Abstract
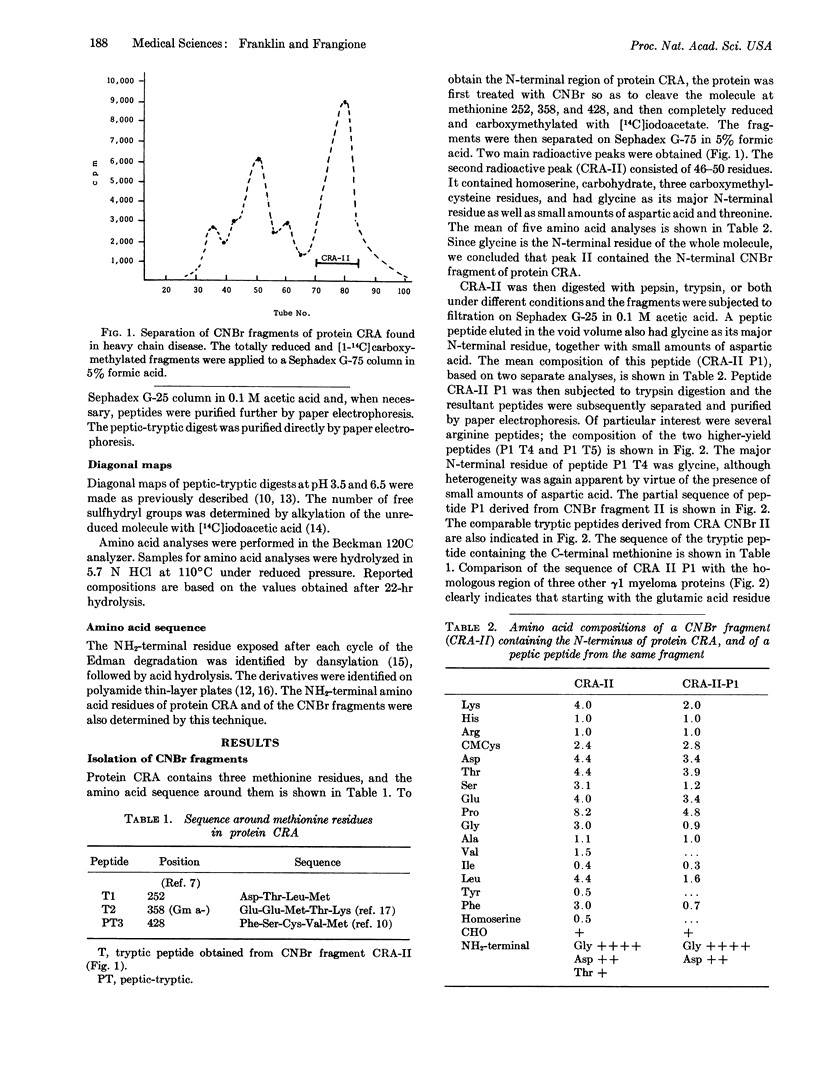
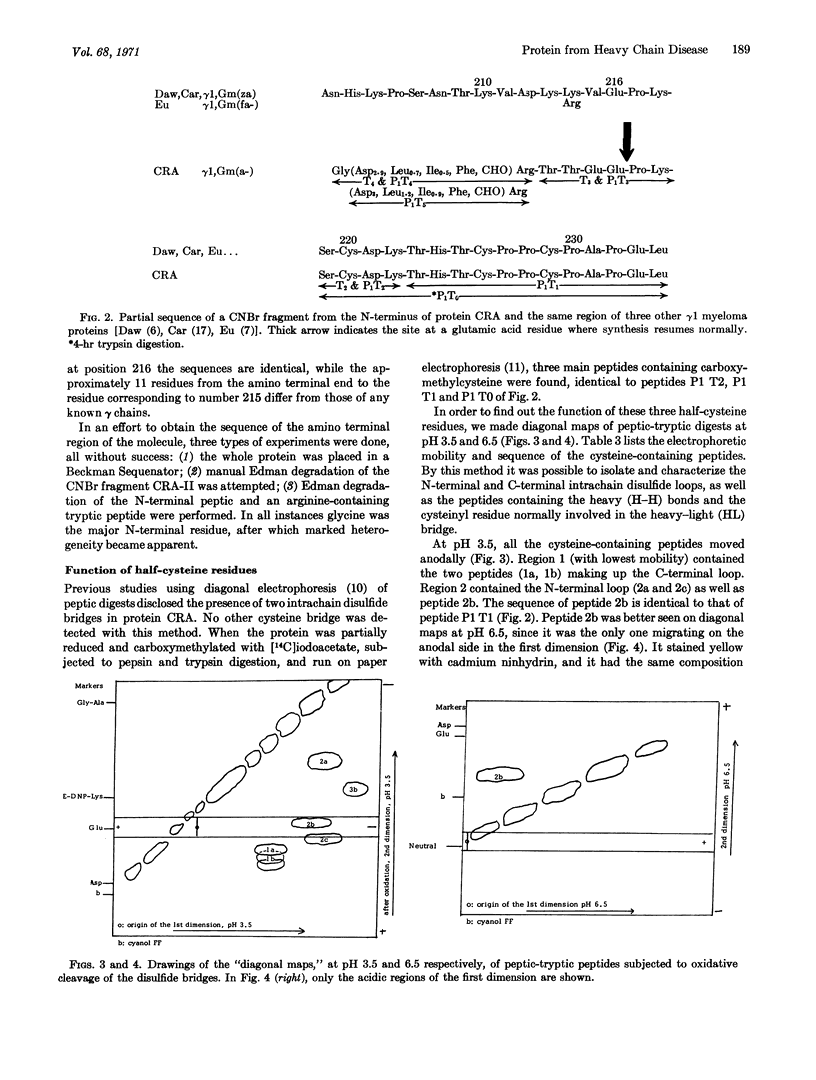
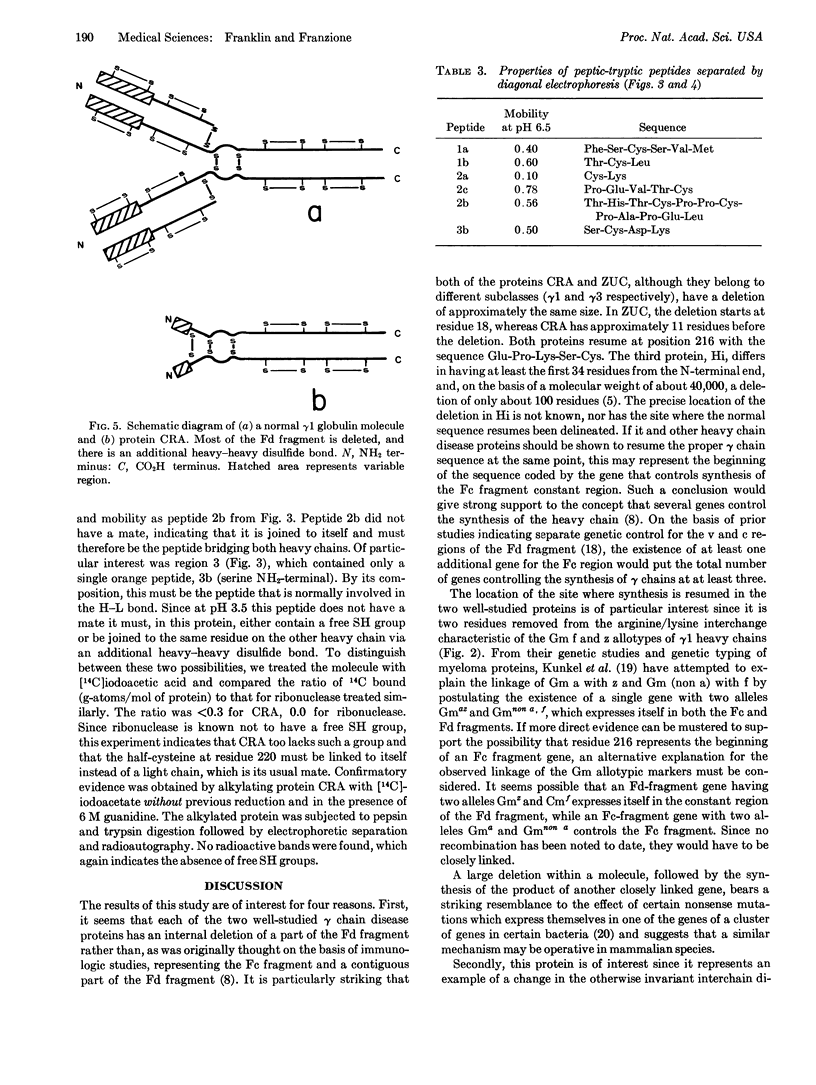
A γ1 protein, designated CRA, found in heavy chain disease contains three inter-heavy disulfide bridges instead of the two normally found in γ1 immunoglobulin heavy chains. Almost the entire Fd fragment is missing. The NH2 terminal region is heterogeneous and contains carbohydrate; after 11 residues that do not resemble any of the known heavy-chain variable-region subclasses, normal synthesis seems to be resumed at the same amino acid residue as in another heavy-chain disease protein (ZUC). This finding raises the possibility that glutamic acid at position 216 represents the beginning of the Fe region, synthesized under the direction of another gene.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN E. C., LOWENSTEIN J., BIGELOW B., MELTZER M. HEAVY CHAIN DISEASE- A NEW DISORDER OF SERUM GAMMA-GLOBULINS : REPORT OF THE FIRST CASE. Am J Med. 1964 Sep;37:332–350. doi: 10.1016/0002-9343(64)90191-3. [DOI] [PubMed] [Google Scholar]
- FRANKLIN E. C. STRUCTURAL STUDIES OF HUMAN 7S GAMMA-GLOBULIN (G IMMUNOGLOBULIN). FURTHER OBSERVATIONS OF A NATURALLY OCCURRING PROTEIN RELATED TO THE CRYSTALLIZABLE (FAST) FRAGMENT. J Exp Med. 1964 Nov 1;120:691–709. doi: 10.1084/jem.120.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B., Franklin E. C., Fudenberg H. H., Koshland M. E. Structural studies of human gamma-G-myeloma proteins of different antigenic subgroups and genetic specificities. J Exp Med. 1966 Oct 1;124(4):715–732. doi: 10.1084/jem.124.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B., Milstein C., Franklin E. C. Intrachain disulphide bridges in immunoglobulin G heavy chains. The Fc fragment. Biochem J. 1968 Jan;106(1):15–21. doi: 10.1042/bj1060015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B., Milstein C. Partial deletion in the heavy chain disease protein ZUC. Nature. 1969 Nov 8;224(5219):597–599. doi: 10.1038/224597a0. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C., Pink J. R. Structural studies of immunoglobulin G. Nature. 1969 Jan 11;221(5176):145–148. doi: 10.1038/221145a0. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C. Variations in the S-S bridges of immunoglobins G: interchain disulfide bridges of gamma G3 myeloma proteins. J Mol Biol. 1968 May 14;33(3):893–906. doi: 10.1016/0022-2836(68)90326-4. [DOI] [PubMed] [Google Scholar]
- Franklin E. C. Heavy-chain diseases. N Engl J Med. 1970 May 7;282(19):1098–1099. doi: 10.1056/NEJM197005072821913. [DOI] [PubMed] [Google Scholar]
- Garen A. Sense and nonsense in the genetic code. Three exceptional triplets can serve as both chain-terminating signals and amino acid codons. Science. 1968 Apr 12;160(3824):149–159. doi: 10.1126/science.160.3824.149. [DOI] [PubMed] [Google Scholar]
- Miller F., Metzger H. Characterization of a human macroglobulin. II. Distribution of the disulfide bonds. J Biol Chem. 1965 Dec;240(12):4740–4745. [PubMed] [Google Scholar]
- Natori S., Garen A. Molecular heterogeneity in the amino-terminal region of alkaline phosphatase. J Mol Biol. 1970 May 14;49(3):577–588. doi: 10.1016/0022-2836(70)90282-2. [DOI] [PubMed] [Google Scholar]
- Terry W. D., Ohms J. Implications of heavy chain disease protein sequences for multiple gene theories of immunoglobulin synthesis. Proc Natl Acad Sci U S A. 1970 Jun;66(2):558–563. doi: 10.1073/pnas.66.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Zawadzki Z. A., Benedek T. G., Ein D., Easton J. M. Rheumatoid arthritis terminating in heavy-chain disease. Ann Intern Med. 1969 Feb;70(2):335–347. doi: 10.7326/0003-4819-70-2-335. [DOI] [PubMed] [Google Scholar]



