Full text
PDF
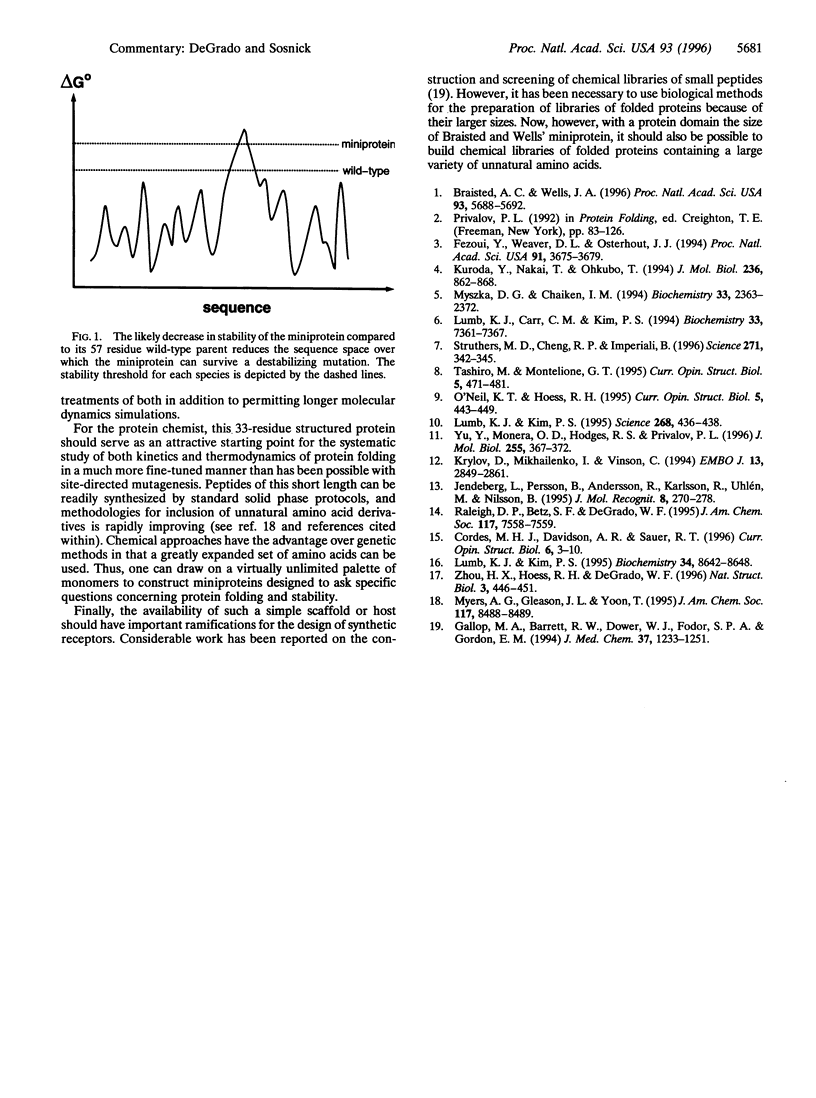
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braisted A. C., Wells J. A. Minimizing a binding domain from protein A. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):5688–5692. doi: 10.1073/pnas.93.12.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes M. H., Davidson A. R., Sauer R. T. Sequence space, folding and protein design. Curr Opin Struct Biol. 1996 Feb;6(1):3–10. doi: 10.1016/s0959-440x(96)80088-1. [DOI] [PubMed] [Google Scholar]
- Fezoui Y., Weaver D. L., Osterhout J. J. De novo design and structural characterization of an alpha-helical hairpin peptide: a model system for the study of protein folding intermediates. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3675–3679. doi: 10.1073/pnas.91.9.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallop M. A., Barrett R. W., Dower W. J., Fodor S. P., Gordon E. M. Applications of combinatorial technologies to drug discovery. 1. Background and peptide combinatorial libraries. J Med Chem. 1994 Apr 29;37(9):1233–1251. doi: 10.1021/jm00035a001. [DOI] [PubMed] [Google Scholar]
- Jendeberg L., Persson B., Andersson R., Karlsson R., Uhlén M., Nilsson B. Kinetic analysis of the interaction between protein A domain variants and human Fc using plasmon resonance detection. J Mol Recognit. 1995 Jul-Aug;8(4):270–278. doi: 10.1002/jmr.300080405. [DOI] [PubMed] [Google Scholar]
- Krylov D., Mikhailenko I., Vinson C. A thermodynamic scale for leucine zipper stability and dimerization specificity: e and g interhelical interactions. EMBO J. 1994 Jun 15;13(12):2849–2861. doi: 10.1002/j.1460-2075.1994.tb06579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y., Nakai T., Ohkubo T. Solution structure of a de novo helical protein by 2D-NMR spectroscopy. J Mol Biol. 1994 Feb 25;236(3):862–868. doi: 10.1006/jmbi.1994.1194. [DOI] [PubMed] [Google Scholar]
- Lumb K. J., Carr C. M., Kim P. S. Subdomain folding of the coiled coil leucine zipper from the bZIP transcriptional activator GCN4. Biochemistry. 1994 Jun 14;33(23):7361–7367. doi: 10.1021/bi00189a042. [DOI] [PubMed] [Google Scholar]
- Lumb K. J., Kim P. S. A buried polar interaction imparts structural uniqueness in a designed heterodimeric coiled coil. Biochemistry. 1995 Jul 11;34(27):8642–8648. doi: 10.1021/bi00027a013. [DOI] [PubMed] [Google Scholar]
- Lumb K. J., Kim P. S. Measurement of interhelical electrostatic interactions in the GCN4 leucine zipper. Science. 1995 Apr 21;268(5209):436–439. doi: 10.1126/science.7716550. [DOI] [PubMed] [Google Scholar]
- Myszka D. G., Chaiken I. M. Design and characterization of an intramolecular antiparallel coiled coil peptide. Biochemistry. 1994 Mar 8;33(9):2363–2372. doi: 10.1021/bi00175a003. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., Hoess R. H. Phage display: protein engineering by directed evolution. Curr Opin Struct Biol. 1995 Aug;5(4):443–449. doi: 10.1016/0959-440x(95)80027-1. [DOI] [PubMed] [Google Scholar]
- Struthers M. D., Cheng R. P., Imperiali B. Design of a monomeric 23-residue polypeptide with defined tertiary structure. Science. 1996 Jan 19;271(5247):342–345. doi: 10.1126/science.271.5247.342. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Montelione G. T. Structures of bacterial immunoglobulin-binding domains and their complexes with immunoglobulins. Curr Opin Struct Biol. 1995 Aug;5(4):471–481. doi: 10.1016/0959-440x(95)80031-x. [DOI] [PubMed] [Google Scholar]
- Yu Y., Monera O. D., Hodges R. S., Privalov P. L. Ion pairs significantly stabilize coiled-coils in the absence of electrolyte. J Mol Biol. 1996 Jan 26;255(3):367–372. doi: 10.1006/jmbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- Zhou H. X., Hoess R. H., DeGrado W. F. In vitro evolution of thermodynamically stable turns. Nat Struct Biol. 1996 May;3(5):446–451. doi: 10.1038/nsb0596-446. [DOI] [PubMed] [Google Scholar]



