Summary
Background:
Given the worldwide epidemic of cardiovascular diseases, a more effective means of dissolving thrombi that cause heart attacks, could markedly reduce death, disability and healthcare costs. Plasminogen activators (PAs) such as streptokinase (SK) and tissue plasminogen activator (TPA) are currently used to dissolve fibrin thrombi. SK is cheaper and more widely available, but it appears less effective because it lacks TPA’s fibrin-targeted properties that focus plasminogen activation on the fibrin surface.
Objective:
We examined whether re-programming SK’s mechanism of action would create PAs with greater fibrin-targeting and potency than TPA.
Methods and Results:
When fibrinogen consumption was measured in human plasma, reprogrammed molecules SKΔ1 and SKΔ59 were 5-fold and > 119-fold more fibrin-dependent than SK (P < 0.0001), and 2-fold and > 50-fold more fibrin-dependent than TPA (P < 0.001). The marked fibrin-targeting of SKΔ59 was due to the fact that: (i) it did not generate plasmin in plasma, (ii) it was rapidly inhibited by α2-antiplasmin, and (iii) it only processed fibrin-bound plasminogen. To assess the fibrin-targeting and therapeutic potential of these PAs in vivo, a novel ‘humanized’ fibrinolysis model was created by reconstituting plasminogen-deficient mice with human plasminogen. When compared with TPA, SKΔ1 and SKΔ59 were 4-fold (P < 0.0001) and 2-fold (P < 0.003) more potent at dissolving blood clots in vivo, respectively, on a mass-dose basis and 2–3 logs more potent than TPA (P < 0.0001) when doses were calibrated by standard activity assays.
Conclusion:
These experiments suggest that reprogramming SK’s mechanism of action markedly enhances fibrin-targeting and creates, in comparison with TPA, activators with greater fibrinolytic potency.
Keywords: fibrin specificity, fibrinolysis, streptokinase, tissue plasminogen activator
Introduction
Heart attacks and strokes are the major causes of death and disability worldwide [1]; both are caused by the occlusion of arteries by a fibrin-containing blood clot. Plasminogen activators (PAs) trigger the dissolution of these fibrin clots (fibrinolysis), thereby restoring blood flow and reducing mortality and disability. All PAs catalyze the conversion of plasminogen (Pg) to the protease plasmin, which digests fibrin to dissolve the clot (Fig. 1). The two PAs approved by the FDA to treat humans, tissue plasminogen activator (TPA) and streptokinase (SK), work through markedly different mechanisms [2,3]. TPA is a serine protease that directly cleaves Pg to plasmin. TPA is considered to be ‘fibrin-targeted or specific’ because its catalytic efficiency is markedly enhanced for Pg that is bound to fibrin in the clot [4,5]. In addition, plasma contains an inhibitor, α2-antiplasmin (α2AP), that inactivates plasmin and may, to a lesser extent, inhibit TPA, further confining the action of these proteases to the clot surface [6-8]. In contrast, SK is a fibrin-independent Pg activator. While SK forms an activator complex with plasmin (SK·plasmin), it also has the unique ability to form an active protease complex with Pg (SK·Pg*) [9,10]. Both SK·plasmin and SK·Pg* are potent PAs in the absence of fibrin and are resistant to α2AP, which further exacerbates the production of plasmin in plasma [11,12]. Treatment with SK degrades clotting factors such as fibrinogen, activates bradykinin (which lowers blood pressure), and depletes circulating Pg [13-16]. These side reactions appear to reduce SK’s efficacy as a clot-dissolving agent in the treatment of heart attack and stroke [17,18].
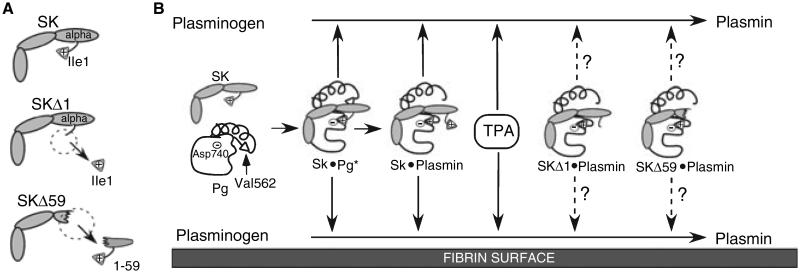
Fig. 1.
Schema of Pg activation by different PAs. (A) Schematic structure of SK, SKΔ1 and SKΔ59. (B) The activation of Pg to plasmin in solution (top) and on the fibrin surface (bottom) is shown. The amino acids involved in non-proteolytic activation of Pg by SK (SK isoleucine 1 (Ile 1), Pg aspartic acid 740 (Asp 740)) and the proteolytic generation of plasmin by other PAs (Pg valine 562 (Val 562) and Pg Asp 740) are indicated. Arrows with solid lines indicate known activity; arrows with dashed lines indicate potential activity.
Two structural elements of the SK molecule may account for its fibrin-independent mechanism of action in vitro [12,19]. First, the formation of the SK·Pg* requires a salt bridge between the N-terminal amino group of Ile1 from SK and Asp740 of Pg [20-23]. This interaction mimics the intramolecular salt bridge that forms when Pg is converted to plasmin. Deletion of Ile1 creates an SK variant (SKΔ1) that can form a functional activator complex with plasmin and not Pg; we have shown that this mutation decreases plasmin formation in plasma by 30-fold (Fig. 1, [20,24]). Active plasmin is typically found only on the fibrin surface, where it is generated by trace amounts of endogenous TPA [25], and it is relatively protected from the inhibitory effects of α2AP. Therefore, formation of the active SKΔ1·plasmin complex, similar to the staphylokinase·plasmin complex [26], may first occur on fibrin, conferring a degree of fibrin-targeting on Pg activation. Catalytic quantities of the SKΔ1·plasmin complex will then, much like the staphylokinase·plasmin complex, activate much larger quantities of Pg that have fibrinolytic potential. However, this initial fibrin-targeting may be counterbalanced by the anticipated resistance of the SKΔ1·plasmin complex to α2AP when it dissociates from the fibrin surface.
The alpha domain of SK is required for resistance to α2AP and it also interacts with the kringle domains of Glu-Pg to induce an open conformation that makes Glu-Pg susceptible to activation [12,19]. SKΔ59 lacks the alpha domain and in the presence of Pg alone is several hundred times less efficient as a PA than SK [12,19]. However, the activity of the SKΔ59·plasmin complex is markedly enhanced for unfolded forms of Pg such as Lys-Pg or Glu-Pg in the presence of fibrin; it is also susceptible to α2AP [12,19]. For these reasons SKΔ59 may act as a fibrin-specific PA like TPA. We examined whether these targeted mechanistic changes in SK could produce PAs with fibrin-targeting and potency comparable with TPA. To permit comparison among these PAs in vivo, we created a novel, humanized model in genetically modified mice to examine blood clot dissolution. In this model, SKΔ1 and SKΔ59 have significantly greater potency than TPA for dissolving clots.
Materials and methods
Materials
Proteins and reagents were obtained from the following suppliers: human fibrinogen, human α2-AP, Calbiochem, Gibbstown, NJ, USA; urokinase and Pg-free human fibrinogen, American Diagnostica, Stamford, CT, USA; TPA, South San Francisco, CA, USA, BioResponse, Hayward, CA, USA; human Lys-plasmin, bovine thrombin, and S-2251 (H-D-valyl-L-leucyl- L -lysine-p-nitroanilide dihydrochloride), Chromogenix AB, Stockholm, Sweden; S-2288 (H-D-isoleucyl- L -prolyl- L -arginine-p-nitroanilide dihydrochloride), Chromogenix AB, Milan, Italy; MUGB (4-methylumbelliferyl p-guanidinobenzoate), Sigma-Aldrich, St Louis, MO, USA; heparin, Elkins-SINN, Cherry Hill, NJ, USA; pooled fresh-frozen human plasma, MCG Blood Bank, Augusta, GA, USA; [125I]NaI, NEN-Dupont, Boston, MA, USA; Lys-Sepharose 4B, Pharmacia Biotech, Uppsala, Sweden.
Proteins
Pg was purified from human plasma using lysine-Sepharose and was ≥ 95% pure when analyzed by reducing SDS-PAGE [27]. Active sites were titrated by MUGB [28]. Purified SKΔ1, SKΔ59, micro-Pg, human and mouse α2AP were prepared as described [19,20,29]. Micro-Pg was activated to microplasmin [30]. Radiodinated human fibrinogen (American Diagnostica) had a specific activity of ~ 2.0 × 106 cpm μg−1 [31]. Soluble fibrin (DD) E fragment was prepared as described [32].
Steady-state Pg activation
The kinetics of Pg activation by TPA or activator complexes were studied as previously described [12,33,34]. Initial reaction rates were determined in triplicate from the slopes of plots of absorbance/time2 [34]. Pg activation parameters, Km (the apparent Michaelis constant for the Pg substrate) and kcat (the catalytic rate constant of activation) for SKΔ1-micro-plasmin and SKΔ59-microplasmin complexes, were calculated as described [33]. An ε1M at 405 nm of 10 000 was employed for p-nitroanilide.
A standard calibration curve for TPA activity (580 000 IU mg−1) was generated using the substrate S-2288 [5].
α2AP inhibition assays
To assess the inhibition of Pg activator complexes by α2AP, human plasmin (12 nM) was incubated with SK, SKΔ1 or SKΔ59 (0–750 nM) in assay buffer (50 mM Tris–HCl, 100 mM NaCl, pH 7.4) for 10 min at 25 °C before human or mouse α2AP (15 nM) was added. S-2251 (0.5 mM) was added 10 min later, and the change of absorbance was monitored at 405 nm to determine the residual plasmin activity. To measure the formation of α2-AP-plasmin complexes in human plasma, TPA (2 or 10 nM), SKΔ1 (2 or 10 nM), SKΔ59 (20 or 100 nM) or SK (2 or 10 nM) were added to human plasma and incubated at 37 °C for 2 h. The reaction was stopped by aprotinin (2000 KIU mL−1 final). The concentration of α2AP-plasmin complexes was determined by an ELISA (American Diagnostica Inc.).
Fibrin plate assay
The fibrin plate method [35,36] was used with slight modification. A mixture of human fibrinogen (2 mg mL−1), human Pg (0.2 mg mL−1), BSA (1 mg mL−1), 1.5% agar and CaCl2 (10 mM) in PBS buffer was clotted with thrombin (1 Unit mL−1), producing a 2-mm fibrin layer in a Petri dish. After 60 min at 25 °C, PAs (0.01–500 nM; 3 μL) were added to the clot surface in duplicate. The plate was incubated for 17 h at 37 °C. The area of lysis was assessed by measuring the perpendicular diameters of the lysis zone and was plotted vs. PA concentration.
Turbidimetric clot lysis assay
The fibrinolytic activities of various PAs were measured using a microtiter plate turbidimetric assay [37]. The time required for 50% loss of turbidity (t1/2) was used as a measure of fibrinolytic rate.
Assays for determination of fibrinogen and Pg in plasma
The concentration of fibrinogen was determined by the sodium sulfite method [38]. The concentration of Pg was determined by a synthetic substrate assay, as described [39].
Humanized in vivo model of experimental fibrinolysis in mice
Human plasma (25 μL) was mixed with trace amounts of human 125I-fibrinogen and clotted in PE50 tubing (Clay Adams, PE-50; Becton Dickinson, Sparks, MD, USA) with CaCl2 (20 mM) and thrombin (0.1 units) at 37 °C for 60 min. Plasminogen-deficient mice (Pg−/−, B6.129P2-Plgtm1Jld/J; Jackson Laboratory, Bar Harbor, ME) weighing 18–26 g were anesthetized by pentobarbital (IP, 70 mg kg−1). Both internal jugular veins were exposed. The left vessel was used to embolize the thrombus and supplement human Pg (14 g kg−1); the right was used for infusion of PA (n = 4 animals/group) and heparin (100 U kg−1). Control animals (n = 11) were similarly treated only with heparin (100 U kg−1). Two hours after embolization mice were euthanized. Blood was collected and centrifuged (at 2000 g, 20 min at 4 °C) to obtain plasma that was stored at −80 °C until used. The heart and lungs were dissected and γ-counted to determine the amount of residual thrombus radioactivity. The amount of lysis was computed using the following equation: percentage of lysis = 100–100 × (embolized clot cpm − residual clot cpm)/(embolized clot cpm). Experiments were performed according to a protocol approved by the IACUC of the Medical College of Georgia.
Statistical analysis
All data are expressed as mean ± SEM. An unpaired Student’s t-test was used to analyze comparisons. Dose response curves were analyzed to obtain EC50s for each agent and compared with PRISM statistical software [40]. A P < 0.05 was considered significant.
Results
Comparisons of fibrin-dependency in human plasma
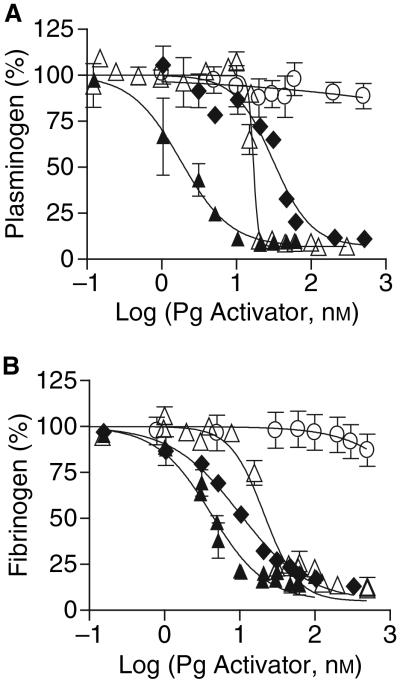
To determine whether these Pg activators required fibrin for efficient Pg activation, we examined how much Pg they activated in human plasma, in the absence of a fibrin clot. Fibrinogen consumption was also measured as a complementary indicator of the amount of plasmin generated. Despite its reputation as a fibrin-targeted PA, TPA caused 50% consumption of Pg and fibrinogen at concentrations of 31 and 10 nM, respectively (the concentration of PA that causes 50% consumption will hereafter be termed EC50; Fig. 2A,B, Table 1). As expected, more Pg and fibrinogen consumption was observed when plasma was treated with SK (EC50 = 1.8 and 4.2 nM, respectively). SKΔ1 was 9-fold and 5-fold more fibrin-dependent than SK in terms of Pg and fibrinogen consumption. SKΔ59 was > 278-fold and 119-fold more fibrin dependent than SK in terms of Pg and fibrinogen consumption. SKΔ1 displayed a slightly greater fibrin-dependent pattern of fibrinogen consumption than TPA and a slightly less fibrin-dependent pattern of Pg consumption (Fig. 2; Table 1). Remarkably, SKΔ59 caused less consumption of Pg and fibrinogen than TPA, by > 16-fold and 50-fold, respectively (EC50 >> 500 nM, Fig. 2, Table 1).
Fig. 2.
Effect of PAs on Pg and fibrinogen consumption in human plasma. PAs (0–500 nM) TPA (◆), SKΔ1 (Δ), SKΔ59 (○) or SK (▲) were added to human plasma and incubated at 37 °C for 2 h. The residual Pg concentration (A) was determined by a synthetic substrate assay (Materials and methods). The residual fibrinogen concentration (B) was determined by precipitation with sodium sulfite. The means ± SEM are shown.
Table 1.
Pg and fibrinogen consumption in human plasma
| PA | Pg consumption (EC50 nM) |
Fibrinogen consumption (EC50 nM) |
|---|---|---|
| TPA | 31.1 ± 1.1 | 10.1 ± 1.1 |
| SK | 1.8 ± 1.2* | 4.2 ± 1.1* |
| SKΔ1 | 16.8 ± 1.0* | 21.1 ± 1.1* |
| SKΔ59 | >500*† | >500*† |
P < 0.001 when compared with TPA.
The calculated EC50 exceeded the highest doses tested.
Effect of a2AP on Pg activation in plasma
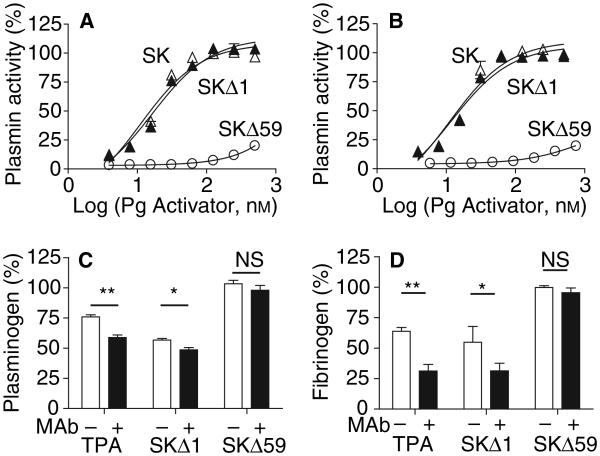
To determine the mechanistic basis for the improved fibrin-targeted properties of the SK variants, we investigated the effects of α2AP on PAs and on the process of plasmin generation in plasma. Previous studies have shown that TPA is inhibited by α2AP in humans [41]. Plasmin is also inhibited by α2AP in plasma [42], but SK·plasmin is resistant to α2AP, disabling this control mechanism [11]. The SKΔ59·plasmin complex was readily inhibited by both human and mouse α2AP; however, the SKΔ1·plasmin complex was resistant to both α2APs, similar to SK·plasmin (Fig. 3A,B). Therefore the susceptibility of the SKΔ59·plasmin complex to α2AP may explain the improved fibrin-targeted properties of SKΔ59, but not of SKΔ1.
Fig. 3.
Effect of α2AP on the activity and fibrin-targeting of SKΔ1·plasmin, SKΔ59·plasmin and SK·plasmin. Resistance to inhibition by human (A) or mouse (B) α2AP. SKΔ1, SKΔ59 or SK (0–750 nM) were preincubated with human plasmin (12 nM) in 50 mM Tris–HCl, 100 mM NaCl, pH 7.4, for 10 min at 25 °C prior to addition of α2-AP (15 nM). After 10 min, residual plasmin activity was measured at 37 °C. (C,D) Effect of α2AP neutralization on Pg (C) and fibrinogen (D) consumption in human plasma treated with PAs. TPA (15 nM), SKΔ1 (15 nM) or SKΔ59 (100 nM) was added to human plasma with (filled bars) or without (clear bars) an antibody inhibitor of α2AP [1 μM, (26)] for 2 h at 37 °C. Residual Pg and fibrinogen concentrations were determined as described above. The means ± SEM are shown. *P < 0.05; **P < 0.01, NS- not significant.
If α2AP plays a role in regulating fibrinogen and Pg consumption in plasma, Pg activators with less fibrin-targeting should generate higher concentrations of α2AP-plasmin complexes. In human plasma only trace amounts of α2AP-plasmin complexes were detected at baseline and in the presence of 2 nM TPA or SKΔ1 (Table 2). Addition of 10 nM TPA or SKΔ1 significantly increased the levels of α2AP-plasmin complexes. SK at 2 nM markedly increased levels of α2AP-plasmin complexes and, at a dose of 10 nM, all α2AP was complexed with plasmin. In contrast, much higher doses of SKΔ59 (20 and 100 nM) did not increase levels of α2AP-plasmin complexes.
Table 2.
Effect of Pg activators on the generation of α2AP-plasmin complexes in human plasma
| Pg activator |
None |
TPA |
SKΔ1 |
SK |
TPA |
SKΔ1 |
SK |
SKΔ59 |
SKΔ59 |
|---|---|---|---|---|---|---|---|---|---|
| Concentration (nM) | 0 | 2 | 2 | 2 | 10 | 10 | 10 | 20 | 100 |
| α2AP-plasmin (μg mL−1) | 0.9 ± 0.6 | 1.5 ± 1.0 | 1.3 ± 0.2 | 62.6 ± 20.4 | 15.2 ± 0.6 | 11.5 ± 6.9 | ≥ 110* | 1.0 ± 0.0 | 0.9 ± 0.7 |
| α2AP-plasmin (%) | 0.8 ± 0.5 | 1.4 ± 0.9 | 1.2 ± 0.2 | 56.9 ± 18.6 | 13.8 ± 0.5 | 10.5 ± 6.3 | 100* | 0.9 ± 0.1 | 0.8 ± 0.6 |
The plasma sample was fully activated and all α2AP was complexed.
To confirm that α2AP plays a direct role in preventing Pg and fibrinogen consumption in plasma, we performed experiments in the presence and absence of a monoclonal antibody that neutralizes α2AP [29]. The α2AP inhibitor increased the consumption of both Pg and fibrinogen by TPA and SKΔ1 (Fig. 3B,C). This indicates that α2AP inhibits the generation of plasmin by both activators in plasma. In addition, α2AP also reduces the consumption of fibrinogen by complexing with and inhibiting plasmin (Table 2). In contrast, the presence of an α2AP inhibitor did not increase the consumption of Pg and fibrinogen by SKΔ59 (Fig. 3B,C) though SKΔ59·plasmin is susceptible to α2AP. This confirms that SKΔ59 achieved fibrin specificity because it did not efficiently produce plasmin in plasma, as indicated by the lack of Pg or fibrinogen consumption (Fig. 2) or generation of α2AP-plasmin complexes (Table 2).
Fibrin differentially modulates Pg activation by SKΔ1, SKΔ59 and TPA
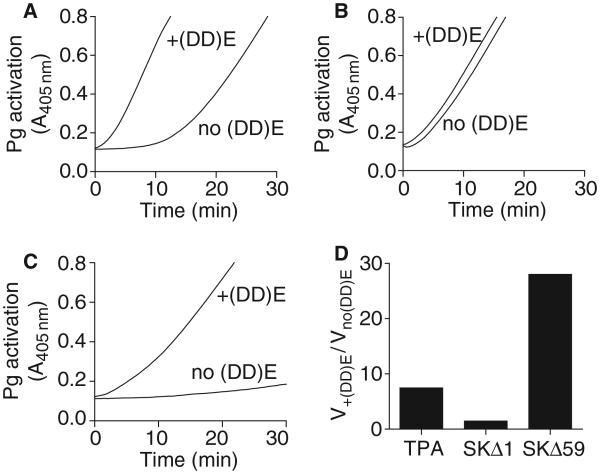
Fibrin unfolds Glu-Pg by interacting with the kringle domains, inducing an open conformation that is more susceptible to cleavage by PAs [24,43]. In order to isolate the effects of fibrin on the Glu-Pg substrate, we compared Pg activation by TPA, with activator complexes formed by SKΔ1 and SKΔ59 with microplasmin, which lacks fibrin-interacting kringle domains. As previously reported, TPA was a relatively poor activator of Glu-Pg in the absence of fibrin [4,5]; the addition of fibrin (DD) E fragments increased the apparent rate of Glu-Pg activation by ~ 9-fold (Fig. 4A). SKΔ1·microplasmin was an efficient activator of human Glu-Pg in the absence of fibrin (DD) E fragments. Activation was only slightly enhanced in the presence of (DD) E (Fig. 4B), largely from a decrease in the Km (Table 3). In contrast, SKΔ59·microplasmin was a poor activator in the absence of (DD) E, which precluded accurate kinetic measurements in the absence of (DD) E (Fig. 4C,D; Table 3). However, the presence of (DD) E increased the apparent rate of Pg activation by ~30-fold.
Fig. 4.
Influence of fibrin on Pg activation by TPA, SKΔ1·microplasmin and SKΔ59·microplasmin. Pg (1000 nM) was added to a microtiter plate containing S-2251 (0.5 mM) with or without 2 μM of soluble (DD) E fragment in assay buffer at 37 °C. After 10 min Pg activation was initiated by t-PA (A; 5 nM), SKΔ1·microplasmin (B; 5 nM) or SKΔ59·microplasmin (C; 20 nM) and detected by the change in absorbance at 405 nm. (D) The ratio between the initial rates of Pg activation in the presence of fibrin (DD) E fragments vs. no fibrin. The initial reaction rates were obtained by plotting V = A405nm min−2.
Table 3.
Effect of fibrin (DD) E fragments on Pg activation by SKΔ1 and SKΔ59
|
kcat min−1 |
Km nM |
kcat/Km μM−1 min−1 |
||||
|---|---|---|---|---|---|---|
| PA | −(DD) E | +(DD) E | −(DD) E | +(DD) E | −(DD) E | +(DD) E |
| SKΔ1 | 4.6 ± 0.6 | 4.9 ± 0.4 | 320 ± 70 | 96 ± 20 | 14.3 | 51.0 |
| SKΔ59 | NA* | 0.3 ± 0.0 | NA* | 400 ± 70 | NA* | 0.7 |
| SK† | 20.0 ± 1.8 | 21.7 ± 0.8 | 458 ± 7 | 354 ± 2 | 43.6 | 61.3 |
| TPA† | 1.6 ± 0.1 | 3.8 ± 0.1 | 331 ± 27 | 122 ± 14 | 4.8 | 31.5 |
NA, no significant activity.
As reported [54].
SKΔ1 and SKΔ59 are potent activators in vivo
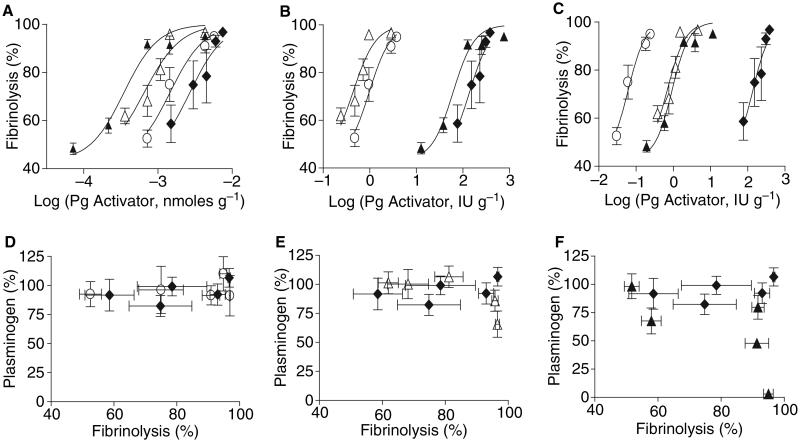
These experiments suggested that mechanistic changes in the SK molecule had made SKΔ1 and SKΔ59 comparable with or better than TPA in terms of fibrin-targeting. To compare the ability of SKΔ1, SKΔ59 and TPA to dissolve blood clots in vivo, we generated a novel humanized model in mice that simulates human pulmonary embolism. Although human TPA and SK have minimal or no activity with mouse Pg, the other molecular components of the mouse fibrinolytic system (e.g. fibrin, α2AP, etc.) are functionally comparable with the human system [2,44,45]. Therefore fibrinolysis experiments were performed in Pg-deficient (Pg−/−) mice that, after supplementation with human Glu-Pg, achieved physiologic levels of this zymogen (2.2 μM). The baseline rate of lysis of the pulmonary clots in the absence of PAs was 43% ± 3% (n = 11), which is comparable with the lysis reported in similar studies (Fig. 5A, [46]). The doses that caused 50% lysis of thromboemboli in vivo (EC50, pmoles g−1) for SKΔ1 (0.65, P < 0.0001), SKΔ59 (1.45, P < 0.003) and SK (0.35, P < 0.0001) were significantly lower than for TPA (2.7). In other words the fibrinolytic potency of SKΔ1 was 4.1-fold higher, the potency of SKΔ59 was 1.9-fold higher, and the potency of SK was 7.7-fold higher than TPA (Fig. 5A).
Fig. 5.
Potency and fibrin-dependency of clot dissolution by TPA, SKΔ1 and SKΔ59 in vivo. The dissolution of clots (fibrinolysis) in vivo is plotted vs. Pg activator dose in nanomoles per gram mouse weight (A), or units gram mouse weight calibrated by turbidimetric clot lysis method (B), or the fibrin plate method (C). After embolization of a 125I-labeled human clot into the lungs of anesthetized Pg-deficient mice, human Pg (14 g kg−1) was administrated via the left jugular vein. Then TPA (◆), SKΔ1 (Δ), SKΔ59 (○) or SK (▲) was injected via the right jugular vein (N = 4 mice per dose). The amount of lysis was measured after 2 h and computed as described in Materials and methods. Pg consumption in vivo by SKΔ59 (C, D), SKΔ1 (Δ, E) or SK (▲, F) was compared with TPA (◆) vs. the fibrinolysis induced by these agents. The means ± SEM are shown.
In classic studies, the dose of PAs is determined not simply by mass alone, but by calibration of the PA’s fibrinolytic activity. To be sure that assay-specific conditions did not differentially affect the function of the PAs, we used two different established fibrinolytic methods: clot turbidity and fibrin plate assays [35-37,47]. When dose response curves were constructed based on the activity of the PAs in clot turbidity assays (Fig. 5B) or the fibrin plate assay (Fig. 5C), the EC50s for TPA were the same as expected. However, the EC50s for SKΔ1 for these two methods (0.43 vs. 0.69, respectively) were 318–159-fold lower than TPA (P < 0.0001). For comparison, the EC50s for SKΔ59 (0.97 vs. 0.06, respectively) were 140–1800-fold lower than TPA (P < 0.0001). Lastly, the EC50s for SK (61 vs. 0.93, respectively) were 2.2–120-fold lower than TPA (P < 0.0001). Thus, using these methods of calibration, the fibrin-dependent SKs (SKΔ1 and SKΔ59) were between 2–3 logs more potent than TPA and significantly more potent than SK (P < 0.01).
SKΔ1, SKΔ59 are fibrin-specific in vivo
The fibrin-dependency of PAs is indicated by how readily they activate Pg in human plasma in the absence of fibrin. We examined the depletion of Pg in vivo for all PAs (Fig. 5D–F). SK induced complete Pg consumption (Fig. 5F). Modest Pg consumption was seen only with the highest doses of SKΔ1 (Fig. 5E). At the fibrinolytic doses tested, neither TPA nor SKΔ59 showed significant Pg depletion (Fig. 5D).
Discussion
The terms fibrin-specificity, fibrin-dependency or fibrin-targeting have been used to describe the tendency of PAs to selectively activate fibrin-bound Pg in preference to circulating Pg in the blood. TPA is considered the prototypical, fibrin-targeted agent because its catalytic activity is enhanced in the presence of fibrin (Fig. 4A and [4,5]). Still, at therapeutic concentrations in humans, TPA activates circulating Pg and degrades fibrinogen [48]. In contrast, SK is considered the prototypical, fibrin-independent agent because it rapidly generates plasmin in the blood at sites distant from fibrin clots. This fibrin-independent action accelerates bradykinin production (which can lower blood pressure), leads to extensive proteolysis of fibrinogen [16] and reduces fibrin clot lysis by depleting Pg [49,50]. Recent studies have linked structural elements in the SK molecule to the mechanisms responsible for SK’s fibrin-independent mode of Pg activation [12,20,21,24]. This report provides the first evidence in vivo that targeted changes in the mechanism of action of SK yield PAs that are more potent and fibrin-targeted than TPA.
Studies of PAs in human plasma in vitro yield valuable mechanistic insights, but it is impossible to model the factors (blood flow, etc.) that may affect the efficacy and targeting of PAs in vivo, for example, when used as therapy in humans. More than 20 studies have examined how structural alterations in SK affect Pg activation, but only a few have examined the effects on fibrinolysis in vitro [12,19,23,51] and none have examined it in vivo. The challenge in constructing an in vivo model for comparing human TPA and SK was that both activators are minimally reactive with mouse Pg and they are variably reactive with other non-human Pgs [44,51-53]. Fortunately, other components of the mouse fibrinolytic system (e.g. α2AP) appear to react normally with human Pg, plasmin [44]. Therefore, to create a ‘humanized’ model of thrombosis, we examined the lysis of human clots in Pg−/− mice supplemented with physiologic levels of human Pg. Pg-deficient (Pg−/−) mice were selected to avoid the potential confounding effects of the mouse Pg on the results [54]. This model simulates human thrombosis (pulmonary thromboembolism) and provides a reproducible system for comparing the activity of PAs on human Pg in vivo. Although these experiments do not assess the potential safety of PAs, with further modifications this system may prove useful for simultaneously estimating the risks of PAs, as well as their fibrinolytic properties.
A key mechanism responsible for the fibrin-independent action of SK is the ability of SK to non-proteolytically activate Pg to form a functional SK·Pg* (Fig. 1). Deletion of the amino-terminal Ile1 (to form SKΔ1) allowed us to assess the contribution of this mechanism to fibrin-targeting. In human plasma, SKΔ1 displayed fibrin-dependency that was roughly comparable with TPA (Fig. 2). However, the fibrin-targeting of both SKΔ1 and TPA was incomplete because higher doses of these agents caused significant fibrinogen and Pg consumption (Fig. 2). Thus both SKΔ1 and TPA are capable of directly activating non-fibrin bound Pg. In addition, the fibrin-targeting of both agents was reduced in the presence of α2AP inhibitors (Fig. 3A,B). For TPA these results may be due to two mechanisms. First, plasmin generated in plasma by TPA is normally rapidly inhibited by α2AP, making TPA appear more fibrin-targeted. Second, although α2AP is not an important inhibitor of TPA under physiologic conditions, when it is given at therapeutic doses, significant amounts of TPA·α2AP complexes are detected in humans [41]. Thus, in addition to its major inhibitor, Pg activator inhibitor-1, α2AP restricts the activity of TPA in plasma. The incomplete fibrin-targeting of SKΔ1 indicates that SKΔ1·plasmin does form in plasma and that it generates free plasmin that can proteolyze fibrinogen. Although α2AP can t efficiently inhibit SKΔ1·plasmin (Fig. 3A), we propose that α2AP reduces the amount of free plasmin available for complex formation with SKΔ1. Through these mechanisms, α2AP contributes to the fibrin-targeting of SKΔ1.
Two key mechanisms contribute to the fibrin-independent action of SK: (i) the resistance of the SK·plasmin complex to α2AP and (ii) the ability of SK·plasmin to efficiently activate Glu-Pg in the absence of fibrin. Truncation of the alpha domain to form SKΔ59 results in the loss of both mechanisms [12,19]. The SKΔ59·plasmin complex was also susceptible to inhibition by α2AP (Fig. 3A). Still this was not the major mechanism responsible for the high fibrin-targeting of SKΔ59, because inhibition of α2AP did not affect plasminogen or fibrinogen consumption by this molecule (Fig. 4-A,B). Instead, one explanation for the extraordinary fibrin-targeting of SKΔ59 may be its high selectivity for fibrin-bound (Fig. 4) or unfolded Pg substrate [12]. Previous studies have shown that the catalytic activity of the SKΔ59 activator complex is markedly enhanced for Lys-Pg [12] and for unfolded forms of Pg that occur after binding to fibrin, in the presence of EACA and in buffers lacking chloride ion [12,19]). These marked increases in the activity of SKΔ59 provide plausible explanations for its fibrinolytic efficacy and specificity. In vivo, SKΔ59 was a more potent fibrinolytic agent than TPA and displayed significantly greater fibrin-targeting.
Acknowledgements
This work was supported in part by NIH Grant HL058496 (to G.L. Reed), American Heart Association SDG Award 0830309N (to I.Y. Sazonova) and Medical College of Georgia Start-up Grant (to I.Y. Sazonova).
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, et al. Heart disease and stroke statistics – 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Gladysheva IP, Turner RB, Sazonova IY, Liu L, Reed GL. Co-evolutionary patterns in plasminogen activation. Proc Natl Acad Sci U S A. 2003;100:9168–72. doi: 10.1073/pnas.1631716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collen D, Lijnen HR, Todd PA, Goa KL. Tissue-type plasminogen activator. A review of its pharmacology and therapeutic use as a thrombolytic agent. Drugs. 1989;38:346–88. doi: 10.2165/00003495-198938030-00003. [DOI] [PubMed] [Google Scholar]
- 4.Thelwell C, Longstaff C. The regulation by fibrinogen and fibrin of tissue plasminogen activator kinetics and inhibition by plasminogen activator inhibitor 1. J Thromb Haemost. 2007;5:804–11. doi: 10.1111/j.1538-7836.2007.02422.x. [DOI] [PubMed] [Google Scholar]
- 5.Fischer BE. Comparison of fibrin-mediated stimulation of plasminogen activation by tissue-type plasminogen activator (t-PA) and fibrin-dependent enhancement of amidolytic activity of t-PA. Blood Coagul Fibrinolysis. 1992;3:197–204. [PubMed] [Google Scholar]
- 6.Wiman B, Boman L, Collen D. On the kinetics of the reaction between human antiplasmin and a low- molecular-weight form of plasmin. Eur J Biochem. 1978;87:143–6. doi: 10.1111/j.1432-1033.1978.tb12360.x. [DOI] [PubMed] [Google Scholar]
- 7.Edy J, Collen D. The interaction in human plasma of antiplasmin, the fast-reacting plasmin inhibitor, with plasmin, thrombin, trypsin and chymotrypsin. Biochim Biophys Acta. 1977;484:423–32. doi: 10.1016/0005-2744(77)90098-5. [DOI] [PubMed] [Google Scholar]
- 8.Robbie LA, Bennett B, Keyt BA, Booth NA. Effective lysis of model thrombi by a t-PA mutant (A473S) that is resistant to alpha2-antiplasmin. Br J Haematol. 2000;111:517–23. doi: 10.1046/j.1365-2141.2000.02365.x. [DOI] [PubMed] [Google Scholar]
- 9.McClintock DK, Bell PH. The mechanism of activation of human plasminogen by streptokinase. Biochem Biophys Res Commun. 1971;43:694–702. doi: 10.1016/0006-291x(71)90670-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Lin X, Loy JA, Tang J, Zhang XC. Crystal structure of the catalytic domain of human plasmin complexed with streptokinase. Science. 1998;281:1662–5. doi: 10.1126/science.281.5383.1662. [DOI] [PubMed] [Google Scholar]
- 11.Cederholm-Williams SA, De Cock F, Lijnen HR, Collen D. Kinetics of the reactions between streptokinase, plasmin and alpha 2-antiplasmin. Eur J Biochem. 1979;100:125–32. doi: 10.1111/j.1432-1033.1979.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 12.Sazonova IY, Robinson BR, Gladysheva IP, Castellino FJ, Reed GL. Alpha domain deletion converts streptokinase into a fibrin-dependent plasminogen activator through mechanisms akin to staphylokinase and tissue plasminogen activator. J Biol Chem. 2004;279:24994–5001. doi: 10.1074/jbc.M400253200. [DOI] [PubMed] [Google Scholar]
- 13.Ohman EM, Harrington RA, Cannon CP, Agnelli G, Cairns JA, Kennedy JW. Intravenous thrombolysis in acute myocardial infarction. Chest. 2001;119:253S–77S. doi: 10.1378/chest.119.1_suppl.253s. [DOI] [PubMed] [Google Scholar]
- 14.Six AJ, Brommer EJ, Muller EJ, Kerkhoff HF. Activation of the fibrinolytic system during intracoronary streptokinase administration. J Am Coll Cardiol. 1987;9:189–96. doi: 10.1016/s0735-1097(87)80100-6. [DOI] [PubMed] [Google Scholar]
- 15.Kunamneni A, Abdelghani TT, Ellaiah P. Streptokinase – the drug of choice for thrombolytic therapy. J Thromb Thrombolysis. 2007;23:9–23. doi: 10.1007/s11239-006-9011-x. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmeister HM, Ruf M, Wendel HP, Heller W, Seipel L. Streptokinase-induced activation of the kallikrein-kinin system and of the contact phase in patients with acute myocardial infarction. J Cardiovasc Pharmacol. 1998;31:764–72. doi: 10.1097/00005344-199805000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Wardlaw JM, Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD000213. CD000213. [DOI] [PubMed] [Google Scholar]
- 18.Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, Walley T, Dickson R. Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation. Health Technol Assess. 2003;7:1–136. doi: 10.3310/hta7150. [DOI] [PubMed] [Google Scholar]
- 19.Reed GL, Houng AK, Liu L, Parhami-Seren B, Matsueda LH, Wang S, Hedstrom L. A catalytic switch and the conversion of streptokinase to a fibrin-targeted plasminogen activator. Proc Natl Acad Sci U S A. 1999;96:8879–83. doi: 10.1073/pnas.96.16.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Reed GL, Hedstrom L. Deletion of Ile1 changes the mechanism of streptokinase: evidence for the molecular sexuality hypothesis. Biochemistry. 1999;38:5232–40. doi: 10.1021/bi981915h. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Reed GL, Hedstrom L. Zymogen activation in the streptokinase-plasminogen complex. Ile1 is required for the formation of a functional active site. Eur J Biochem. 2000;267:3994–4001. doi: 10.1046/j.1432-1327.2000.01434.x. [DOI] [PubMed] [Google Scholar]
- 22.Boxrud PD, Verhamme IM, Fay WP, Bock PE. Streptokinase triggers conformational activation of plasminogen through specific interactions of the amino-terminal sequence and stabilizes the active zymogen conformation. J Biol Chem. 2001;276:26084–9. doi: 10.1074/jbc.M101966200. [DOI] [PubMed] [Google Scholar]
- 23.Mundada LV, Prorok M, DeFord ME, Figuera M, Castellino FJ, Fay WP. Structure-function analysis of the streptokinase amino terminus (residues 1-59) J Biol Chem. 2003;278:24421–7. doi: 10.1074/jbc.M301825200. [DOI] [PubMed] [Google Scholar]
- 24.Gladysheva IP, Sazonova IY, Houng A, Hedstrom L, Reed GL. Regulation of nonproteolytic active site formation in plasminogen. Biochemistry. 2007;46:8879–87. doi: 10.1021/bi602591g. [DOI] [PubMed] [Google Scholar]
- 25.Aoki N, Sakata Y, Ichinose A. Fibrin-associated plasminogen activation in alpha 2-plasmin inhibitor deficiency. Blood. 1983;62:1118–22. [PubMed] [Google Scholar]
- 26.Collen D, Schlott B, Engelborghs Y, van Hoef B, Hartmann M, Lijnen HR, Behnke D. On the mechanism of the activation of human plasminogen by recombinant staphylokinase. J Biol Chem. 1993;268:8284–9. [PubMed] [Google Scholar]
- 27.Deutsch DG, Mertz ET. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970;170:1095–6. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- 28.Reed GL, Lin LF, Parhami SB, Kussie P. Identification of a plasminogen binding region in streptokinase that is necessary for the creation of a functional streptokinase-plasminogen activator complex. Biochemistry. 1995;34:10266–71. doi: 10.1021/bi00032a021. [DOI] [PubMed] [Google Scholar]
- 29.Sazonova IY, Thomas BM, Gladysheva IP, Houng AK, Reed GL. Fibrinolysis is amplified by converting alpha-antiplasmin from a plasmin inhibitor to a substrate. J Thromb Haemost. 2007;5:2087–94. doi: 10.1111/j.1538-7836.2007.02652.x. [DOI] [PubMed] [Google Scholar]
- 30.Turner RB, Liu L, Sazonova IY, Reed GL. Structural elements that govern the substrate specificity of the clot dissolving enzyme plasmin. J Biol Chem. 2002;277:33068–74. doi: 10.1074/jbc.M203782200. [DOI] [PubMed] [Google Scholar]
- 31.Lukacova D, Matsueda GR, Haber E, Reed GL. Inhibition of Factor XIII activation by an anti-peptide monoclonal antibody. Biochemistry. 1991;30:10164–70. doi: 10.1021/bi00106a013. [DOI] [PubMed] [Google Scholar]
- 32.Wiman B, Ranby M. Determination of soluble fibrin in plasma by a rapid and quantitative spectrophotometric assay. Thromb Haemost. 1986;55:189–93. [PubMed] [Google Scholar]
- 33.Gladysheva IP, Sazonova IY, Chowdhry SA, Liu L, Turner RB, Reed GL. Chimerism reveals a role for the streptokinase Beta -domain in nonproteolytic active site formation, substrate, and inhibitor interactions. J Biol Chem. 2002;277:26846–51. doi: 10.1074/jbc.M202999200. [DOI] [PubMed] [Google Scholar]
- 34.Wohl RC, Summaria L, Robbins KC. Kinetics of activation of human plasminogen by different activator species at pH 7.4 and 37 degrees C. J Biol Chem. 1980;255:2005–13. [PubMed] [Google Scholar]
- 35.Jespersen J, Astrup T. A study of the fibrin plate assay of fibrinolytic agents. Optimal conditions, reproducibility and precision. Haemostasis. 1983;13:301–15. doi: 10.1159/000214769. [DOI] [PubMed] [Google Scholar]
- 36.Astrup T, Mullerts S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys. 1952;40:346–51. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- 37.Urano S, Metzger AR, Castellino FJ. Plasmin-mediated fibrinolysis by variant recombinant tissue plasminogen activators. Proc Natl Acad Sci U S A. 1989;86:2568–71. doi: 10.1073/pnas.86.8.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rampling MW, Gaffney PJ. The sulphite precipitation method for fibrinogen measurement; its use on small samples in the presence of fibrinogen degradation products. Clin Chim Acta. 1976;67:43–52. doi: 10.1016/0009-8981(76)90215-1. [DOI] [PubMed] [Google Scholar]
- 39.Friberger P, Knos M, Gustavsson S, Aurell L, Claeson G. Methods for determination of plasmin, antiplasmin and plasminogen by means of substrate S-2251. Haemostasis. 1978;7:138–45. doi: 10.1159/000214252. [DOI] [PubMed] [Google Scholar]
- 40.Motulsky HG, Spannard P, Neubig R. Analyzing Data with GraphPad Prism [computer program] GraphPad Prim Software, GraphPad Prim Software, San Diego; San Diego: 1994. Version. [Google Scholar]
- 41.Lucore CL, Sobel BE. Interactions of tissue-type plasminogen activator with plasma inhibitors and their pharmacologic implications. Circulation. 1988;77:660–9. doi: 10.1161/01.cir.77.3.660. [DOI] [PubMed] [Google Scholar]
- 42.Schneider M, Nesheim M. A study of the protection of plasmin from antiplasmin inhibition within an intact fibrin clot during the course of clot lysis. J Biol Chem. 2004;279:13333–9. doi: 10.1074/jbc.M313164200. [DOI] [PubMed] [Google Scholar]
- 43.Dunn FW, Deguchi K, Soria J, Soria C, Lijnen HR, Tobelem G, Caen J. Importance of the interaction between plasminogen and fibrin for plasminogen activation by tissue-type plasminogen activator. Thromb Res. 1984;36:345–51. doi: 10.1016/0049-3848(84)90326-8. [DOI] [PubMed] [Google Scholar]
- 44.Lijnen HR, van Hoef B, Beelen V, Collen D. Characterization of the murine plasma fibrinolytic system. Eur J Biochem. 1994;224:863–71. doi: 10.1111/j.1432-1033.1994.00863.x. [DOI] [PubMed] [Google Scholar]
- 45.Wohl RC, Sinio L, Summaria L, Robbins KC. Comparative activation kinetics of mammalian plasminogens. Biochim Biophys Acta. 1983;745:20–31. doi: 10.1016/0167-4838(83)90165-6. [DOI] [PubMed] [Google Scholar]
- 46.Lijnen HR, Carmeliet P, Bouche A, Moons L, Ploplis VA, Plow EF, Collen D. Restoration of thrombolytic potential in plasminogen-deficient mice by bolus administration of plasminogen. Blood. 1996;88:870–6. [PubMed] [Google Scholar]
- 47.Robbie LA, Booth NA, Croll AM, Bennett B. The roles of alpha 2-antiplasmin and plasminogen activator inhibitor 1 (PAI-1) in the inhibition of clot lysis. Thromb Haemost. 1993;70:301–6. [PubMed] [Google Scholar]
- 48.Rao AK, Pratt C, Berke A, Jaffe A, Ockene I, Schreiber TL, Bell WR, Knatterud G, Robertson TL, Terrin ML. Thrombolysis in Myocardial Infarction (TIMI) Trial–phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11:1–11. doi: 10.1016/0735-1097(88)90158-1. [DOI] [PubMed] [Google Scholar]
- 49.Torr SR, Nachowiak DA, Fujii S, Sobel BE. “Plasminogen steal” and clot lysis. J Am Coll Cardiol. 1992;19:1085–90. doi: 10.1016/0735-1097(92)90300-c. [DOI] [PubMed] [Google Scholar]
- 50.Onundarson PT, Haraldsson HM, Bergmann L, Francis CW, Marder VJ. Plasminogen depletion during streptokinase treatment or two-chain urokinase incubation correlates with decreased clot lysability ex vivo and in vitro. Thromb Haemost. 1993;70:998–1004. [PubMed] [Google Scholar]
- 51.Fay WP, Garg N, Sunkar M. Vascular functions of the plasminogen activation system. Arterioscler Thromb Vasc Biol. 2007;27:1231–7. doi: 10.1161/ATVBAHA.107.140046. [DOI] [PubMed] [Google Scholar]
- 52.Collen D, van Hoef B, Schlott B, Hartmann M, Guhrs KH, Lijnen HR. Mechanisms of activation of mammalian plasma fibrinolytic systems with streptokinase and with recombinant staphylokinase. Eur J Biochem. 1993;216:307–14. doi: 10.1111/j.1432-1033.1993.tb18147.x. [DOI] [PubMed] [Google Scholar]
- 53.Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–5. [PubMed] [Google Scholar]
- 54.Bugge TH, Flick MJ, Daugherty CC, Degen JL. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]