dorsalization and rostralization in this region and produces a characteristic axis duplication (10, 11). Overexpression or misexpression of genes in tissues where they are not normally expressed may thus provide clues to their normal function, depending on the effect that they have on an established molecular pathway or patterning event by changing downstream gene expression or morphology. Indeed, many genes of unknown function have been identified as naturally occurring agonists or antagonists of BMP function based on the dorsalized or ventralized phenotype that is observed when they are ectopically expressed.
A disadvantage of using injection of mRNAs is that there is poor control of transcriptional timing. Injected mRNAs are immediately translated and persist for many hours, and up to several days in some cases, in the embryo. Injection of cDNA expression constructs with tissue- or temporal-specific promoters is an alternative method for overexpression. cDNAs containing ubiquitously expressed viral promoters, such as the cytomegalovirus promoter, will not be transcribed until zygotic transcription initiates during the mid-blastula transition, so this technique may be useful for determining the effect of later gene induction. However, this approach has the major drawback that expression of cDNA constructs is of variable efficiency and results in highly mosaic expression (12, 13).
While different anti-sense technologies continue to be developed in a variety of systems, morpholinos have proven the most effective method for attaining reproducible knockdown of a specific gene (14). Blocking a known signaling pathway may also be accomplished by injection of a dominant negative protein (as described above for BMP). It is important to note, however, that dominant-negative proteins (as is the case with a dominant negative BMP receptor) may affect other related proteins and family members and thus phenotypic effects may not be a result of inhibiting a single target.
Once gene expression has been altered by injection or by chemical exposure, tissues within individual treatment groups can be easily pooled, subject to microarray analysis (as discussed below) and analyzed for changes in global gene expression due to a specific perturbation. Although lack of a sequenced genome still poses a significant challenge when working with Xenopus laevis, the availability of genetic resources continues to be improved.
Considerations when performing a microarray analysis in Xenopus laevis
Xenopus laevis is an excellent system for controlling early gene expression (i.e. knockdown, upregulation and/or mis-regulation of specific genes) in a whole animal system and for gaining access to tissues at very early time points. Because many embryos are obtained in a single spawning event, a large amount of material may be generated in a relatively short period of time. Experiments can thus be performed, and adequate quantities of sample for a microarray can be collected rapidly without relying on amplification techniques. However, because the genomic resources are scarce there tends to be very little or poor annotation for most genes on commercial X. laevis chips. It is therefore important to consider the annotation status for a particular chip before embarking on a resource-intensive study such as a microarray project. One may want to consider using a chip from the related organism Xenopus tropicalis, which shares a high degree homology with X. laevis. The X. tropicalis genome is not duplicated and it has thus been fully sequenced. Despite these drawbacks, a number of genetic resources have been developed lately (see (15) for a good review and comprehensive list) and progress continues to be made in understanding the X. laevis genome. Finally, because Xenopus laevis is not a clonal species, it is also important to recognize that there will be a high degree of background variability between individual frogs. It is therefore important to have enough biological replicates to identify significant targets above the background, and to have a good statistician on hand. In spite of these genomic limitations, its large degree of genetic conservation with higher organisms, short developmental timeline, and overall accessibility make it a very useful model system with which to efficiently study early events in vertebrate development.
2. Materials
Unless otherwise indicated, solutions should be prepared and stored at room temperature.
2.1 Generation and Testing of Morpholino Oligonucleotides
Morpholino oligonucleotides (GeneTools, LLC; see Methods for considerations regarding morpholino ordering and appropriate control morpholinos)
2.2 Generation of Synthetic mRNA for Microinjection
mMESSAGE mMACHINE High Yield Capped RNA Transcription Kit (Ambion). Polymerase-specific kits are available and selection depends on which RNA polymerase promoter is upstream of the gene of interest.
Equilibrated Phenol pH 8.0 (USB)
Sevag [Chloroform:Isoamylalcohol (24:1)]
10M Ammonium Acetate
Ethanol
Isopropanol
Sephadex G-50 spin column (IBI Scientific). Optional but recommended
Linearized template cDNA
2.3 Collection of Testes
Tricaine (Sigma). 0.2% (w/v) dissolved in dH2O (prepare fresh)
Modified Barth’s Saline (MBS). For 10X stock: 880mM NaCl, 10mM KCl, 25mM NaHCO3, 100mM HEPES (pH 7.5), 10mM MgSO4, 0.14mM Ca(NO3)2, 0.41mM CaCl2; Adjust to pH 7.5 with NaOH, filter sterilize and store at 4°C
Testis Buffer: 10% Fetal Bovine Serum, 1% Pen/Strep (100U/mL Penicillin, 100μg/mL Streptomycin; Sigma) in 1X MBS. Divide into 10mL aliquots and store at −20°C
2.4 Collection and Fertilization of Embryos
Powder-free, Latex-free Vinyl Gloves for handling frogs (see Note 1)
1mL sterile syringe (Kendall)
27 and 20 gauge sterile syringe needles (Kendall)
Human Chorionic Gonadotropin (Sigma). 4,000U/mL dissolved in sterile dH2O; store at 4°C
Holtfreter’s Frog Water. For 200X stock: 3M NaCl, 34mM KCl, 12.5mM NaHCO3 33.7mM CaCl2. For 4L, dissolve NaCl, KCl, NaHCO3 in 3L dH2O. Dissolve 19.8g CaCl2·2H2O in 500mL dH2O, add slowly to above and bring to 4L
Conical Tissue Grinder (Research Products International)
Aged Tap Water (Tap water, allowed to sit for at least 24 hours for chlorine to evaporate; optional as 0.1X MBS may be used instead)
Dejellying Solution (prepare fresh): 2% (w/v) Cysteine dissolved in dH2O; Adjust to pH 7.8–8.0 with NaOH
DeBoer’s Pond Water. For 20X stock: 100mM NaCl, 1.3mM KCl, 0.44mM CaCl2; Adjust to pH 7.4 with NaHCO3, and store at 4°C
Glass Petri dish (100 × 15mm)
2.5 Targeted Embryo Injection and Culture
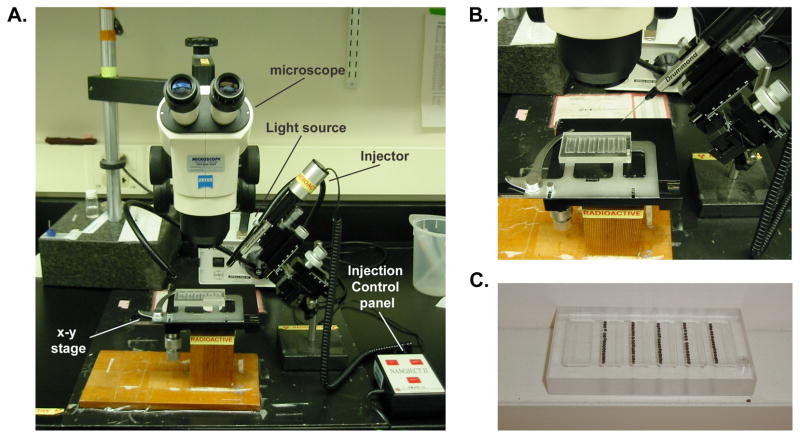
Dissecting Microscope and transmitted light source with double gooseneck arms. A manually operated X–Y stage is optional but recommended, particularly if homemade injection trays (Item 7 below) are to be used (see Figure 3.2A,B)
Microinjector that can accurately deliver 10–100nL volumes. Narishige and Drummond make two good options. For this protocol, we describe injections using the Drummond Nanoject II.
Pulled glass capillaries (micropipettes; injection needles) used for sample injection; obtainable from companies that make microinjectors (Narishige L = 90mm, OD = 1mm, ID = 0.6mm; Drummond L = 3.5 inches, OD = 1.14mm, ID = 0.53mm)
Micropipette puller (Sutter)
26 gauge Hamilton Syringe to be used for backfilling the micropipette needle with mineral oil (for use with Drummond Nanoject)
Low temperature (14–25°C) bioincubator is useful but not required
Homemade Injection Trays. These are optional but highly recommended to facilitate injection (see Note 2 and Figure 3.2C)
Wooden applicator sticks for manipulating and sorting embryos. Sticks can be shaped to a point using a boxcutter and then blunted slightly to avoid puncturing embryos during manipulation
Sterile Mineral Oil (Sigma; for use with Drummond Nanoject)
Ficoll solution. Ficoll (type 400 DL; Sigma) is dissolved to 5% w/v in 0.1X MBS; Adjust to pH 7.5
Gentamicin (50mg/mL; Gibco)
Figure 3.2. Microinjection apparatus.
(A) Example of a microinjection set-up using a dissecting microscope, fiberoptic light source and an X–Y stage (B) Close-up view of the X–Y stage with injection tray and injection apparatus (right). (C) Homemade injection tray with embryos prepared for injection in each of the five lanes.
3. Methods
3.1 Selection and Testing of Morpholino Oligonucleotides
Antisense morpholino oligonucleotides (MOs) block gene function by either binding to sequence near the ATG start codon of an mRNA and inhibiting its translation, or by binding to the splice junction of a gene and preventing splicing. Because complete sequence information, including splice junctions, is not available for X. laevis, translation blocking morpholinos are more commonly used in this species. X. laevis is pseudotetraploid, having undergone a partial duplication of the genome and thus it is important to search available data bases [e.g. Xenbase (http://xenbase.org/genomes/blast.do?); Entrez (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene); The Gene Index Project (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/Blast/index.cgi)] to determine whether there are multiple copies of your gene of interest and, if so, to identify sequences corresponding to both before designing MOs. MOs are purchased from GeneTools. The company provides instructions for optimal MO design on their website (http://www.gene-tools.com/node/18), or customers can upload relevant sequence information and GeneTools will identify the most appropriate target sequence. In most cases, it is necessary to either design one oligo that will block both copies of your gene of interest or, if this is not possible, to use a mixture of two oligos that will target both copies of the gene. A non-overlapping oligo may be used as a positive control, for additional verification of phenotype specificity. A standard negative control MO is available from GeneTools. Alternatively, mismatched MOs containing five mismatched bases distributed throughout the oligo sequence are a more stringent test of specificity. The appropriate dose is dependent upon the individual MO and must be determined empirically. To minimize potential toxic effects, we recommend starting at a low dose (1–5ng) and increasing as necessary. We have found that high doses of MO may be tolerated reasonably well (up to 100ng), depending on the particular MO.
-
1
MO solubility is an ongoing issue and appears to be dependent upon the individual oligonucleotide (see Note 3). MOs are shipped as lyophilized pellets. The MO is then resuspended, at a concentration appropriate to the particular use, in sterile (not DEPC-treated) dH2O, and heated at 65°C for 10 minutes to bring it into solution. For Xenopus injections, we have found that 1mM stock dilutions are sufficiently concentrated for all required applications. Create appropriate working dilutions and store in individual aliquots at −80°C (see Note 3). Prior to use, heat aliquots at 65°C for 10 minutes and vortex briefly to ensure that all MO is in solution to obtain an accurate concentration. Spin tubes at top speed for 5 minutes in a microcentrifuge to remove insoluble material that may clog the injection needle.
-
2
There are several methods outlined below to verify that MOs specifically knockdown the intended target.
Demonstrate reduction in protein synthesized by Immunohistochemistry (IHC) or Western blotting in morphants compared to control embryos.
In the absence of an adequate gene-specific antibody, demonstrate reduction of an overexpressed epitope-tagged protein in morphants compared to control embryos by IHC or Western blotting.
If splice-blocking morpholinos are used, demonstrate that the targeted splicing event is inhibited via polymerase chain reaction (PCR) to detect loss of the spliced form in morphants.
Ultimately the best test of specificity is to demonstrate that phenotype(s) attributed to knockdown of a particular gene are rescued by co-injection of a gene-specific mRNA that harbors silent mutations that prevent morpholino recognition.
3.2 Generation of Synthetic RNA for microinjection
mRNAs require a 5′ 7-methylguanosine cap, and a 3′ polyadenylate [poly(A)] tail to prevent degradation and promote efficient translation in vivo. For in vitro synthesized RNAs, the former is accomplished by addition of cap analog [m7G(5′)ppp(5′)G] to the synthesis reaction. A poly(A) tail can be added following the RNA synthesis reaction using a commercially available kit (e.g. Ambion poly(A) tailing kit) but is more commonly encoded in the cDNA or in the expression vector (e.g. pSP64T, obtainable from http://faculty.washington.edu/rtmoon/XE40.html (16)). Alternatively, polyadenylation can be accomplished in vivo by the inclusion of an SV40 polyadenylation signal in the expression vector. pCS2+ is a commonly used multipurpose expression vector that includes an SP6 promoter upstream, and an SV40 poly(A) signal downstream of the multiple cloning site, as well as a second polylinker with unique restriction sites for linearizing the template DNA downstream of the SV40 poly(A) signal (17, 18). A number of pCS2+ derivatives have been constructed that allow fusions to epitope tags or reporter proteins such as β-galactosidase to facilitate detection in downstream applications. As with MOs, the dose for a particular mRNA must also be empirically determined. RNA tends to be more toxic than MOs, thus much lower amounts (1–5pg) should be used initially when determining the appropriate dose.
-
1
Linearized DNA template should be prepared in advance and resuspended in H2O or TE at 0.5μg/μL. An example protocol is as follows: Cut 20μg of template DNA in 100μL reaction volume with the appropriate restriction enzyme. Run 5μL on an agarose gel to verify that cutting is complete. Extract once with Phenol:Sevag (1:1) and once with Sevag alone. Add Ammonium Acetate to 0.4M and 2 volumes 100% EtOH. Precipitate overnight at −20°C, or for 15 minutes at −80°C. Spin 10 minutes at 4°C at top speed, rinse with 70% EtOH. Vacuum dry DNA pellet and resuspend in 36μL DEPC dH2O. This will give a concentration of roughly 0.5μg/μL, assuming a 10% loss of input.
-
2
Capped mRNAs for overexpression and/or morpholino rescue are generated using Ambion’s mMESSAGE mMACHINE High Yield Capped RNA transcription kit.
-
3
To prepare capped RNAs, add the following ingredients, in the order listed below, to a sterile 1.5mL tube at room temperature (if tube is kept on ice, the spermidine in the buffer can precipitate the template DNA).
4μl DEPC treated dH2O
10μl 2X rNTP Mix [includes m7G(5′)ppp(5′)G cap analog]
2μl 10X transcription buffer
2μl linearized template DNA (0.5μg/μl)
2μl T7 or T3 RNA polymerase for a total volume of 20μl.
-
4
Tap tube to mix and spin briefly to collect contents at the bottom of the tube.
-
5
Incubate at 37°C for two to four hours (see Note 4).
-
6
Add 1μl DNase (from kit), incubate at 37°C for 15 minutes.
-
7
Before use in injection, the capped mRNA must be purified from the reaction components, particularly from the cap analog, which will compete with transcription of full-length product (see Note 5). Bring up reaction volume to 50μL with 30μL DEPC dH2O to minimize loss during the purification and do a single Phenol:Sevag (1:1) extraction.
-
8
During centrifugation for the Phenol:Sevag extraction, prepare sephadex G-50 spin columns according to manufacturer’s instructions. Briefly, invert column to resuspend the gel. Remove caps on both ends of the column, place in a collection tube (provided) and spin at 1100 × g for 1 minute in a swinging bucket centrifuge to drain excess buffer. Empty collection tube and repeat. Place column in new collection tube.
-
9
Following the Phenol:Sevag extraction, transfer the aqueous phase of the extraction (~50μL) to the center of the gel bed of the sephadex G-50 column and spin at 1100 × g for 4 minutes.
-
10
Transfer the G-50 column eluate to a new 1.5mL tube and precipitate the capped mRNA with 0.2M Ammonium acetate and two volumes of 100% EtOH at −80°C for 15 minutes. Spin at top speed in a microcentrifuge at 4°C for 10 minutes. Rinse pellet with ice-cold 70% EtOH, and vacuum dry briefly. Do not over dry.
-
11
Resuspend the small RNA pellet in 25μL DEPC dH2O and determine mRNA concentration by UV spectroscopy. Run 300ng of capped mRNA on a 1X MOPS gel to determine that a single full-length transcript has been synthesized and to verify expected concentration (see Note 6).
3.3 Collection of Testes
To harvest testes for fertilization, a male is anesthetized in 0.2% Tricaine for 20–40 minutes until unresponsive when its claw is pinched firmly between your thumb and forefinger.
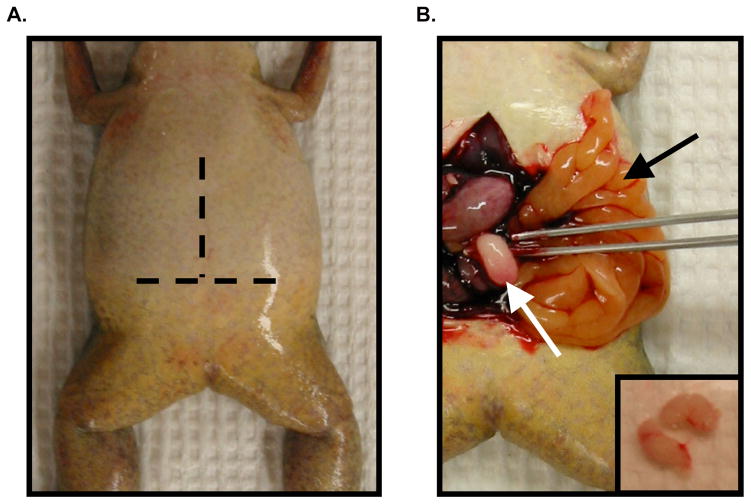
Use a pair of blunt forceps to pull the skin up from the body wall. A tissue placed over the prospective incision site to remove excess moisture may aid in grasping the slippery skin. Using either a surgical knife or a pair of fine-pointed scissors, make a horizontal incision in the skin across the abdomen (see Fig. 3.3A). Make a parallel incision in the underlying body wall, being careful not to damage the underlying organs. Make a third, this time vertical incision along the length of the abdomen, beginning at the midpoint of the horizontal incision and continuing anteriorly up the midline through both layers (see Fig. 3.3A). Fold back the resulting flaps like the pages of a book (see Fig. 3.3B). Clip ventricle of the heart with the scissors to exsanguinate and ensure death. To prevent contamination of the testes, take particular care to avoid perforating the gut during the dissection.
Using a blunt pair of forceps, move the overlying organs aside and pull the fat bodies out (Fig. 3.3B, black arrow), following them back to either side to find the testes (Fig. 3.3B, white arrow). Remove each testis by snipping it away from the fat bodies with scissors.
Once the testes are removed, carefully snip away any adherent fat bodies and vasculature and rinse in a petri dish filled with cold 1X MBS to remove remaining blood before storing in cold Testis Buffer.
For optimum results, use freshly isolated testes. Remaining testes may be stored at 4°C and used for up to two weeks, although sperm viability will decrease with time (see Note 7).
Dispose of the carcass appropriately according to IACUC regulations.
Figure 3.3. Harvesting testes from a male frog.
(A) Ventral view of an anesthetized male frog. Dashed lines represent the approximate location of horizontal and vertical incisions to be made. (B) Testis removal following incisions in panel A and exanguination (described in Methods). The testis (white arrow, and inset) is located at the end of the fat bodies (black arrow) on the left and right side of the abdominal cavity.
3.4 Collection and Fertilization of Embryos
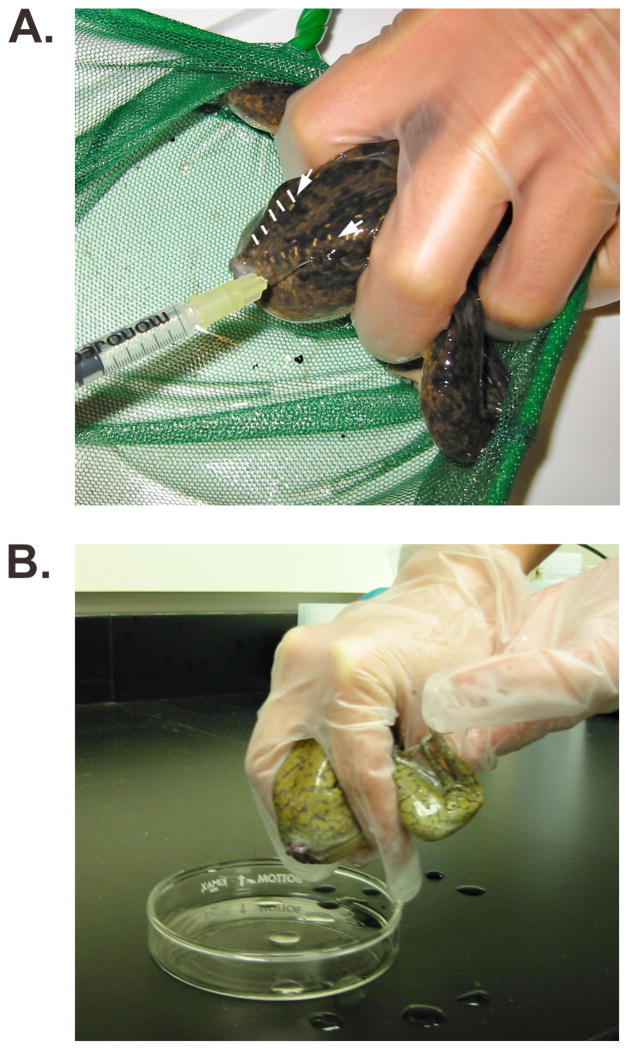
To induce spawning, prime three mature females with 1200U human chorionic gonadotropin (HCG) solution by injecting 0.3mL of 400U/mL HCG per frog into the dorsal lymph sac (see Figure 3.4A and Note 8). Injected females are housed in plastic drawers or buckets in a 15°C bioincubator in 1X Holtfreter’s Frog Bath overnight (see Note 9). Spawning will begin 12–14 hours later if they are kept at 15°C. It may be necessary to exchange the Frog Bath to bring the frogs up to room temperature more quickly if spawning does not begin. Alternatively, females can be injected the day eggs will be used, housed at room temperature and will begin spawning within approximately six hours.
Collect eggs in a glass petri dish containing 1X MBS (see Note 10) To induce spawning, pick up the female in one hand, holding the rear legs anteriorly against her body with the index and middle fingers on either side of her torso (see Figure 3.4B). To keep the frog calm, cover the eyes and head with the palm of the same hand. Gently apply pressure to the abdomen with thumb. The other hand may be used to stabilize the animal on the ventral side and to apply gentle pressure laterally (see Note 11). More than one dish may be used if necessary to distinguish the eggs from individual frogs.
Just prior to fertilization, cut a small piece of testis (~1/5, from Section 3.3) and place it into a clean 1.5mL tube containing ~1mL 1X MBS. Crush with a conical tissue grinder to make a testis slurry, and store on ice until used up (~3 fertilizations). Alternatively, a small piece of whole testis may be used, as described below.
Remove as much of the 1X MBS as possible from the dish of eggs with a plastic transfer pipet, taking care to avoid touching the eggs, which may damage them as they are fragile at this stage. The jelly-like consistency of the egg mass will protect the eggs from excess surface tension until they are fully dejellied (step 5, this section). Add ~300μL testis slurry and ~1mL 0.1X MBS and agitate dish to ensure that solution reaches all eggs. The lower salt solution facilitates fertilization by increasing sperm activity (see Note 12). Allow eggs and sperm to incubate together for 2–3 minutes in the ~1mL of 0.1X MBS to permit fertilization to take place. After fertilization, fill the dish with aged tap water or 0.1X MBS and let stand for about 20 minutes. During this time, the jelly coat will form such that the eggs adhere to each other and to the dish. Eggs that have been fertilized will rotate within the fertilization membrane such that the pigmented animal pole faces upward. From this time on, embryos may be kept at varying temperatures, ranging from 14–25°C, to control the rate of development as necessary (see Note 13). An approximation of the changes in developmental timing based on temperature are as follows: 25°C = 100%, 20°C ≈ 75%, 16°C ≈ 50%, and 14°C ≈ 25% normal developmental rate. Timing of normal development is based on staging by Nieuwkoop and Faber (19).
To prevent the eggs from adhering to one another and to the needle during injection, it is important to fully dejelly the eggs. Once rotation is complete (~20 minutes) and prior to injection, embryos may be dejellied at any time. It should be noted, however, that empirical evidence suggests that cleavage patterns may be more regular if they are dejellied after they have cleaved at least once. Pour off the aged tap water or 0.1X MBS, and fill the dish with 2% cysteine solution (pH 7.8–8.0). Very gently agitate on an orbital shaking platform for 3–5 minutes until the jelly coat is completely removed and the eggs are clustered tightly together in the center of the dish.
Once the eggs are dejellied, remove the cysteine solution using a plastic transfer pipet or by tilting the dish and pouring into a waste container. The eggs are free-floating and fragile at this point and thus it is important to tilt the dish in order to keep the eggs covered with a small volume of fluid at all times to reduce damage from surface tension. Using the same technique, rinse the eggs 2–3 times with 1X De Boer’s solution and then 2 times with 0.1X MBS. Embryos can be cultured in 0.1X MBS throughout early development.
Figure 3.4. Spawning eggs from a female frog.
(A) Priming a mature female by injection of human chorionic gonadotropin into the dorsal lymph sac on the posterior aspect of the frog. The injection needle is inserted just lateral to the V-shaped lateral line sutures (white arrows; dashed white lines demarcate the sutures on the left side of the frog’s back). The head and eyes of the frog are covered with the palm of the hand (out of view) to reduce stress and the frog is immobilized by using a net to prevent forward motion and by holding the legs forward with two fingers. (B) Spawning of a female frog. The frog is held as described above, using the opposite hand, instead of the net, to provide additional stability.
3.5 Targeted Embryo Injection and Culture
There are many different small volume microinjectors available, but two commonly used apparati for injection of Xenopus eggs and oocytes are the Pico-injector (Medical Systems) and the Nanoject (Drummond Scientific). The Pico-injector uses compressed gas to reliably deliver nanoliter volumes through micropipettes by applying a regulated pressure for a digitally set period of time. For this method, the tip diameter of the micropipette is irrelevant, and thus finely tapered micropipettes with very small tip openings can be used to minimize damage to embryos, which is especially important when injecting single cells at late cleavage stages. A disadvantage is that injection volume must be calibrated for each new micropipette by measuring the size of the drop delivered using a stage micrometer. The Nanoject uses positive volume displacement to dispense preset volumes. This instrument is less costly, and has the advantage that one does not need to calculate drop size for each micropipette. Micropipettes must exceed a certain minimal tip diameter, however, and must also be back-filled with mineral oil prior to use. Below, we describe a protocol for use of the mineral oil-based volume displacement method. For a detailed description of the gas pressure-based injector see (20).
To generate needles for microinjection, glass capillaries are heated and pulled to a fine point using a needle puller. Specific settings will vary between needle pullers and some experimentation with the various parameters (i.e. heat, pull, velocity, etc.) is required. However, once the appropriate settings for a given capillary have been established, many needles may be pulled quickly in case replacement is required during the injection day or for future experiments.
A good needle is sharp with a small bore at an angle of approximately 45°. Under a dissecting microscope, clip the tip of the needle using a pair of sharp forceps. The needle should be sharp but not so thin that the tip is flexible and will bend when it comes in contact with the embryo.
Backfill the needle with mineral oil using a Hamilton Syringe. Insert the syringe all the way into the tip of the needle. Applying constant pressure to the plunger, eject the oil while slowly withdrawing the syringe to avoid introducing bubbles, which will compromise the accuracy of the volume displacement method used by the injector (see Note 14).
Load the needle onto the injector taking care not to introduce bubbles. Be sure that the needle is loaded straight to avoid bending the recessed plunger on the injector, and apply gentle but firm pressure until the needle pops snugly into the rubber o-ring.
The injection control box has three buttons labeled “empty,” “fill,” and “inject.” The inject button may also be operated using a foot pedal, allowing for hands-free injection. Lay a piece of parafilm on the injection stage, clean side up. Depress the “empty” button to eject one third to one half the volume of oil onto the parafilm in order to make room for the injection solution (see Note 15). Pipet a few microliters of the sample solution onto the parafilm and depress the fill button to draw sample into the needle, taking care to avoid air bubbles by keeping the needle submerged (see Note 16). When preparing to inject, depress the “inject” button to eject several droplets into air to determine if the injector is yielding droplets of roughly equivalent size.
Mount the injection tray on the injection stage (see Figure 3.2B) and fill with 5% Ficoll solution. Check that the injector is aligned properly with the stage such that the needle is positioned over the tray and tracks appropriately along each lane.
Using a disposable plastic transfer pipet, select and transfer embryos to the injection tray. When selecting embryos be sure to choose ones that look healthy (i.e. cleavage and pigmentation patterns are regular).
Arrange embryos of the desired stage in the injection tray, with the appropriate side facing the needle (e.g. if dorsal injection is desired, align the embryos with the dorsal side facing the injector).
The injection volume and speed are controlled by the orientation of a panel of dipsticks located on the right side of the injection control box. Find the key for dipstick settings and their corresponding volumes and speeds on the bottom of the control box and set them appropriately.
Proceed with injection, periodically injecting into air to ensure that the needle is not clogged and droplets are of expected size.
Once injections are complete, culture the embryos in Ficoll solution for several hours to overnight to allow them to heal (see Note 17).
Rinse 2–3 times in 0.1X MBS and remove any unhealthy or dying embryos as they will compromise the health of the others in the dish, then culture embryos in 0.1X MBS. 50ug/mL gentamicin may be added in order to inhibit bacterial growth (optional).
Continue to culture embryos in 0.1X MBS (+/− gentamicin) until desired stage is reached. Culture solution should remain clear and be kept free of debris.
Acknowledgments
This work was supported by grants from the NIH (RO3HD058841 and RO1HD37976) and Shriners’ Hospital Research Foundation to JLC. MM was supported by NIH grant T32HD049309.
Footnotes
Frogs may be handled with clean hands or with vinyl gloves. Gloves made from other materials (e.g. latex) will damage the frogs’ fragile skin. If gloves are not used, hands should be washed thoroughly before and after contact with the frogs to ensure that they are free from lotions and trace detergent, which can be damaging to the frog, and to remove bacteria that may have transferred from the skin of the frogs.
Though not required, we have found it extremely useful to use homemade injection trays (Fig. 3.2C) to hold embryos and oocytes for injection. These trays can be ordered from any custom acrylic fabricator and will be manufactured to specification. Our trays consist of a x x x cm rectangular hollowed out acrylic block with narrow strips of acrylic used to generate troughs for holding eggs. Acrylic strips (~4 mm in width) are placed 1.5–2mm apart, alternating strips that are 2 mm and 4 mm in height, to create a trough in which one wall is taller than the other; this allows the embryo to rest against the taller wall while being injected from the opposite side. Embryos can be lined up in recessed lanes, allowing them to be completely submerged in Ficoll solution without floating about. This ensures that they are not subject to excess surface tension, yet are still held in place so that keeping track of which embryos have been injected is not an issue. A number of other techniques have also been used to hold embryos in place during injection. In the most basic setting, embryos can be placed in a petri dish and, using a pair of blunt forceps, positioned and stabilized appropriately for injection. Following injection, the embryos are placed to one side of the dish to keep track of which ones have been injected. Alternatively, a plastic petri dish may be fitted with a nylon mesh and used to hold embryos in place (20).
GeneTools recommends storing MO stocks and working dilutions at room temperature and, if necessary, heating them at 65°C for 10 minutes prior to use (http://www.gene-tools.com/files/Essential%20INFO%2002-09.pdf). We have found that while some oligos are stable (i.e. they generate reproducible results) when stored at room temperature, we have also found that both the more concentrated stocks and working dilutions of certain oligos have given more reproducible results when stored at −80°C and heated at 65°C for 10 minutes prior to use.
It is necessary to determine the appropriate cap analog:GTP ratio for your particular transcript. Increasing cap analog increases the fraction of capped transcripts but reduces yield. Yield is also dependent on the efficiency of the particular transcription enzyme (i.e. SP6, T7 or T3). We bias the reaction toward increasing the amount of capped transcript, as uncapped mRNA is rapidly degraded in the embryo. Ambion provides a very detailed protocol entitled “Synthesis of Capped RNA Transcripts” in the mMESSAGE mMACHINE manual which describes the potential benefits to varying cap analog:GTP ratio and incubation time which may be useful.
As an alternative to purification of capped mRNA on a sephadex column, nucleotide removal may also be accomplished by isopropanol precipitation after DNase treatment as follows: Add 115μl RNase free water and 15μl Ammonium acetate stop solution (from kit). In a fume hood, add 75μl Tris-buffered phenol and 75μl Sevag. Vortex well to mix. Microcentrifuge at 4°C for 5 minutes. Remove the aqueous top layer to a new 1.5mL tube. Discard the bottom organic layer into an appropriate organic waste container. Add 150μl 100% isopropanol, mix by inverting tube and allow RNA to precipitate at −20°C for 15 minutes to 1 hour. Spin at 4°C in a microcentrifuge for 15 minutes at top speed. Remove and discard the supernatant. Rinse pellet with 70% ethanol, spin briefly, remove all supernatant and resuspend in 25μL DEPC-treated dH2O.
The expected yield for a single reaction starting with 0.5μg cDNA template is 15–20μg capped mRNA. The Ambion manual provided with the mMESSAGE mMACHINE kit provides several useful sections on troubleshooting various issues with capped RNA synthesis. Low RNA yield may be due to several factors. Increasing the incubation time to 4–6 hours, the amount of template, and/or the amount of polymerase are simple first steps to try. We have found that the quality and purity of the cDNA template is very important. Preparing new plasmid DNA or repurifying the cut template by phenol/chloroform extraction and ethanol precipitation often resolves yield issues for established transcripts. For transcripts that are being used for the first time, it may be necessary to empirically determine the appropriate ratio of cap analog:GTP as addition of the cap analog dramatically reduces yield. Some reactions may yield multiple products. Multiple bands on the gel may be due to persistent mRNA secondary structure from incomplete denaturation on the gel or to an artifact of electrophoresis, or to premature termination of the mRNA synthesis reaction. In the case of the latter, the incomplete product will lack a polyA tail and will likely be degraded once injected. It is, however, important to adjust the concentration for injection appropriately so that it reflects only the full-length product.
To check sperm viability, the testis may homogenized in 1X MBS and a small amount of sperm may be diluted in 0.1X MBS, transferred to a glass slide and examined for motility under a light microscope.
We prime three females per experiment day to ensure that enough eggs are produced for a particular day’s experiments. We recommend priming at least two frogs, as there is significant variability between individual frogs as to how well they spawn and the quality of eggs laid. When performing an experiment (such as a microarray analysis) in which minimizing background variability is essential, we recommend using the eggs from a single female.
If older females are used, ovary development may be stimulated by pre-priming frogs 3–10 days prior to desired injection day with 50U Pregnant Mare Serum Gonadotropin (PMSG; Sigma) injected into the dorsal lymph sac. Females will need to be temporarily kept in a separate holding tank to distinguish them from females that have not been pre-primed
In the wild, eggs are spawned into fresh water which induces formation of a jelly coat around the embryos. The jelly coat serves several functions, including providing mechanical support, acting as a block to polyspermy and activating the acrosomal reaction required for fertilization. To facilitate in vitro fertilization, eggs are spawned into a high salt solution (1X MBS) to prevent formation of the jelly coat. Eggs may also be spawned into a dry petri dish but care must be taken to avoid splashing water into the dish as this will induce instantaneous formation of a jelly coat and inhibit subsequent fertilization.
The cloaca of a female that is spawning will be dark red or pink in color. Females that are not spawning should not be squeezed. To induce spawning it may necessary to gently squeeze the frog as described in the methods. However, it is absolutely necessary to be gentle when squeezing and one must be sure not to press on the back of the animal as this may cause injury. Alternatively, the frog may push out the eggs when you pick her up. In this case, it is only necessary to stabilize her, using the same technique described above, while holding her over the egg collection dish.
Alternatively, testis may be used directly to fertilize eggs individually. Remove 1X MBS, add ~1mL 0.1X and use a small piece of testis to touch each of the eggs. Proceed as described
Embryos may be allowed to develop at a range of temperatures, from 14–23°C. This allows for control of the rate of development to facilitate injections at a particular stage, or to produce embryos at varying stages simultaneously. While changing the incubation temperature does not cause problems for most applications, it is important to include the appropriate controls to ensure that your particular experimental output is not sensitive to changes in temperature.
Introduction of air bubbles into the injection needle is a common issue with this apparatus. Some tips to avoid introducing bubbles are as follows: 1) When backfilling the needle with mineral oil, be sure that the tip of the syringe is inserted as far as possible into the tip of the needle. Begin ejecting oil firmly before beginning to withdraw the syringe and continue ejecting oil until the syringe is completely withdrawn. Excess oil spilling out of the back of the needle can be blotted off with a tissue later. 2) When loading the needle onto the injector use a single upward movement. Avoid moving the needle up and down onto the plunger as this will also introduce unwanted bubbles. When seating the needle in the orings, take care not to chip the back of the needle as this will compromise the seal between the needle and the rubber o-ring.
A very small tip on the needle may prevent the mineral oil from being ejected effectively, if at all. If this is the case, carefully clip the needle back with sharp forceps to a 45° angle (this can be done on the injector if you’re careful) and attempt to eject the mineral oil. Repeat until the mineral oil is able to be ejected consistently
When filling the needle with sample, keep the tip submerged in the sample and fill slowly. The plunger withdraws more quickly than either the solution or the mineral oil can pass through the needle, particularly when the tip is very small. This creates a vacuum, which can result in bubbles if the sample is depleted too quickly during filling. Pausing occasionally to allow the mineral oil and sample to catch up prevents this issue. A very small bubble or two may be unavoidable. If this is the case, be sure that the size of the bubble is minimized by depressing the eject button until sample starts to exit the tip of the needle. This will ensure that compression of the gas in the bubble does not affect the volume of oil displaced by the plunger during injection. A few small bubbles may not pose a significant problem, but if they become too numerous or too large, the needle will have to be reloaded.
Prolonged culture in Ficoll, while not detrimental, may affect the rate of development. Therefore, it may be necessary to also incubate controls in Ficoll. We recommend prompt removal from Ficoll solution once embryos have healed for several hours.
References
- 1.Dale L, Slack JM. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987;99:527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- 2.Moody SA. Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Dev Biol. 1987;122:300–319. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- 3.Moody SA. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev Biol. 1987;119:560–578. doi: 10.1016/0012-1606(87)90059-5. [DOI] [PubMed] [Google Scholar]
- 4.Green JB, Smith JC, Gerhart JC. Slow emergence of a multithreshold response to activin requires cell-contact-dependent sharpening but not prepattern. Development. 1994;120:2271–2278. doi: 10.1242/dev.120.8.2271. [DOI] [PubMed] [Google Scholar]
- 5.Galli A, Roure A, Zeller R, Dono R. Glypican 4 modulates FGF signalling and regulates dorsoventral forebrain patterning in Xenopus embryos. Development. 2003;130:4919–4929. doi: 10.1242/dev.00706. [DOI] [PubMed] [Google Scholar]
- 6.Scharf SR, Gerhart JC. Axis determination in eggs of Xenopus laevis: a critical period before first cleavage, identified by the common effects of cold, pressure and ultraviolet irradiation. Dev Biol. 1983;99:75–87. doi: 10.1016/0012-1606(83)90255-5. [DOI] [PubMed] [Google Scholar]
- 7.Kao KR, Masui Y, Elinson RP. Lithium-induced respecification of pattern in Xenopus laevis embryos. Nature. 1986;322:371–373. doi: 10.1038/322371a0. [DOI] [PubMed] [Google Scholar]
- 8.Dale L, Howes G, Price BM, Smith JC. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992;115:573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- 9.Jones CM, Lyons KM, Lapan PM, Wright CV, Hogan BL. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992;115:639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- 10.Maeno M, Ong RC, Suzuki A, Ueno N, Kung HF. A truncated bone morphogenetic protein 4 receptor alters the fate of ventral mesoderm to dorsal mesoderm: roles of animal pole tissue in the development of ventral mesoderm. Proc Natl Acad Sci U S A. 1994;91:10260–10264. doi: 10.1073/pnas.91.22.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki A, Thies RS, Yamaji N, Song JJ, Wozney JM, Murakami K, Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sargent TD, Mathers PH. Analysis of class II gene regulation. Methods Cell Biol. 1991;36:347–365. doi: 10.1016/s0091-679x(08)60287-3. [DOI] [PubMed] [Google Scholar]
- 13.Vize PD, Melton DA, Hemmati-Brivanlou A, Harland RM. Assays for gene function in developing Xenopus embryos. Methods Cell Biol. 1991;36:367–387. doi: 10.1016/s0091-679x(08)60288-5. [DOI] [PubMed] [Google Scholar]
- 14.Heasman J. Morpholino oligos: making sense of antisense? Dev Biol. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- 15.Ogino H, Ochi H. Resources and transgenesis techniques for functional genomics in Xenopus. Dev Growth Differ. 2009;51:387–401. doi: 10.1111/j.1440-169X.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 16.Zelus BD, Giebelhaus DH, Eib DW, Kenner KA, Moon RT. Expression of the poly(A)-binding protein during development of Xenopus laevis. Mol Cell Biol. 1989;9:2756–2760. doi: 10.1128/mcb.9.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rupp RA, Snider L, Weintraub H. Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev. 1994;8:1311–1323. doi: 10.1101/gad.8.11.1311. [DOI] [PubMed] [Google Scholar]
- 18.Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- 19.Nieuwkoop PD, Faber J Hubrecht-Laboratory. A systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. 2. North-Holland Pub. Co; Amsterdam: 1967. Normal table of Xenopus laevis (Daudin) [Google Scholar]
- 20.Sive Hazel L, RMG, Harland Richard M. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- 21.Lane MC, Sheets MD. Heading in a new direction: implications of the revised fate map for understanding Xenopus laevis development. Dev Biol. 2006;296:12–28. doi: 10.1016/j.ydbio.2006.04.447. [DOI] [PubMed] [Google Scholar]