Abstract
Mitochondria are highly dynamic and undergo constant fusion and fission that are essential for maintaining physiological functions of cells. Although dysfunction of mitochondria has been implicated in tumorigenesis, little is known about the roles of mitochondrial dynamics in metastasis, the major cause of cancer death. In the present study, we found a marked upregulation of mitochondrial fission protein dynamin-related protein 1 (Drp1) expression in human invasive breast carcinoma and metastases to lymph nodes. Compared to non-metastatic breast cancer cells, mitochondria also were more fragmented in metastatic breast cancer cells that express higher levels of total and active Drp1 and less mitochondrial fusion protein 1 (Mfn1). Silencing Drp1 or overexpression of Mfn1 resulted in mitochondria elongation or clusters, respectively, and significantly suppressed metastatic abilities of breast cancer cells. In contrast, silencing Mfn proteins led to mitochondrial fragmentation and enhanced metastatic abilities of breast cancer cells. Interestingly, these manipulations of mitochondrial dynamics altered the subcellular distribution of mitochondria in breast cancer cells. For example, silencing Drp1 or overexpression of Mfn1 inhibited lamellipodia formation, a key step for cancer metastasis, and suppressed chemoattractant-induced recruitment of mitochondria to lamellipodial regions. Conversely, silencing Mfn proteins resulted in more cell spreading and lamellipodia formation, causing accumulation of more mitochondria in lamollipodia regions. More importantly, treatment with a mitochondrial uncoupling agent or ATP synthesis inhibitor reduced lamellipodia formation and decreased breast cancer cell migration and invasion, suggesting a functional importance of mitochondria in breast cancer metastasis. Together, our findings show a new role and mechanism for regulation of cancer cell migration and invasion by mitochondrial dynamics. Thus targeting dysregulated Drp1-dependent mitochondrial fission may provide a novel strategy for suppressing breast cancer metastasis.
Keywords: Breast cancer, metastasis, lamellipodia, Drp1, mitochondrial dynamics, fission and fusion
Introduction
Breast cancer is the most common cancer in women and the second leading cause of cancer death (1). Patients who are not cured are those in whom breast cancer has metastasized. Metastasis begins with the migration and invasion of cancer cells into surrounding tissues and lymphatics, and then to target organs. One of the key steps in this directed migration and invasion is formation of lamellipodia at the leading edge of cells (2). Lamellipodia formation, triggered by chemoattractants (3), is dependent on the reorganization and reassembly of the actin cytoskeleton, which needs an abundance of ATP (4).
Mitochondria are organelles that provide the majority of the energy in most cells due to their synthesis of ATP by oxidative phosphorylation (5). They also have other roles including a contribution to intracellular calcium homeostasis (6), and are critical for many cellular functions including growth, division, energy metabolism and apoptosis in cells. Mitochondria exist as dynamic networks that often change size and subcellular distribution, and these dynamics are maintained by two opposing processes: fission and fusion (7), regulated by dynamin-related protein 1 (Drp1) and mitofusins (Mfns) (8), respectively. Mitochondrial dynamics is strictly controlled by the cell because of its vital role in maintaining mitochondrial functions (7–11). In quiescent cells, mitochondria tend to exist as a meshwork of interconnected tubes. However, the energy-producing mitochondria need to be redistributed to those regions of the cell with the greatest energy demands (12). Thus, this meshwork of mitochondria needs to be sectioned via fission as it is being repositioned (13). Indeed, mitochondrial fission directs mitochondria to concentrate in neuronal areas that are expected to have higher ATP consumption and is critical for neurite growth (14).
Altered mitochondrial dynamics has been linked to altered mitochondrial physiology and abnormal cell functions (15, 16), which has been implicated in many human diseases (17). Unbalanced mitochondrial fission or fusion events dysregulate key cellular processes, potentially contributing to tumorigenesis (18,19). A very recent study showed that human lung cancer cell lines exhibited excess mitochondrial fission and impaired mitochondrial fusion due to an imbalance of Drp1/Mfn expression, and that this was important for cell cycle progression (20). However, whether dysregulated mitochondrial dynamics contributes to breast cancer metastasis is unknown.
In the present study, we found marked upregulation of Drp1 protein expression in human invasive breast carcinoma and metastases to lymph nodes. We therefore characterized the molecular basis of mitochondrial dynamics in three breast cancer cell lines with various metastatic abilities. Our data show for the first time that Drp1-dependent mitochondrial fission is required for mitochondria redistribution to lamellipodial regions at the leading edge of breast cancer cells, which is critical for their migration and invasion in response to a chemotactic gradient. Thus, targeting dysregulated Drp1-dependent mitochondrial fission may provide a novel strategy for suppressing breast cancer metastasis.
Results
Increased Drp1 protein levels in human invasive breast carcinomas and metastases to lymph nodes
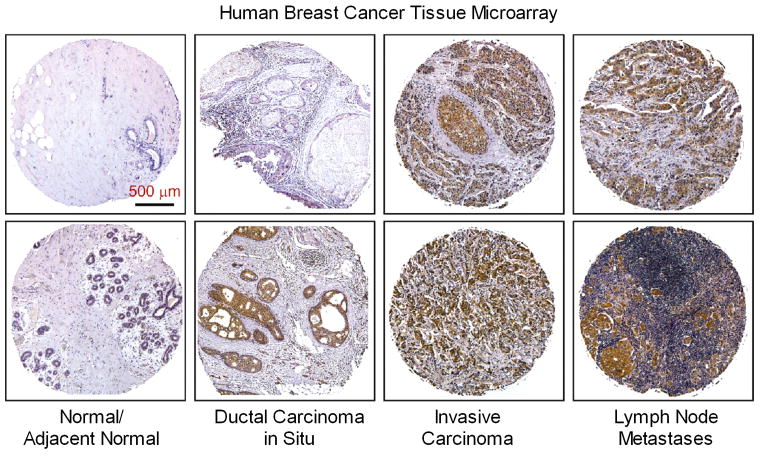
We performed immunohistochemical analysis of mitochondrial fission protein Drp1 expression in commercial microarrays of 184 human breast cancer specimens or normal/adjacent normal tissues. Fig. 1 shows that Drp1 immunostaining was very weak in normal breast tissue. There was a varied increase in Drp1 staining in noninvasive ductal carcinoma in situ, which was much more intense in invasive breast carcinoma and metastases to lymph nodes. To semi-quantify these differences, expression levels of Drp1 protein in all microarray cases were graded from 1 – 4 based on overall staining intensity. As shown in Table 1, average Drp1 staining intensities in ductal carcinoma in situ were increased as compared to normal or adjacent normal breast tissues (1.92 ± 0.12 vs. 1.27 ± 0.07, p<0.01). There was no significant difference in Drp1 protein expression between invasive breast carcinoma and lymph node metastases, but both were significantly higher than ductal carcinoma in situ (2.49 ± 0.12 and 2.78 ± 0.15 vs. 1.92 ± 0.12, #p<0.05 and ##p<0.001, respectively). These data suggest that up-regulation of Drp1 mitochondrial fission protein is proportional to the degree of invasiveness and metastasis of these breast cancers, and raised the possibility that this has functional relevance.
Figure 1. Upregulation of Drp1 protein expression in human breast carcinomas and metastases to lymph node.
Representative immunostaining of Drp1 protein in human breast cancer tissue microarrays using a mouse anti-Drp1 antibody as described under “Materials and Methods”.
Table 1.
Drp1 expression by immunohistochemistry staining in breast cancer specimens
| Breast specimens | n | Staining intensity
|
Average ± SE | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Normal/Adjacent normal | 41 | 30 | 11 | 0 | 0 | 1.27 ± 0.07 |
| Ductal carcinoma in situ | 50 | 18 | 21 | 8 | 3 | 1.92 ± 0.12* |
| Invasive carcinoma | 53 | 4 | 27 | 13 | 9 | 2.49 ± 0.12**, # |
| Lymph node metastasis | 40 | 2 | 17 | 9 | 12 | 2.78 ± 0.15**, ## |
Statistical significance was determined using a Kruskal-Wallis test and Dunn posttest.
p<0.01,
p<0.001 vs. Normal/Adjacent normal.
p<0.05,
p<0.001 vs. Ductal carcinoma in situ
Mitochondria are more fragmented in metastatic breast cancer cells
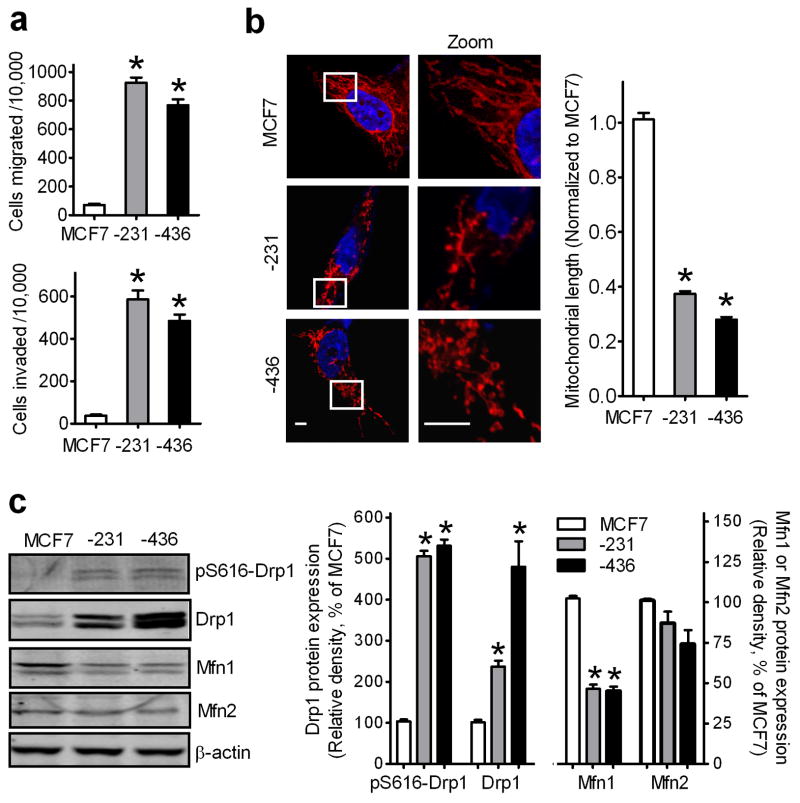
We therefore characterized the molecular basis of mitochondrial dynamics in three breast cancer cell lines with various metastatic abilities. Transwell migration and invasion assays using NIH-3T3 fibroblast conditioned medium (CM) as a chemoattractant (21) demonstrated that the non-metastatic breast cancer MCF7 cell line has at least 10-fold lower migratory and invasive abilities when compared to two metastatic breast cancer cell lines, MDA-MB-231 and MDA-MB-436 (Fig. 2a). Fig. 2b showed that mitochondria are tubular network-like structures in MCF7 cells whereas in MDA-MB-231 and MDA-MB-436, mitochondria are short tubules and spheres with an average length that was 63–73% shorter than that in MCF7 cells. Western blot assays showed that Drp1 was significantly increased by 2.5- and 5-fold in MDA-MB-231 and MDA-MB-436 cells, respectively, when compared to that in MCF7 cells, whereas mitochondrial fusion protein Mfn1, but not Mfn2, was decreased by about 50% (Fig. 2c). Thus, metastatic breast cancer cells have enhanced mitochondrial fission associated with increased expression of Drp1 and decreased expression of Mfn1.
Figure 2. Mitochondria are more fragmented in metastatic breast cancer cells.
(a) Comparison of migration and invasion abilities of breast cancer MCF7, MDA-MB-231 and MDA-MB-436 cells. n=5, mean ± s.e.m., *p<0.01. (b) Representative images of MCF7, MDA-MB-231 and MDA-MB-436 cells, stained with MitoTracker Red, show mitochondrial morphology (left panel), analyzed by measuring mitochondrial length (right panel). Scale bars, 5 μm. (c) Western blot analysis of Drp1, pS616-Drp1, Mfn1 and Mfn2 expression levels in MCF7, MDA-MB-231 and MDA-MB-436 cells using anti-Drp1, -pS616-Drp1, -Mfn1 and -Mfn2 antibodies (left panel), analyzed by measuring band density (right panel). β-Actin was used as an internal control. n=3, mean ± s.e.m., *p<0.01 as compared to that of MCF7 cells.
Since phosphorylation of Drp1 regulates mitochondrial fission, we also examined Drp1 phosphorylation at Ser-616 (pS616-Drp1), which enhances its activity (22). Compared to MCF7 cells, both MDA-MB-231 and MDA-MB-436 cells had 5-fold higher levels of pS616-Drp1 (Fig. 2c), suggesting that metastatic breast cancer cells manifest higher levels of active Drp1.
Mitochondrial fission is required for breast cancer cell migration and invasion
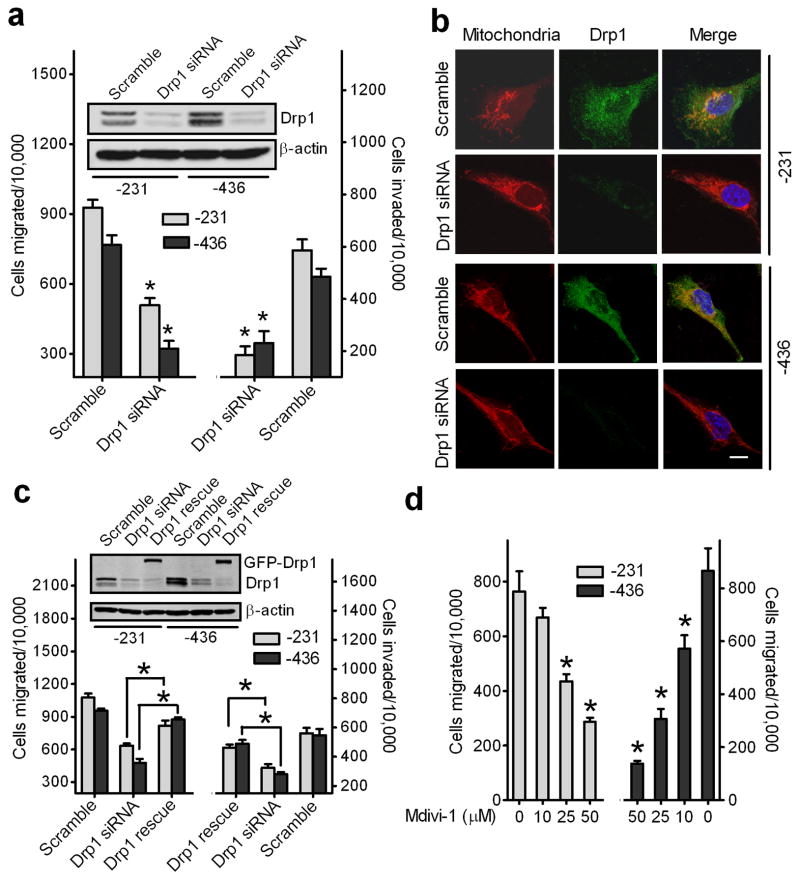
We then examined if silencing endogenous Drp1 to decrease mitochondrial fission could attenuate breast cancer cell migration and invasion abilities. As shown in Fig. 3a (inset), transfection of Drp1-targeted siRNAs caused over 85% reductions in endogenous Drp1 protein in MDA-MB-231 and MDA-MB-436 cells when compared to cells transfected with scramble siRNAs, which was confirmed by immunofluorescence staining (Fig. 3b). As expected, mitochondria became tubular and elongated in Drp1-silenced breast cancer cells.
Figure 3. Reduction of mitochondrial fission suppresses migration and invasion abilities of breast cancer cells.
(a) Knockdown of endogenous Drp1 inhibits migration and invasion abilities of breast cancer MDA-MB-231 and MDA-MB-436 cells. n=4, mean ± s.e.m., *p<0.01. Inset: Western blot analysis of Drp1 expression in cells transfected with scramble or Drp1 siRNAs. (b) Representative confocal images of MDA-MB-231 cells (upper) and MDA-MB-436 cells (lower), transfected with scramble or Drp1 siRNAs and stained with Mito Tracker Red, show endogenous expression of Drp1 and mitochondrial morphology. Scale bar, 10 μm. Mitochondria are in red, Drp1 is in green, and the nucleus is in dark blue. (c) A GFP-tagged Drp1 mutant, insensitive to Drp1 siRNAs, was expressed in Drp1-silenced breast cancer cells for 48h and cells were then collected for Western blot analysis of Drp1 expression (Inset) and Transwell migration and invasion assays. n=3, mean ± s.e.m., *p<0.01. (d) A selective inhibitor of Drp1, Mdivi-1, inhibits migration of breast cancer cells. MDA-MB-231 and MDA-MB-436 cells, pretreated with different concentrations of Mdivi-1 (Sigma) for 30 min, were subjected to Transwell migration assays in response to NIH-3T3 CM. n=3, mean ± s.e.m., *p<0.01.
We further examined the effects of silencing Drp1 on the metastatic abilities of breast cancer cells in vitro. As shown in Fig. 3a, when compared to cells transfected with scramble siRNA, knockdown of Drp1 reduced CM-induced migration and invasion of MDA-MB-231 and MDA-MB-436 cells by about 50% and 70%, respectively (Fig. 3a).
To rule out “off-target” effects of siRNA, we carried out rescue experiments by re-expressing, in Drp1-silenced breast cancer cells, green fluorescent protein (GFP)-tagged Drp1 with a mutation that is insensitive to Drp1 siRNAs. As shown in Fig. 3c, expression of GFP-tagged Drp1 attenuated the defects in migration and invasion of Drp1-silenced MDA-MB-231 and MDA-MB-436 cells.
To further determine the importance of Drp1 in breast cancer cell migration and invasion, we treated MDA-MB-231 and MDA-MB-436 cells with Mdivi-1, a Drp1 specific inhibitor that allows for unopposed fusion (23). As shown in Fig. 3d, Mdivi-1 treatment induced a dose-dependent inhibition of cell migration. Collectively, these data demonstrate that targeting Drp1 expression or activity suppresses breast cancer cell migration and invasion.
Increased mitochondrial fusion inhibits migration and invasion of breast cancer cells
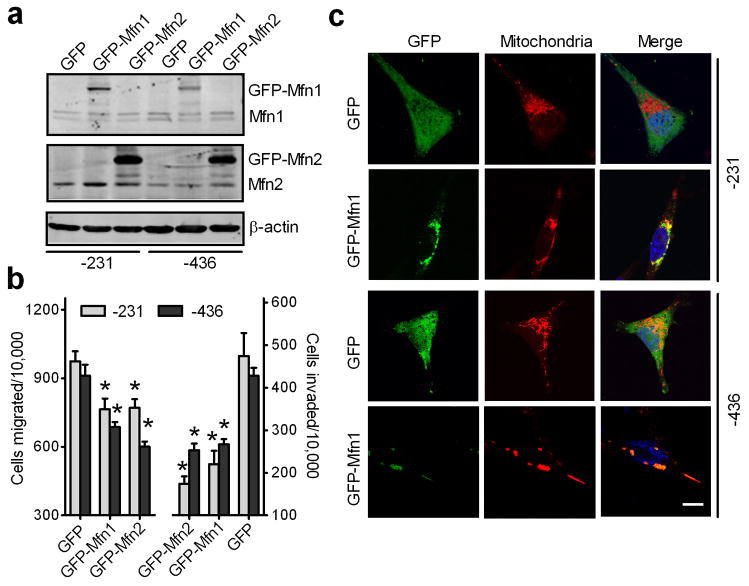
Mitofusion proteins are also important in regulation of mitochondrial dynamics (24, 25). Given that Mfn1 expression levels in MDA-MB-231 and MDA-MB-436 cells were lower than that in MCF7 cells, exogenous GFP-tagged Mfn1 was overexpressed in MDA-MB-231 and MDA-MB-436 cells (Fig. 4a). Overexpression of GFP-tagged Mfn1 significantly reduced MDA-MB-231 cell migration and invasion by 25% and 50%, respectively. Similarly, MDA-MB-436 cells transfected with GFP-tagged Mfn1 had 30–40% lower migration and invasion abilities as compared to control cells expressing GFP (Fig. 4b). Since counting GFP-positive cells revealed a transfection efficiency of approximately 70%, this partial inhibition of in vitro cell migration and invasion by GFP-tagged Mfn1 is likely underestimated. As expected, mitochondria were aggregated to form clusters in MDA-MB-231 and MDA-MB-436 cells expressing GFP-tagged Mfn1 when compared to control cells expressing GFP (Fig. 4c). Interestingly, overexpression of GFP-tagged Mfn2 in MDA-MB-231 and MDA-MB-436 cells also significantly reduced cell migration and invasion (Fig. 4a and 4b).
Figure 4. Increased mitochondrial fusion inhibits migration and invasion of breast cancer cells.
MDA-MB-231 and MDA-MB-436 cells, transfected with GFP, GFP-tagged Mfn1 or Mfn2 for 24h, were collected for Western blot analysis of Mfn1 and Mfn2 (a), or subjected to migration and invasion assays (b) n=3, mean ± s.e.m., *p<0.05. (c) Representative confocal images of MDA-MB-231 and MDA-MB-436 cells, expressing GFP or GFP-tagged Mfn1, stained with MitoTracker Red, show mitochondrial morphology. Mitochondria are in red, GFP is in green, and the nucleus is in dark blue. Scale bar, 10 μm.
Suppression of mitochondrial fusion increases breast cancer cell migration and invasion
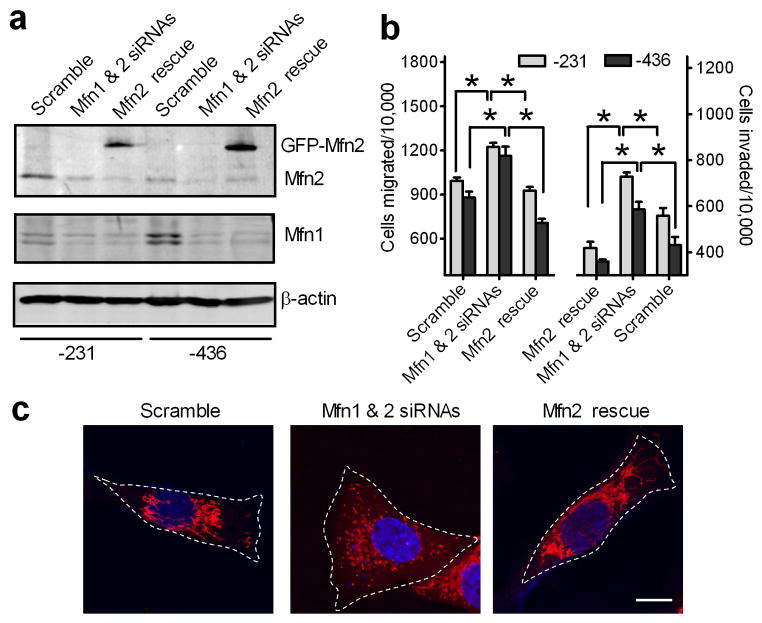
We further investigated whether the reduction of mitochondrial fusion will increase metastatic abilities of breast cancer cells. Compared with control cells transfected with scramble siRNAs, MDA-MB-231 and MDA-MB-463 cells transfected with Mfn1 and Mfn2 siRNAs had a 50% reduction of Mfn1 and Mfn2 proteins (Fig. 5a) and a 30% increase in cell migration and invasion abilities (Fig. 5b). This increase was largely abolished (Fig. 5b) when Mfn1- and Mfn2-silenced cells were co-transfected with siRNA-insensitive GFP-tagged Mfn2 (Fig. 5a) and fragmented mitochondria were restored to an elongated state (Fig. 5c). Similarly, overexpression of siRNA-insensitive GFP-tagged Mfn1 also attenuated increased migration and invasion abilities of Mfn1- and Mfn2-silenced breast cancer cells (data not shown). Thus, suppression of mitochondrial fusion enhances migration and invasion of breast cancer cells.
Figure 5. Suppression of mitochondrial fusion increases migration and invasion of breast cancer cells.
MDA-MB-231 and MDA-MB-436 cells were transfected with scramble or Mfn1 and Mfn2 siRNAs. Mfn1 and Mfn2-silenced cells were then transfected with GFP-tagged, siRNA-insensitive Mfn2 mutant for 24h. Cells were collected for Western blot analysis of Mfn1 and Mfn2 (a), and Tanswell migration and invasion assays (b). n=3, mean ± s.e.m., *p<0.05. (c) Representative confocal images of MDA-MB-231 cells, transfected with scramble, Mfn1 and Mfn2 siRNAs without (Mfn1 & 2 siRNAs) or with GFP-tagged, siRNA-insensitive Mfn2 mutant (Mfn2 rescue), stained with MitoTracker Red, show mitochondrial morphology. Mitochondria are in red, and the nucleus is in dark blue. Scale bar, 10 μm.
Manipulations of mitochondrial dynamics in breast cancer cells had no effect on cell viability and cell cycle
As shown in Supplemental Fig. 1a, neither Drp1 knockdown nor overexpression of Mfn1 in MDA-MB-231 and MDA-MB-463 cells significantly increased cell apoptosis when compared to control cells. The effects of altering mitochondrial dynamics on the cell cycle of these cells was determined with flow cytometric analysis. Our results indicated that silencing Drp1 or silencing Mfn1 and Mfn2 in MDA-MB-231 and MDA-MB-436 cells caused no significant changes in the percent of cells in the G0/G1, S and G2/M phase when compared to cells transfected with scramble siRNAs (Supplemental Fig. 1b and 1c). Together, these data suggest that the changes in migration and invasion abilities of breast cancer cells seen when their mitochondrial dynamics were dysregulated were not due to changes in their viability or cell cycle.
Prolonged downregulation of Mfns interferes with mitochondrial DNA (mtDNA) maintenance (26) and this may lead to changes in mitochondria-dependent cell migration and invasion. MDA-MB-231 cells were double-stained using Mitotracker Red and dsDNA dye, Picogreen. Supplemental Fig. 2 shows no reduction in picogreen colocalization with the mitochondria in cells treated with Mfn1 and Mfn2 siRNAs for 48h, and only a modest reduction (<15%) after 96h treatment. Thus, the impact of mtDNA loss over the typical timecourse of our experiments appears to be negligible.
Lamellipodia formation in breast cancer cells depends on mitochondrial dynamics
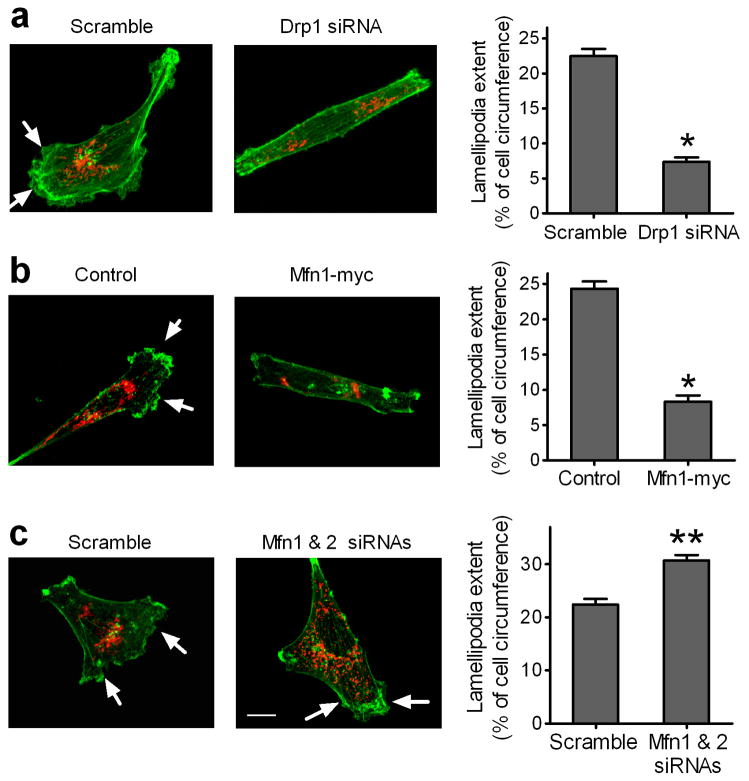
We further determined if mitochondrial dynamics is involved in the formation of lamellipodia, the flattened F-actin-rich leading edge of migrating cells, which is a key structure for cancer cell migration and invasion (27). As shown in Fig. 6a and 6b, silencing Drp1 or Mfn1 overexpression caused less cell spreading and reduced the extent of lamellipodia, indicated by arrows, in MDA-MB-231 cells by 60%. In contrast, silencing Mfn1 and Mfn2 resulted in more cell spreading and increased the extent of lamellipodia in MDA-MB-231 cells by 40% (Fig. 6c). These data suggest that mitochondrial fission promotes lamellipodia formation whereas mitochondrial fusion suppresses lamellipodia formation in breast cancer cells.
Figure 6. Mitochondrial dynamics regulates lamellipodia formation in breast cancer cells.
Decrease in mitochondrial fission (a) or increase in mitochondrial fusion (b) inhibits lamellipodia formation, whereas a decrease in mitochondrial fusion (c) promotes lamellipodia formation, in MDA-MB-231 cells. (a) Cells transfected with scramble or Drp1 siRNAs were seeded on coverslips for 24h. (b) Cells on coverslips were transfected with control vector or vector encoding myc-tagged Mfn1 for 24h. (c) Cells transfected with scramble or Mfn1 and Mfn2 siRNAs were seeded on coverslips for 24h. Cells were stained with Alexa-Fluor 488-labeled phalloidin dyes for F-action (green) and MitoTracker Red for mitochondria (Red), and then visualized by Z-Stack imaging with a confocal microscope. Scale bar, 10 μm. Arrows indicate lamellipodia at cell edges. Lamellipodia extent at cell edges was quantified as a percentage of the cell circumference on 50 randomly selected cells in each group. Columns, means; bar, s.e.m. (n = 4). *p<0.01 and **p<0.05 compared with the scramble or control group.
Mitochondrial fission directs mitochondrial distribution to lamellipodia without effects on the membrane potential of mitochondria
During lamellipodia formation of breast cancer cells induced by the chemoattractant NIH-3T3 CM, we observed that mitochondria change from a perinuclear aggregated state to an extended and scattered state, and more mitochondria are distributed to the lamellipodia region at the leading edge of cells (Fig. 7a, left panel).
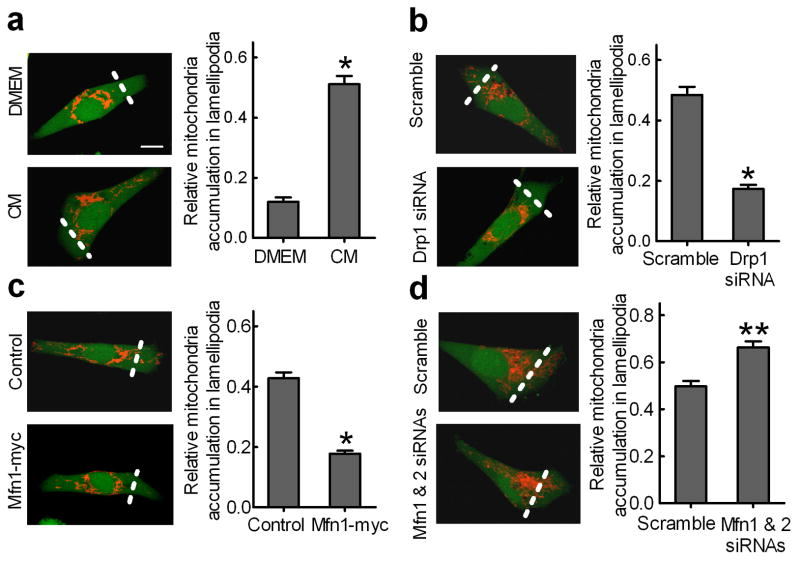
Figure 7. Mitochondrial fission promotes mitochondrial distribution to lamellipodia.
MDA-MB-231 cells stained with MitoTracker Red and CellTracker Green were visualized with a confocal microscope and the integrated fluorescent intensity was analyzed by Image-Pro Plus software. We defined the lamellipodia region as the area from the leading edge of a cell to half of the distance to the nucleus, indicated by the dish line. Scale bar, 10 μm. Relative MitoTracker and CellTracker fluorescence intensities in the lamellipodia region were first normalized to that of the whole cell. The relative abundance of mitochondria in the lamellipodia region was calculated using the ratio of normalized MitoTracker fluorescence intensity vs. normalized CellTracker fluorescence intensity in the lamellipodial region of 50 randomly selected cells in each group. Columns, means; bar, s.e.m. (n = 4). *p<0.01 and **p<0.05 compared with the DMEM, scramble or control group. (a) Chemoattractant NIH-3T3 CM induced distribution of mitochondria to the lamellipodial region in MDA-MB-231 cells. Silencing Drp1 (b) or Mfn1 overexpression (c) blocked mitochondria distribution to lamellipodia in MDA-MB-231 cells. (d) Silencing Mfn1 and Mfn2 directs more mitochondria to lamellipodia in MDA-MB-231 cells.
To quantify these mitochondrial changes, we loaded cells with the cytosolic dye, CellTracker Green, which clearly delineated the edges of the cell. Data shown in Fig. 7a (right panel) indicated that the NIH-3T3 CM induced 4-fold more mitochondria distributed to the lamellipodia region. Silencing Drp1 or Mfn1 overexpression reduced mitochondria accumulation in the lamellipodia region by 60% (Fig. 7b and 7c) whereas silencing Mfn1 and Mfn2 increased mitochondria accumulation in the lamellipodial region by 35% (Fig. 7d).
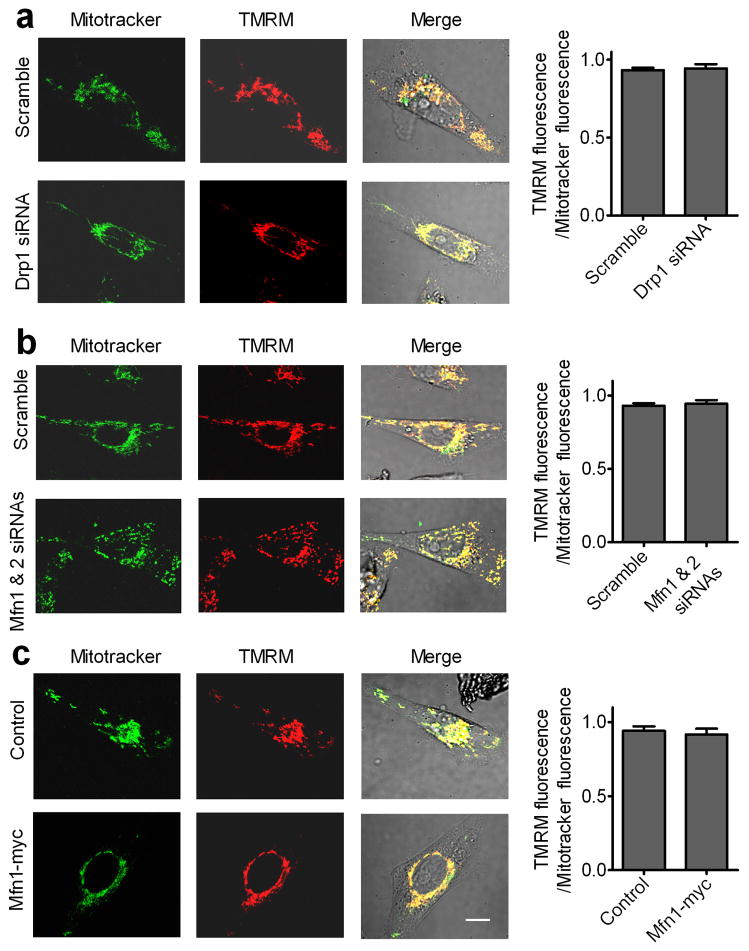
We further investigated whether manipulations of mitochondrial dynamics affected the mitochondrial membrane potential that is important for mitochondrial functions (28). Tetramethylrhodamine methyl ester (TMRM) stains polarized mitochondria (29). A ratio of TMRM and the mitochondrial marker was used as an index of mitochondrial membrane potential. As shown in Fig. 8, silencing Drp1, Mfn1 overexpression, or silencing Mfn1 and Mfn2 had no significant effect on mitochondrial membrane potential. Thus, manipulations of mitochondrial dynamics affect mitochondrial localization but not mitochondrial membrane potential.
Figure 8. Manipulations of Drp1 and Mfn proteins had no significant effects on the membrane potential of mitochondria in MDA-MB-231 cells.
MDA-MB-231 cells stained with TMRM and MitoTracker Green were visualized with a confocal microscope and the integrated fluorescent intensity was analyzed by Image-Pro Plus software. Scale bar, 10 μm. A ratio of TMRM fluorescence and MitoTracker fluorescence was used as an index of mitochondrial membrane potential. Columns, means; bar, s.e.m. of 50 randomly selected cells in each group. (a) Silencing Drp1; (b) silencing Mfn1 and Mfn2; (c) Mfn1 overexpression.
Functional importance of mitochondria in lamellipodia formation and breast cancer cell migration and invasion
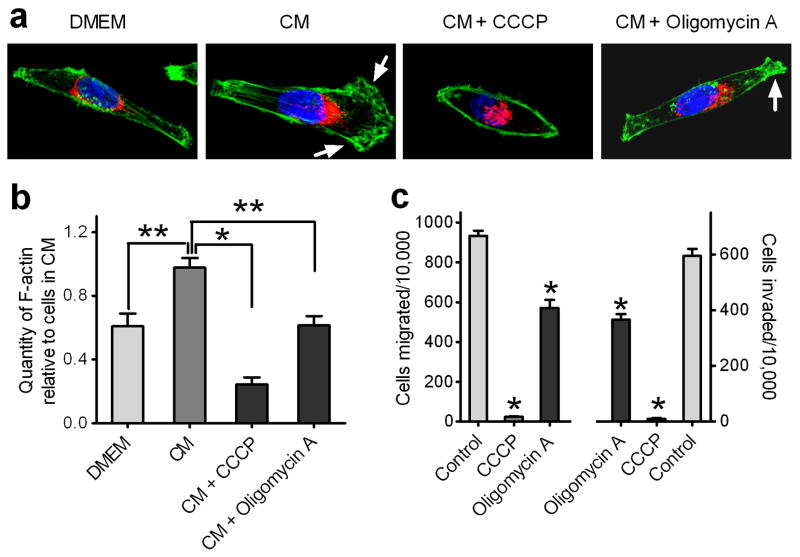
F-actin assembly is a major cellular process that consumes metabolic energy in cells. As shown in Fig. 9a, NIH-3T3 CM induced mitochondria redistribution and lamellipodia formation in MDA-MB-231 cells. The amount of F-actin in MDA-MB-231 cells treated with NIH-3T3 CM was almost 2-fold greater than that in cells cultured in serum-free DMEM (Fig. 9b).
Figure 9. Functional importance of mitochondria in lamellipodia formation and breast cancer cell migration and invasion.
MDA-MB-231 cells were incubated with serum-free DMEM or NIH-3T3 CM in the absence or presence of CCCP (100 μM) or oligomycin A (1 μg/ml) for 30 min. (a) Representative confocal images of cells stained with Alexa-Fluor 488-labeled phalloidin dyes for F-action (green) and MitoTracker Red for mitochondria (Red). Scale bar, 10 μm. Arrow points to the lamellipodia. (b) The content of F-actin in cells stained with Alexa-Fluor 488-labeled phalloidin dyes was quantified as described in Materials and Methods. n=3, mean ± s.e.m., *p<0.01 and **p<0.05. (c) MDA-MB-231 cells pretreated without (Control) or with CCCP or oligomycin A were subjected to Transwell migration and invasion assays. n=3, mean ± s.e.m., *p<0.01.
Mitochondrial ATP synthesis is driven by a proton gradient across the inner mitochondrial membrane. The proton ionophore m-chlorophenylhydrazone (CCCP) disperses this proton gradient, thereby uncoupling mitochondrial respiration from ATP synthesis (30). CCCP completely blocked NIH-3T3 CM-induced lamellipodia formation in MDA-MB-231 cells (Fig. 9a), while decreasing F-actin polymerization (Fig. 9b), and obliterating the migration and invasion abilities of the treated breast cancer cells (Fig. 9c). We also examined the effect of oligomycin A, which blocks mitochondrial ATP synthesis without dissipating the mitochondrial membrane potential (31). Oligomycin A caused a 40% reduction of F-actin polymerization (Fig. 9b) and partially ablates cell migration and invasion (Fig. 9c). Altogether, these results show that mitochondria play an important role in the migration and invasion of breast cancer cells.
Discussion
We previously described some of the intracellular regulators of breast cancer cell metastasis (32, 33), particularly as they relate to the cytoskeletal rearrangement critical for cancer cell motility, which requires an abundance of ATP. Mitochondria are the organelles responsible for aerobic ATP synthesis. They form a tubular meshwork that interdigitates with the other organelles. As the energy demands in different regions of a cell change, the mitochondria are cleaved by Drp1 into more mobile segments, which are repositioned to areas of greatest need. Thus, mitochondrial fission is essential for maintaining physiological functions of normal cells. However, cancer cells are unusual with regard to their bioenergetics, since they often derive a significant portion of their energy from aerobic glycolysis (34), even in the presence of oxygen. With two pathways for deriving energy, the functional significance of mitochondrial dynamics in a migrating cancer cell is unknown. Our study is the first to demonstrate a critical role of mitochondrial fission in breast cancer metastasis.
By analyzing human breast cancer specimens, we found that Drp1 protein expression was modestly increased in noninvasive ductal carcinoma in situ as compared to normal breast tissues, but was markedly increased in invasive breast carcinoma and cancer that had metastasized to lymph nodes. These results suggest that Drp1 upregulation may be an early event in the development of an invasive breast cancer phenotype. However, Drp1 protein expression in breast cancer specimens varied significantly, even within the same category. Since this may be related to breast cancer sub-type, we are currently examining the expression of ER, PR and HER2 to classify these breast cancer specimens. If Drp1 over-expression is more common in a particular sub-type of breast cancer, this may become an important marker for planning treatment and developing new therapies.
A recent study showed different mitochondrial protein profiles in various breast cancer cell lines with different tumorigenicity and metastatic abilities (35). However, no information is available regarding the difference in the molecular machinery of mitochondrial dynamics among these cells. Since breast cancer is a complex and heterogeneous disease, choosing the right breast cancer cell lines as experimental models is critical for defining the pathological importance of Drp1 unregulation in breast cancer. MDA MB-231 and MDA MB-436 cells have claudin-low basal phenotypes enriched with epithelial-to-mesenchymal transition markers, and form metastatic tumors in nude mice (36). These were contrasted with MCF7 cells, a luminal A phenotype that forms tumors without metastases in nude mice. These cell lines are representative of types of cells seen in breast cancer. Our data show that mitochondrial architecture in MCF7 cells differs from that of MDA-MB-231 and MDA-MB-436 cells. The elongated tubular mitochondrial structure of MCF7 cells is consistent with their comparatively high reliance on oxidative phosphorylation (37) coupled with their lower degree of motility. In contrast, the mitochondria of MDA-MB-231 and MDA-MB-436 cells were cleaved into short tubular segments. This also seems logical given the greater migratory and invasive capabilities of these two cell lines (38), and their increased random movements when not exposed to a chemotactic gradient (39). Thus, our data provided the first evidence for a difference in resting mitochondrial dynamics between these cell lines. To establish a molecular basis for this difference, we performed western blot analysis for Drp1, Mfn1 and Mfn2 on these cell lines. As might be expected, MDA-MB-231 and MDA-MB-436 cells had significantly increased levels of total and active Drp1 protein and significantly lower amounts of Mfn1 in comparison to MCF7 cells. Surprisingly, Mfn2 levels were similar between these cell lines. However, this could reflect the additional role of Mfn2 in tethering the endoplasmic reticulum to the mitochondria (40), something that presumably would be maintained irrespective of the mitochondrial segment length.
While the mitochondria of more motile MDA-MB-231 and MDA-MB-436 cells were also more fragmented, these two findings might be unrelated. However, we found that silencing Drp1 resulted in elongation of the mitochondrial tubules. Under these circumstances, the ability of the cells to migrate and invade was significantly reduced to half or less of control values. This inhibition could be reversed by expression of a siRNA-insensitive Drp1 protein, confirming that this was a Drp1-mediated effect. We also performed the converse experiments by transfecting the cells with recombinant Mfn1 or Mfn2, and again observed a significant reduction of the migration and invasion abilities of the cells. It should be noted that downregulation of Drp1 affects both the outer and inner membranes and maintains a continuous mitochondrial structure whereas Mfn overexpression tethers outer mitochondrial membranes together to form mitochondrial clusters with the inner membrane not fused. However, these two manipulations have a similar impact on cell migration and invasion, suggesting that mitochondrial fission is required for breast cancer cell migration and invasion. This also suggests that knockdown of mitochondrial fusion proteins would enhance migration and invasion, which we demonstrated in both MDA-MB-231 and MDA-MB-436 cells.
Whenever cell migration or invasion is studied, there is always a concern that the results could be impacted by changes in cell viability or proliferation. We found that under the conditions of our experiments, altering Drp1, Mfn1 and Mfn2 expression had no significant effect on breast cancer cell cycle or cell viability. These data suggest that the changes in migration and invasion we observed were not due to alternations in cell viability or proliferation. A very recent study reported that Drp1 knockdown significantly increased apoptosis in A549 lung cancer cells from 0.5% to 2% and the Drp1 inhibitor Mdivi-1 markedly reduced lung cancer cell proliferation (20). Although we found that silencing Drp1 also slightly increased breast cancer cell apoptosis, this was not statistically significant. Our data also do not show a direct link between Drp1 levels and cell cycle in breast cancer. One possible explanation is that Drp1 protein was only downregulated by 85% in our studies. The residual Drp1 protein may be enough to maintain the cell cycle but not cell migration and invasion. In fact, although MCF7 cells express significantly lower levels of Drp1 compared to MDA-MB-231 and MDA-MB-436 cells, their proliferation rate is similar.
Past work in our laboratory showed that lamellipodia formation at the leading edge of migrating cells is crucial for chemoattractant-induced breast cancer cell migration and invasion (32, 33). The present study shows that mitochondrial fission is necessary for the redistribution of mitochondria to the leading edge in response to chemoattractants, and that their presence enhances formation of lamellipodia. This was an intriguing finding since such a phenomenon has not been reported in other migrating cells that have a much greater reliance on oxidative phosphorylation (41) or in leukocytes which have a high capacity for glycolysis (42, 43). It is possible that accumulation of mitochondria in the lamellipodia region is an important first step for breast cancer cell migration and invasion. Thus it was important to determine the role of these mitochondria in lamellipodia regions of breast cancer cells.
The mitochondrial protonmotive force is determined by substrate oxidation and proton currents due to leak and ATP turnover (28). The mitochondrial membrane potential typically decreases when ATP synthesis is rapid (e.g., respiration near state 3), but is also influenced in intact cells by substrate availability. We did not observe any differences in mitochondrial membrane potential in response to Drp1 or Mfn1/-2 alterations that influenced mitochondrial dynamics and the redistribution of mitochondria to the lamellipodia region of migrating breast cancer cells. However, the inhibition of cell migration and invasion by oligomycin A suggests that mitochondrial ATP production is an important local energy source that is required for breast cancer cell migration and invasion. The more complete inhibition of migration and invasion caused by CCCP is consistent with known additional roles of the mitochondrial membrane potential, such as providing the driving force for calcium sequestration given that localized calcium oscillations may also be important for cell movement (44). A further understanding of the role and mechanisms of mitochondria in lamellipodia formation and in migration and invasion of breast cancer cells will require a subcellular assessment of ATP concentrations combined with studies of local mitochondrial-dependent calcium fluxes in metastatic breast cancer cells.
In conclusion, our data show for the first time that mitochondrial dynamics play an important role in breast cancer cell migration and invasion. Fission facilitates and fusion inhibits these processes, likely due to the physical impediments to repositioning created by long intertwined tubules. Since our data suggest that upregulation of Drp1, a protein controlling mitochondrial fission, is an early event in development of metastatic breast cancer, identifying and targeting the mechanism(s) underlying Drp1 upregulation in breast cancer patients will be a first step toward identifying new approaches to prevent metastasis.
Materials and Methods
Immunohistochemistry analysis of human breast tissues
Breast tissue microarrays were from US Biomax Inc. (BR1008, BR8011, and BR243F). Immunohistochemistry was performed as we described (32) using a mouse anti-Drp1 antibody (BD Biosciences). The negative control used non-immune mouse IgG as the primary antibody. Expression levels of Drp1 protein were graded from 1 – 4 based on overall staining intensity.
Cell culture and transfection
Human breast cancer MDA-MB-231, MDA-MB-436, and mouse embryonic NIH-3T3 fibroblast cells from American Type Culture Collection (ATCC) were cultured in DMEM with 10% fetal bovine serum (FBS). MCF7 cells from ATCC were cultured in IMEM, 10% FBS, and 10 μg/ml insulin. Plasmids were transfected into cells using Amaxa nucleofector kits (33), and cells harvested after 24h transfection were subjected to Western blot analysis and Transwell assays. Transfection efficiency with the control GFP vector system was approximately 70%.
Plasmid construction and siRNAs
Plasmids encoding GFP-tagged Drp1, Mfn1 or Mfn2 were gifts from Dr. Quan Chen (Institute of Zoology, Chinese Academy of Sciences). To silence Drp1, 27-base nucleotides were chemically synthesized (5′-ACUAUUGAAGGAACUGCAAAAUAUA-dAdG-3′ and 5′-UAUAUUUUGCAGUUCCUUCAAUAGU-dAdT-3′) (22). siRNAs for Mfn1 and Mfn2 were designed to target the sequence of Mfn1 (GATACTAGCTACTGTGAAA), and Mfn2 (GGAAGAGCACCGTGATCAA). The annealed siRNAs were transfected into cells using an Amaxa nucleofector kit. Cells were harvested after 48h transfection for Western blot analysis and Transwell assays. The siRNA-insensitive mutant of GFP-Drp1 or GFP-Mfn2 was constructed by PCR using Quickchange site-directed mutagenesis kit (Stratagene) with the wild-type GFP-Drp1 or GFP-Mfn2 plasmid as the template. The primer was designed to contain three mutated nucleotide sites on the siRNA targeting sequence of Drp1 (CTGTTGAAAGAACTACAAAATAT) or Mfn2 (GGAAAAGCACGGTGATAAA).
Western blot analysis
Cell lysates were subjected to SDS–PAGE before transfer to Immobilon-FL. Primary antibodies were used to identify the relevant protein and loading control (β-actin). IRDye-labeled secondary antibodies were used for band detection with an Odyssey infrared imaging system (LI-COR Biosciences).
Transwell invasion and migration assays
Matrigel invasion assays were carried out at 37°C for 16h using 24-well Transwell inserts (Corning) coated with 30 μg of Matrigel (BD Biosciences). Cells (50,000) suspended in 200 μl of serum-free medium were seeded into the upper chamber and 600 μl of NIH-3T3 CM were placed in the lower chamber. Cells that migrated and invaded through the membrane were counted and normalized relative to 10,000 seeded cells. Transwell cell migration assays were performed similarly, but only for 5h and without Matrigel. CM from NIH-3T3 cells was collected and used as a chemoattractant as we previously reported (32,33).
Immunofluorescence and confocal microscopy
Cells on coverslips were fixed with 4% paraformaldehyde in PBS, permeabilized with 0.1% Triton X-100, blocked with 1% BSA and 10% horse serum, and then incubated with primary antibodies and Rhodamine- or FITC-conjugated secondary antibodies. To determine subcellular distribution of mitochondria, cells were loaded with 50nM MitoTracker Red and 5μM CellTracker Green (Life Technologies) for 30min to stain mitochondria and cell cytosol. Cells were visualized by Z-Stack imaging with a confocal microscope (Olympus, FV1000) and processed using Fluoview software (Olympus). The length of mitochondria was measured using Image-Pro Plus software. The ratio of the mitochondrial marker vs. the cytsolic dye in the lamellipodial region was used to eliminate the possibility that the abundance of mitochondria in lamellipodial were simply a reflection of cytosol accumulation in these areas. For lamellipodia staining, cells were fixed and stained with Alexa-Fluor 488-labelled phalloidin. Lamellipodia were identified as a convex stretch of perpendicular actin stain at the peripheral edge of the cell as visualized by the Alexa-Fluor 488-labeled phalloidin stain (32).
Determination of mitochondrial membrane potential
The mitochondrial membrane potential was measured using the fluorescent dye TMRM (29). In brief, cells were exposed to 20nM TMRM (Invitrogen) and 50nM MitoTracker Green for 20 min at 37°C to allow dye equilibration across the plasma and inner mitochondrial membranes. For imaging, the medium was replaced with medium containing 5 nM TMRM. The ratio of the TMRM fluorescence vs. MitoTracker Green fluorescence was used as an indicator of mitochondrial membrane potential.
Measurement of cellular F-actin content
As previously reported (45), cells in plates were incubated with fixative containing 2 mM Alexa-Fluor 488-labelled phalloidin, transferred to a 1.5-ml tube, and incubated for 1h at room temperature. Cells were pelleted by centrifugation, and incubated with methanol for 1h to extract the Alexa-Fluor 488 phalloidin. Alexa-Fluor 488 phalloidin binding to F-action in each sample was measured using a Hitachi F4500 spectrophotometer with excitation and emission wavelength of 488 and 502 nm, respectively.
Statistical analysis
Tissue microarray scoring was analyzed with a Kruskal–Wallis test and Dunn posttest. Other results are mean ± s.e.m of at least three determinations, and statistical comparisons used a Student’s t test, or a two-way ANOVA with the Bonferroni correction where there were multiple comparisons. p < 0.05 was considered to be significant.
Supplementary Material
Acknowledgments
We thank Profs. Fuyu Yang and Quan Chen for their valuable suggestions. This work was supported by the National Institutes of Health (CA125661), Nebraska LB595 program, and the National Basic Research Program of China (2010CB833701, 2012CB934003). Dr. Juan Zhang is a grant recipient of the National Natural Science Foundation of China (31100973).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Condeelis J, Singer RH, Segall JE. The great escape: when cancer cells hijack the genes for chemotaxis and motility. Annu Rev Cell Dev Biol. 2005;21:695–718. doi: 10.1146/annurev.cellbio.21.122303.120306. [DOI] [PubMed] [Google Scholar]
- 4.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Jagow G, Engel WD. Structure and function of the energy-converting system of mitochondria. Angew Chem Int Ed Engl. 1980;19:659–675. doi: 10.1002/anie.198006593. [DOI] [PubMed] [Google Scholar]
- 6.Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology. 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 7.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 8.Chan DC. Dissecting mitochondrial fusion. Dev Cell. 2006;11:592–594. doi: 10.1016/j.devcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Werth JL, Thayer SA. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci. 1994;14:348–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Liu W, Liu J, Xiao W, Liu L, Jiang C, et al. G-protein beta2 subunit interacts with mitofusin 1 to regulate mitochondrial fusion. Nat Commun. 2010;1:101. doi: 10.1038/ncomms1099. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Madrid F, Serrador JM. Bringing up the rear: defining the roles of the uropod. Nat Rev Mol Cell Biol. 2009;10:353–359. doi: 10.1038/nrm2680. [DOI] [PubMed] [Google Scholar]
- 13.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu CY, Lee CF, Hong CH, Wei YH. Mitochondrial DNA mutation and depletion increase the susceptibility of human cells to apoptosis. Ann N Y Acad Sci. 2004;1011:133–145. doi: 10.1007/978-3-662-41088-2_14. [DOI] [PubMed] [Google Scholar]
- 16.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 18.Grandemange S, Herzig S, Martinou JC. Mitochondrial dynamics and cancer. Semin Cancer Biol. 2009;19:50–56. doi: 10.1016/j.semcancer.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, et al. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012;26:2175–2186. doi: 10.1096/fj.11-196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albini A, Benelli R. The chemoinvasion assay: a method to assess tumor and endothelial cell invasion and its modulation. Nat Protoc. 2007;2:504–511. doi: 10.1038/nprot.2006.466. [DOI] [PubMed] [Google Scholar]
- 22.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 23.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, et al. Mitochondrial Fusion Is Required for mtDNA Stability in Skeletal Muscle and Tolerance of mtDNA Mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Condeelis JS, Wyckoff JB, Bailly M, Pestell R, Lawrence D, Backer J, et al. Lamellipodia in invasion. Semin Cancer Biol. 2001;11:119–128. doi: 10.1006/scbi.2000.0363. [DOI] [PubMed] [Google Scholar]
- 28.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verburg J, Hollenbeck PJ. Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J Neurosci. 2008;28:8306–8315. doi: 10.1523/JNEUROSCI.2614-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- 31.Bertina RM, Steenstra JA, Slater EC. The mechanism of inhibition by oligomycin of oxidative phosphorylation in mitochondria. Biochim Biophys Acta. 1974;368:279–297. doi: 10.1016/0005-2728(74)90175-3. [DOI] [PubMed] [Google Scholar]
- 32.Xie Y, Wolff DW, Wei T, Wang B, Deng C, Kirui JK, et al. Breast cancer migration and invasion depend on proteasome degradation of regulator of G-protein signaling 4. Cancer Res. 2009;69:5743–5751. doi: 10.1158/0008-5472.CAN-08-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirui JK, Xie Y, Wolff DW, Jiang H, Abel PW, Tu Y. Gbetagamma signaling promotes breast cancer cell migration and invasion. J Pharmacol Exp Ther. 2010;333:393–403. doi: 10.1124/jpet.109.164814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim Biophys Acta. 2011;1807:552–561. doi: 10.1016/j.bbabio.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Chen YW, Chou HC, Lyu PC, Yin HS, Huang FL, Chang WS, et al. Mitochondrial proteomics analysis of tumorigenic and metastatic breast cancer markers. Funct Integr Genomics. 2011;11:225–239. doi: 10.1007/s10142-011-0210-y. [DOI] [PubMed] [Google Scholar]
- 36.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guppy M, Leedman P, Zu X, Russell V. Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF7 breast cancer cells. Biochem J. 2002;364:309–315. doi: 10.1042/bj3640309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 39.Platet N, Prevostel C, Derocq D, Joubert D, Rochefort H, Garcia M. Breast cancer cell invasiveness: correlation with protein kinase C activity and differential regulation by phorbol ester in estrogen receptor-positive and -negative cells. Int J Cancer. 1998;75:750–756. doi: 10.1002/(sici)1097-0215(19980302)75:5<750::aid-ijc14>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 40.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 41.Couchman JR, Rees DA. Organelle-cytoskeleton relationships in fibroblasts: mitochondria, Golgi apparatus, and endoplasmic reticulum in phases of movement and growth. Eur J Cell Biol. 1982;27:47–54. [PubMed] [Google Scholar]
- 42.Biswas S, Ray M, Misra S, Dutta DP, Ray S. Is absence of pyruvate dehydrogenase complex in mitochondria a possible explanation of significant aerobic glycolysis by normal human leukocytes? FEBS Lett. 1998;425:411–414. doi: 10.1016/s0014-5793(98)00273-7. [DOI] [PubMed] [Google Scholar]
- 43.Campello S, Lacalle RA, Bettella M, Manes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203:2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conklin MW, Lin MS, Spitzer NC. Local calcium transients contribute to disappearance of pFAK, focal complex removal and deadhesion of neuronal growth cones and fibroblasts. Dev Biol. 2005;287:201–212. doi: 10.1016/j.ydbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J Cell Biol. 1997;138:913–926. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.