Abstract
Background
Homeodomain-leucine zipper (HD-Zip) proteins, a group of homeobox transcription factors, participate in various aspects of normal plant growth and developmental processes as well as environmental responses. To date, no overall analysis or expression profiling of the HD-Zip gene family in soybean (Glycine max) has been reported.
Methods and Findings
An investigation of the soybean genome revealed 88 putative HD-Zip genes. These genes were classified into four subfamilies, I to IV, based on phylogenetic analysis. In each subfamily, the constituent parts of gene structure and motif were relatively conserved. A total of 87 out of 88 genes were distributed unequally on 20 chromosomes with 36 segmental duplication events, indicating that segmental duplication is important for the expansion of the HD-Zip family. Analysis of the Ka/Ks ratios showed that the duplicated genes of the HD-Zip family basically underwent purifying selection with restrictive functional divergence after the duplication events. Analysis of expression profiles showed that 80 genes differentially expressed across 14 tissues, and 59 HD-Zip genes are differentially expressed under salinity and drought stress, with 20 paralogous pairs showing nearly identical expression patterns and three paralogous pairs diversifying significantly under drought stress. Quantitative real-time RT-PCR (qRT-PCR) analysis of six paralogous pairs of 12 selected soybean HD-Zip genes under both drought and salinity stress confirmed their stress-inducible expression patterns.
Conclusions
This study presents a thorough overview of the soybean HD-Zip gene family and provides a new perspective on the evolution of this gene family. The results indicate that HD-Zip family genes may be involved in many plant responses to stress conditions. Additionally, this study provides a solid foundation for uncovering the biological roles of HD-Zip genes in soybean growth and development.
Introduction
Transcription factors (TFs) are types of proteins that affect many biological processes such as growth, development and cell division and respond to environmental stimuli and stressors in cells or organisms [1]. TFs bind to DNA and either activate or repress gene expression at the level of mRNA transcription [2]. Typical TFs mainly contain a DNA binding domain and a transcriptional activation domain; the former recognizes target DNA sequences while the latter initiates transcription [3]. Homeobox (HB), one of the key transcription factors families, was first identified in a set of Drosophila genes controlling development [4]. Each HB gene encodes a conserved 61 amino acid sequence known as the homeodomain (HD) [5], [6], which is responsible for sequence-specific DNA binding. Subsequently, HDs have also been discovered in invertebrates and vertebrates, plants and fungi [7]. In plants, KNOTTED1, which was isolated from maize (Zea mays L.), was the first HD-containing protein [8]. Since the isolation of this protein, more and more plant HD-containing genes have been isolated. According to the sequence differences and location of their HD domains, homology of the flanking sequences and other correlative domains, HD-containing proteins were classified into six families, including HD-Zip (homeodomain-leucine-zipper), KNOX (KNOTTED1-like homeobox), PHD-Finger (homeodomain-finger), Bell (bell domain), WOX (Wuschel-related homeobox) and ZF-HD (zinc finger-homeodomain) [9], [10].
Among these families, HD-Zip proteins are ubiquitous in plants and carry out essential roles in various processes of plant growth and development [11]. HD-Zip proteins contain a leucine motif adjacent to the N-terminus of the homeodomain [4], [12], [13]. The Arabidopsis (Arabidopsis thaliana) genome encompasses 48 genes believed to encode HD-Zips, which are clustered into four subfamilies based on their additional conserved domains, structures and physiological functions [14]. Members of group I and II recognize similar pseudopalindromic binding sites (BSs; CAATNATTG) [15]–[18], while HD-Zip III and IV proteins interact with slightly different sequences (GTAAT[G/C]ATTAC and TAAATG[C/T]A, respectively) [19], [20]. Members of the HD-Zip gene family contain a special conserved HD and a common conserved LZ domain [21]. The difference between the four subfamilies mainly arose in the region downstream of the LZ domain, which contains different domains. Encoded proteins of the HD-Zip II subfamily can be distinguished from HD-Zip I proteins by the presence of a conserved “CPSCE” motif, named after the five conserved amino acids Cys, Pro, Ser, Cys, Glu (in one letter code), which is located next to the LZ domain and near the C-terminus [22]. Both the HD-Zip III and HD-Zip IV subfamily proteins can be distinguished from other subfamily proteins by the presence of a StAR (steroidogenic acute regulatory protein)-related lipid-transfer (START) domain followed by an HD-START-associated domain (HD-SAD) [23], [24]. However, the HD-Zip III subfamily proteins contain an additional C-terminal MEKHLA domain, while HD-Zip IV subfamily proteins lack this motif [25].
Many members of the HD-Zip protein family have been found in a wide variety of plant species, and many efforts have been undertaken to elucidate the functions of HD-Zip genes. HD-Zip I proteins are mainly involved in responses to abiotic stress, auxin, de-etiolation, blue light signaling and the regulation of organ growth and developmental process [11]. For example, ATHB7 and ATHB12 from Arabidopsis subfamily I, which are both strongly induced by abscisic acid (ABA) and water-deficit, function as negative primary regulators of the ABA response mechanism in Arabidopsis [26]. Transcription of HB1 from Medicago truncatula subfamily I, which is strongly induced by salt stress in root apices, regulates root architecture and lateral root emergence [27]. HD-Zip II proteins are involved in responses to illumination conditions, shade avoidance and auxin signaling [28]–[32]. ATHB2 is the first gene that is specifically and reversibly regulated by changes in the R/FR ratio in green plants, which induces the shade avoidance response in most angiosperms [33]. HAT2, another member of this subfamily, was identified as an auxin-inducible gene in seedlings through DNA microarray screening [30]. HAHB10, a sunflower HD-ZIP II gene, participates in the induction of flowering and in the regulate of phytohormone-mediated responses to biotic stress [34].
HD-Zip III proteins control apical meristem development, embryogenesis, leaf polarity, lateral organ initiation and vascular bundle development [35]–[37]. ATHB8 and ATHB15 are thought to direct vascular development [38], [39]. Several studies have elucidated the mode of action of REV, PHB and PHV, along with KANADI, in controlling abaxial-adaxial patterning of lateral organs [40]. PopREVOLUTA(PRE), a populus class III HD-ZIP gene demonstrates in regulating the development of cambia and secondary vascular tissues [41]. HD-Zip IV proteins play crucial roles in anthocyanin accumulation, epidermal cell differentiation, trichome formation, root development and cuticle development [11], [42]. The gl2 (GLABRA2) mutant shows unusual trichome expansion and ectopic root hair differentiation [13]. Recent studies have demonstrated that upregulating the expression of HDG11, one of the HD-Zip IV genes, allows this gene to perform novel functions in drought tolerance. This finding may help reveal how drought tolerance has evolved, as altering the expression pattern of HDG11 may be a way in which drought tolerance evolves in nature [43]. OCL1 (OUTER CELL LAYER1) encodes a maize HD-ZIP class IV transcription, ectopic expression of OCL1 leads to pleiotropic phenotypic aberrations in transgenic maize plants, the most conspicuous one being a strong delay in flowering time [44].
Soybean (Glycine max) is one of the most economically and nutritionally crucial crops. It provides not only vegetable protein and edible oil but also essential amino acids for humans and animals. However, soybean production is threatened by drought, salinity and other environmental stresses. For example, drought reduces the yield of soybean by about 40%, affecting all stages of plant development from germination to flowering and reducing the quality of the seeds [45]. Salinity inhibit soybean growth and production and together with drought cause osmotic stress in plant [46], [47]. Thus, there is a great need to study the soybean in order to improve sustenance and better yield. To date, the soybean genome has been sequenced, which has enabled gene prediction tools and annotation to become publicly available [48]. A series of transcription factors have been studied in soybean, such as ERF, WRKY, BURP, MADS-box, MYB, NAC and so on [49]–[54]. HD-Zip family genes have been characterized in Arabidopsis, rice, Populus, maize and other species [55], [56], but no genome-wide characterization of the HD-Zip family has been performed in soybean to date. In the current study, 88 putative genes of the HD-Zip family were identified. After examining the publicly reported expression patterns of the paralogous pairs in soybean under stress, we investigated the transcript levels of six paralogous pairs under stress treatment. The results presented in this study show that the expression of the 12 soybean HD-Zip genes is stress-responsive. Our findings lay the foundation for further investigations into the biological and molecular functions of HD-Zip transcription factors in soybean.
Results
Identification of HD-Zip gene family in soybean
The HD-Zip genes, characterized by the existence of HD and LZ domains, have previously been systematically analyzed in Arabidopsis, rice, Populus and maize. In the present study, to gain insight into the HD-Zip gene family in soybean, we first used the HD-Zip genes of Arabidopsis to perform a BLASTP search against the soybean genome database (release 1.0). According to the features of each HD-Zip subfamily, four representatives were randomly chosen as secondary queries from the resulting sequences. Using this method, a total of 100 putative HD-Zip genes were obtained. SMART and Pfam analysis were performed to retain those putative genes that included both HD and LZ domains. This analysis revealed 88 members in soybean, which is greater than that identified in other representative species, including Arabidopsis (48), rice (48), maize (55) and Populus (63) [55], [56]. The 88 identified soybean HD-Zip genes in our study were designated Gmhdz1 to Gmhdz88 following the nomenclature proposed in a previous study [55]. The encoded proteins varied from 206 to 853 amino acids (aa) in length, with an average of 462 aa, which is similar to that reported in Populus (465 aa) [57]. The details about other parameters of the nucleic acid and protein sequences are provided in Table 1.
Table 1. List of 88 HD-Zip genes identified in soybean and their sequence characteristics (bp, base pair; aa, amino acids; D, Dalton).
| Arabidopsis | Protein | Chr. | ORF length | Exons | ||||
| Name | Sequenced ID | orthologs locus | Length(a.a.) | Mol.Wt.(Da) | PI | (bp) | ||
| Gmhdz1 | Glyma0041s0035 | HAT14 | 309 | 34425.2 | 6.33 | scaffold_41 | 930 | 3 |
| Gmhdz2 | Glyma01g04890 | ATHB1 | 345 | 39174 | 4.63 | 1 | 1038 | 3 |
| Gmhdz3 | Glyma01g05230 | ATHB13 | 283 | 32185.9 | 6.08 | 1 | 852 | 3 |
| Gmhdz4 | Glyma01g38390 | ATHB21 | 214 | 25074.2 | 6.26 | 1 | 645 | 3 |
| Gmhdz5 | Glyma01g40450 | HAT22 | 283 | 31863.1 | 8.5 | 1 | 852 | 3 |
| Gmhdz6 | Glyma01g41581 | ATHB2 | 268 | 30100.9 | 8.16 | 1 | 807 | 4 |
| Gmhdz7 | Glyma01g45070 | PDF2 | 731 | 80339.9 | 5.92 | 1 | 2211 | 10 |
| Gmhdz8 | Glyma02g02290 | ATHB13 | 295 | 33680.6 | 6.19 | 2 | 888 | 3 |
| Gmhdz9 | Glyma02g02630 | ATHB1 | 345 | 39145.3 | 4.67 | 2 | 1038 | 3 |
| Gmhdz10 | Glyma02g06560 | ATHB21 | 212 | 25015.1 | 5.82 | 2 | 639 | 3 |
| Gmhdz11 | Glyma02g28860 | HAT14 | 309 | 34206.3 | 8.44 | 2 | 990 | 4 |
| Gmhdz12 | Glyma03g01860 | ANL2 | 835 | 91244.1 | 5.97 | 3 | 2508 | 9 |
| Gmhdz13 | Glyma03g26701 | HAT3 | 310 | 34415.1 | 6.41 | 3 | 933 | 4 |
| Gmhdz14 | Glyma03g30200 | HAT14 | 280 | 30983.9 | 8.03 | 3 | 867 | 4 |
| Gmhdz15 | Glyma03g34710 | ATHB51 | 233 | 27315.9 | 6.03 | 3 | 702 | 3 |
| Gmhdz16 | Glyma04g05200 | HAT14 | 247 | 28321.2 | 9.32 | 4 | 873 | 3 |
| Gmhdz17 | Glyma04g09000 | CNA1 | 844 | 92741 | 6.03 | 4 | 2535 | 18 |
| Gmhdz18 | Glyma04g34341 | ATHB1 | 289 | 32133.2 | 4.64 | 4 | 870 | 4 |
| Gmhdz19 | Glyma04g40960 | ATHB7 | 245 | 28360.5 | 5.44 | 4 | 738 | 2 |
| Gmhdz20 | Glyma05g01390 | ATHB1 | 331 | 37413.2 | 4.77 | 5 | 996 | 3 |
| Gmhdz21 | Glyma05g04990 | ATHB2 | 298 | 33329.2 | 7.68 | 5 | 897 | 4 |
| Gmhdz22 | Glyma05g23150 | HAT22 | 305 | 33662.1 | 8.67 | 5 | 918 | 3 |
| Gmhdz23 | Glyma05g30000 | PHB | 853 | 93958.1 | 6.06 | 5 | 2562 | 18 |
| Gmhdz24 | Glyma05g30940 | ATHB16 | 345 | 38838.9 | 4.89 | 5 | 1038 | 4 |
| Gmhdz25 | Glyma05g33520 | HDG11 | 713 | 79304.9 | 6.37 | 5 | 2142 | 10 |
| Gmhdz26 | Glyma06g09100 | CNA1 | 845 | 92923.2 | 6.03 | 6 | 2538 | 18 |
| Gmhdz27 | Glyma06g13890 | ATHB7 | 251 | 29036.2 | 5.53 | 6 | 756 | 2 |
| Gmhdz28 | Glyma06g20230 | ATHB1 | 326 | 36832.4 | 4.71 | 6 | 981 | 3 |
| Gmhdz29 | Glyma07g01940 | CNA1 | 838 | 92450.7 | 6.06 | 7 | 2517 | 18 |
| Gmhdz30 | Glyma07g01950 | CNA1 | 841 | 92947.1 | 6.12 | 7 | 2526 | 11 |
| Gmhdz31 | Glyma07g02220 | GL2 | 751 | 84101 | 5.65 | 7 | 2256 | 11 |
| Gmhdz32 | Glyma07g05800 | ATHB12 | 238 | 27335.2 | 5.11 | 7 | 717 | 2 |
| Gmhdz33 | Glyma07g08340 | ANL2 | 829 | 90529.4 | 5.97 | 7 | 2490 | 9 |
| Gmhdz34 | Glyma07g14270 | HAT3 | 308 | 34006.9 | 7.66 | 7 | 954 | 5 |
| Gmhdz35 | Glyma07g34230 | ATHB17 | 206 | 23270.4 | 8.62 | 7 | 642 | 4 |
| Gmhdz36 | Glyma08g06190 | HDG11 | 721 | 80097.8 | 6.21 | 8 | 2166 | 10 |
| Gmhdz37 | Glyma08g13110 | PHB | 833 | 91561.5 | 6.15 | 8 | 2550 | 18 |
| Gmhdz38 | Glyma08g14130 | ATHB16 | 312 | 35437 | 4.93 | 8 | 939 | 3 |
| Gmhdz39 | Glyma08g15771 | HAT14 | 377 | 41903 | 6.1 | 8 | 1134 | 4 |
| Gmhdz40 | Glyma08g21610 | CNA1 | 838 | 92386.5 | 6.06 | 8 | 2517 | 18 |
| Gmhdz41 | Glyma08g21620 | CNA1 | 843 | 93335.6 | 6.17 | 8 | 2532 | 18 |
| Gmhdz42 | Glyma08g21890 | GL2 | 748 | 83710.8 | 5.66 | 8 | 2247 | 11 |
| Gmhdz43 | Glyma08g40705 | ATHB1 | 320 | 36687.7 | 4.79 | 8 | 963 | 3 |
| Gmhdz44 | Glyma08g40970 | ATHB13 | 280 | 31882.6 | 5.68 | 8 | 843 | 3 |
| Gmhdz45 | Glyma09g02750 | PHB | 852 | 93475.4 | 5.89 | 9 | 2559 | 18 |
| Gmhdz46 | Glyma09g07051 | HAT14 | 237 | 26645.3 | 8.49 | 9 | 714 | 3 |
| Gmhdz47 | Glyma09g16790 | HAT14 | 327 | 36191.6 | 8.09 | 9 | 984 | 4 |
| Gmhdz48 | Glyma09g26600 | ANL2 | 776 | 85098.6 | 5.53 | 9 | 2331 | 9 |
| Gmhdz49 | Glyma09g29810 | HDG11 | 722 | 79550.4 | 6.25 | 9 | 2169 | 10 |
| Gmhdz50 | Glyma09g34061 | HDG5 | 800 | 88979.7 | 5.69 | 9 | 2403 | 11 |
| Gmhdz51 | Glyma09g37410 | ATHB20 | 270 | 30941.7 | 6.35 | 9 | 813 | 3 |
| Gmhdz52 | Glyma09g37680 | ATHB2 | 229 | 25443.4 | 8.52 | 9 | 690 | 3 |
| Gmhdz53 | Glyma09g40130 | ANL2 | 820 | 89611.68 | 6.01 | 9 | 2463 | 9 |
| Gmhdz54 | Glyma10g38280 | ANL2 | 765 | 83880.5 | 5.77 | 10 | 2298 | 9 |
| Gmhdz55 | Glyma10g39720 | PDF2 | 740 | 82507.11 | 6.25 | 10 | 2334 | 10 |
| Gmhdz56 | Glyma11g00570 | PDF2 | 732 | 80460.95 | 5.86 | 11 | 2199 | 10 |
| Gmhdz57 | Glyma11g03850 | ATHB2 | 285 | 31968 | 8.47 | 11 | 858 | 4 |
| Gmhdz58 | Glyma11g04840 | HAT22 | 283 | 31683 | 8.78 | 11 | 852 | 3 |
| Gmhdz59 | Glyma11g20520 | REV | 842 | 92062.9 | 5.75 | 11 | 2529 | 18 |
| Gmhdz60 | Glyma11g37920 | ATHB5 | 314 | 35762.3 | 4.87 | 11 | 945 | 3 |
| Gmhdz61 | Glyma12g08080 | REV | 841 | 92012.8 | 5.75 | 12 | 2526 | 18 |
| Gmhdz62 | Glyma12g10710 | HDG2 | 727 | 79323.7 | 5.56 | 12 | 2286 | 11 |
| Gmhdz63 | Glyma12g32050 | HDG2 | 781 | 84642.9 | 5.74 | 12 | 2346 | 11 |
| Gmhdz64 | Glyma13g00310 | HAT3 | 213 | 24498.9 | 8.92 | 13 | 642 | 3 |
| Gmhdz65 | Glyma13g05270 | ATHB20 | 291 | 33146.1 | 7.25 | 13 | 876 | 3 |
| Gmhdz66 | Glyma13g23890 | ATHB1 | 285 | 32676.2 | 4.74 | 13 | 858 | 4 |
| Gmhdz67 | Glyma14g10370 | HAT14 | 305 | 34410.3 | 6.23 | 14 | 918 | 3 |
| Gmhdz68 | Glyma15g01960 | GL2 | 751 | 83589.59 | 5.84 | 15 | 2256 | 11 |
| Gmhdz69 | Glyma15g13640 | PHB | 846 | 92842 | 5.89 | 15 | 2541 | 18 |
| Gmhdz70 | Glyma15g18320 | HAT22 | 226 | 25323.8 | 6.95 | 15 | 681 | 3 |
| Gmhdz71 | Glyma15g42380 | HAT14 | 384 | 42520.6 | 5.63 | 15 | 1155 | 4 |
| Gmhdz72 | Glyma16g02390 | ATHB12 | 245 | 28334.2 | 5.14 | 16 | 738 | 2 |
| Gmhdz73 | Glyma16g34350 | HDG11 | 718 | 79148.95 | 6.3 | 16 | 2157 | 10 |
| Gmhdz74 | Glyma17g06380 | HAT14 | 209 | 23720.1 | 9.27 | 17 | 630 | 3 |
| Gmhdz75 | Glyma17g10490 | ATHB1 | 329 | 37381.1 | 4.82 | 17 | 990 | 3 |
| Gmhdz76 | Glyma17g15380 | ATHB2 | 299 | 33672.6 | 7.67 | 17 | 900 | 4 |
| Gmhdz77 | Glyma17g16930 | HAT22 | 312 | 34422.8 | 8.05 | 17 | 939 | 3 |
| Gmhdz78 | Glyma18g01830 | ATHB5 | 322 | 36683.3 | 4.82 | 18 | 969 | 3 |
| Gmhdz79 | Glyma18g15970 | ATHB13 | 279 | 31693.4 | 5.67 | 18 | 840 | 3 |
| Gmhdz80 | Glyma18g16390 | ATHB1 | 264 | 30554.6 | 4.71 | 18 | 975 | 3 |
| Gmhdz81 | Glyma18g45970 | ANL2 | 773 | 84777.39 | 6.14 | 18 | 2469 | 9 |
| Gmhdz82 | Glyma18g48880 | HAT3 | 289 | 31924.6 | 6.46 | 18 | 870 | 4 |
| Gmhdz83 | Glyma18g49290 | ATHB20 | 268 | 30527.3 | 7.17 | 18 | 807 | 3 |
| Gmhdz84 | Glyma19g01300 | ATHB1 | 284 | 32387.9 | 4.71 | 19 | 855 | 4 |
| Gmhdz85 | Glyma19g02490 | ATHB20 | 292 | 33500.3 | 6.9 | 19 | 879 | 3 |
| Gmhdz86 | Glyma19g33100 | HAT14 | 270 | 30215.3 | 8.51 | 19 | 879 | 4 |
| Gmhdz87 | Glyma19g37380 | ATHB51 | 214 | 25143.3 | 7.76 | 19 | 645 | 3 |
| Gmhdz88 | Glyma20g01770 | ATHB17 | 218 | 24720.2 | 9.13 | 20 | 660 | 4 |
Phylogenetic and structural analyses of the HD-Zip proteins in soybean
To evaluate the evolutionary relationships among soybean HD-Zip proteins, an unrooted phylogenetic tree of the 88 soybean protein sequences was generated, with 1,000 bootstrap replicates. The soybean HD-Zip family was further divided into four major subfamilies (I to IV) with >50% bootstrap values (Figure 1A). Subfamily III has the fewest HD-Zip gene members (12), while subfamily I contains the most members (30), followed by subfamily II (27) and IV (19). This distribution is similar to that observed for HD-Zip genes in other species. Based on phylogenetic analysis, we identified 41 sister pairs (Table 2), all of which had high bootstrap support (>94%).
Figure 1. Phylogenetic relationships, gene structure and motif compositions of soybean HD-Zip genes.
A. The unrooted tree was generated with the MEGA5.0 program using the full-length amino acid sequences of the 88 soybean HD-Zip proteins by the Neighbor-Joining (NJ) method, with 1,000 bootstrap replicates. The percentage bootstrap scores higher than 50% are indicated on the nodes. The tree shows four major phylogenetic subfamilies (subfamily I to IV) indicated with different colored backgrounds. B. Exon/intron organization of soybean HD-Zip genes. Green boxes represent exons and black lines represent introns. The untranslated regions (UTRs) are indicated by blue boxes. The numbers 0, 1 and 2 represents the splicing phases. The sizes of exons and introns can be estimated using the scale at the bottom. C. Schematic representation of the conserved motifs in soybean HD-Zip proteins elucidated from publicly available data. Each colored box represents a motif in the protein, with the motif name indicated in the box on the right. The length of the protein and motif can be estimated using the scale at the bottom.
Table 2. Divergence between HD-Zip genes pairs in soybean.
| Paralogous pairs | Ks | Ka | Ka/Ks | Duplication Date(MY) | Duplicate type |
| Gmhdz2-Gmhdz9 | 0.101 | 0.028 | 0.275 | 8.25 | Segmental |
| Gmhdz3-Gmhdz8 | 0.089 | 0.111 | 0.214 | 7.3 | Segmental |
| Gmhdz5-Gmhdz58 | 0.139 | 0.038 | 0.274 | 11.37 | Segmental |
| Gmhdz6-Gmhdz57 | 0.201 | 0.027 | 0.136 | 16.44 | Segmental |
| Gmhdz7-Gmhdz56 | 0.109 | 0.021 | 0.189 | 8.96 | Segmental |
| Gmhdz11-Gmhdz47 | 0.224 | 0.043 | 0.19 | 18.39 | Segmental |
| Gmhdz12-Gmhdz33 | 0.142 | 0.015 | 0.109 | 11.6 | Segmental |
| Gmhdz13-Gmhdz34 | 0.145 | 0.044 | 0.3 | 11.9 | Segmental |
| Gmhdz14-Gmhdz86 | 0.154 | 0.068 | 0.442 | 12.63 | Segmental |
| Gmhdz15-Gmhdz87 | 0.127 | 0.043 | 0.336 | 10.44 | Segmental |
| Gmhdz17-Gmhdz26 | 0.115 | 0.006 | 0.054 | 9.4 | Segmental |
| Gmhdz18-Gmhdz28 | 0.181 | 0.047 | 0.257 | 14.87 | Segmental |
| Gmhdz19-Gmhdz27 | 0.117 | 0.021 | 0.182 | 9.62 | Segmental |
| Gmhdz20-Gmhdz75 | 0.156 | 0.061 | 0.388 | 12.81 | Segmental |
| Gmhdz21-Gmhdz76 | 0.13 | 0.038 | 0.292 | 10.61 | Segmental |
| Gmhdz22-Gmhdz77 | 0.142 | 0.047 | 0.334 | 11.64 | Segmental |
| Gmhdz23-Gmhdz37 | 0.084 | 0.017 | 0.205 | 6.85 | Segmental |
| Gmhdz24-Gmhdz38 | 0.145 | 0.038 | 0.265 | 11.88 | Segmental |
| Gmhdz25-Gmhdz36 | 0.098 | 0.025 | 0.256 | 8.02 | Segmental |
| Gmhdz31-Gmhdz42 | 0.118 | 0.042 | 0.353 | 9.63 | Segmental |
| Gmhdz32-Gmhdz72 | 0.074 | 0.034 | 0.461 | 6.03 | Segmental |
| Gmhdz35-Gmhdz88 | 0.132 | 0.02 | 0.149 | 10.78 | Segmental |
| Gmhdz39-Gmhdz71 | 0.207 | 0.036 | 0.173 | 16.97 | Segmental |
| Gmhdz43-Gmhdz80 | 0.218 | 0.064 | 0.295 | 17.83 | Segmental |
| Gmhdz44-Gmhdz79 | 0.106 | 0.011 | 0.102 | 8.68 | Segmental |
| Gmhdz45-Gmhdz69 | 0.188 | 0.013 | 0.069 | 15.37 | Segmental |
| Gmhdz46-Gmhdz70 | 0.114 | 0.024 | 0.214 | 9.35 | Segmental |
| Gmhdz49-Gmhdz73 | 0.093 | 0.009 | 0.096 | 7.64 | Segmental |
| Gmhdz51-Gmhdz83 | 0.112 | 0.058 | 0.518 | 9.2 | Segmental |
| Gmhdz52-Gmhdz82 | 0.134 | 0.038 | 0.287 | 10.97 | Segmental |
| Gmhdz59-Gmhdz61 | 0.115 | 0.007 | 0.062 | 9.44 | Segmental |
| Gmhdz60-Gmhdz78 | 0.163 | 0.033 | 0.199 | 13.39 | Segmental |
| Gmhdz64-Gmhdz74 | 0.131 | 0.055 | 0.42 | 10.74 | Segmental |
| Gmhdz65-Gmhdz85 | 0.205 | 0.046 | 0.223 | 16.83 | Segmental |
| Gmhdz53-Gmhdz81 | 0.14 | 0.017 | 0.12 | 11.47 | Segmental |
| Gmhdz66-Gmhdz84 | 0.093 | 0.024 | 0.257 | 7.66 | Segmental |
| Gmhdz29-Gmhdz40 | 0.088 | 0.005 | 0.062 | 7.18 | Segmental |
| Gmhdz40-Gmhdz41 | 0.1092 | 0.0226 | 0.2065 | 8.95 | Tandem |
| Gmhdz29-Gmhdz30 | 0.112 | 0.0138 | 0.1231 | 9.18 | Tandem |
To gain further insights into the structural diversity of the HD-Zip genes, we compared the exon/intron organization in the coding sequences of individual HD-Zip genes in soybean (Figure 1B). Most closely related members in the same subfamilies share similar exon/intron structures and intron numbers, which was consistent with the characteristics defined in the above phylogenetic analysis. For instance, the HD-Zip genes in subfamily I and II contain two to five exons, while those in subfamily IV contain 9 to 11 exons and those in subfamily III possess 18 exons, with the exception of Gmhdz30, which harbors 11 exons.
To obtain intron gain/loss information for all sister pairs, we also compared the intron/exon structures of the genes that clustered together at the terminal branch of the phylogenetic tree. Among these, five pairs showed changes in their intron/exon structure, including Gmhdz18/-28, Gmhdz20/-75, Gmhdz24-/38, Gmhdz52/-82 and Gmhdz13/-34 (Fig. 1B), which only occurred in subfamily I and II. Through comparison of the five pairs with neighbouring genes, we found that Gmhdz75 and Gmhdz82 lost one intron during the long evolutionary period, while Gmhdz24, Gmhdz18 and Gmhdz34 gained one intron.
A total of 88 HD-Zip genes from soybean were subjected to analysis with MEME to reveal conserved motifs shared among related proteins. Thirty conserved motifs were identified (Fig. 1C); the details are shown in Figure S1 and Table S1. Each of the putative motifs obtained from MEME was annotated by searching Pfam and SMART. To simplify the MEME results, if two or more motifs among the 30 motifs identified represented the same domain and stayed close, they were merged and displayed as a domain district, while the motifs that were not annotated were not shown in Figure 1C. After this process was completed, it became clear that most of the closely related members had common motif compositions, suggesting functional similarities among HD-Zip proteins within the same subfamily.
Among these, the conserved motifs encoding the HD and LZ domain were found in all soybean HD-Zip genes; these were the most conserved motifs that were identified. The CPSCE motif was found in the majority of members of the HD-Zip III and HD-Zip II subfamilies with the exception of Gmhdz74. HD-Zip_N is less conserved within the HD-Zip II subfamily, as it was not found in Gmhdz35, -46, -64, -70, -74 or -88. The MEKHLA domain, which is specific to subfamily III, was found only in subfamily III proteins (12 members). Motifs representing the START region were identified in subfamily III and IV genes.
Chromosomal location and gene duplication
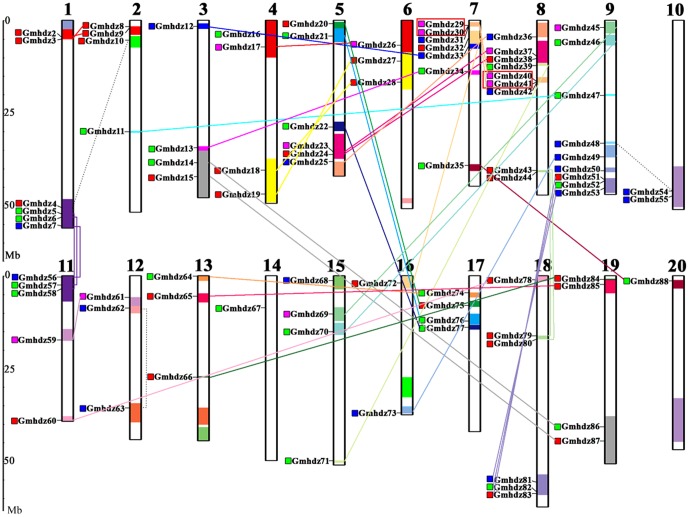
A total of 87 of the 88 soybean HD-Zip genes were mapped to the 20 soybean chromosomes, while only one gene (Gmhdz1) was mapped to as yet unattributed scaffolds (Figure 2). The soybean HD-Zip genes are unevenly distributed among all chromosomes; chromosomes 14 and 20 contain only one HD-Zip gene, while chromosomes 8 and 9 contain nine, the maximum number HD-Zip genes per chromosome. Among the HD-Zip subfamilies, HD-Zip III and HD-Zip IV are mainly located on chromosomes 7, 8 and 9, while HD-Zip I and II are located on chromosomes 1, 2, 3, 18 and 19.
Figure 2. Chromosomal locations of soybean HD-Zip genes.
Colored boxes ahead of the gene names represent the classes to which each HD-Zip gene belongs (HD-Zip I, red; HD-Zip II, green; HD-Zip III, pink; HD-Zip IV, blue). The 88 HD-Zip genes were mapped to the 20 chromosomes, while only one gene (Gmhdz1) resides on unassembled scaffolds. The data used to generate the schematic diagram of the genome-wide chromosome organization were obtained from the SoyBase browser. Only segmental duplicated blocks including HD-Zip genes are indicated with the same colors. Small boxes connected by colored line (two types) indicate corresponding sister gene pairs, of which the genes connected by solid lines are located in the homologous duplicated blocks, while genes connected by the dashed line were observed in the duplicated blocks shown with different colors. Tandemly duplicated genes are indicated with red boxes. The scale represents the length of the chromosome.
Previous analysis of the soybean genome has revealed that the paralogs within a gene family were mainly derived from genome duplications that occurred approximately 59 and 13 million years ago(Mya) [48]. In this study, we identified 37 related duplicated blocks (Table S2). Of the 87 mapped HD-Zips, only one gene (Gmhdz67) is located outside of a duplicated block. Moreover, 27 block pairs cover 37 HD-Zip sister pairs, and 10 duplicated blocks only harbor HD-Zips on one of the blocks and lack the corresponding duplicates, suggesting that dynamic changes may have occurred after segmental duplication, leading to the loss of some genes.
It is noteworthy that among the 87 genes, two gene pairs (Gmhdz29/-30, Gmhdz40/-41) were detected within a distance of less than 5 kb (<100 kb) on chromosomes 7 and 8, which may have resulted from tandem duplication (Figure 2). Alignment analysis of protein sequences using the Smith-Waterman algorithm (http://www.ebi.ac.uk/Tools/psa/) showed that two pairs (Gmhdz29/-30, Gmhdz40/-41) have high sequence similarities (the former is 98%, the latter is 96.3%) between two counterparts of each gene pair and therefore meet the standards of tandem duplicates, the sequence similarities of the other paralogous pairs were showed in Table S3. Analysis of HD-Zip paralogous pairs showed that 37 out of 41 gene pairs remain in conserved positions on segmental duplicated blocks, providing strong evidence that gene duplication has made an important contribution to soybean HD-Zip gene expansion (Figure 2 and Table 2). According to the ratio of nonsynonymous to synonymous substitutions (Ka/Ks), the history of selection acting on coding sequences can be measured [58]. A pair of sequences will have Ka/Ks<1 if one sequence has been under purifying selection but the other has been drifting neutrally, while Ka/Ks = 1 if both sequences are drifting neutrally and rarely, Ka/Ks>1 at specific sites that are under positive selection [59]. A summary of Ka/Ks for 39 HD-Zip duplicated pairs is shown in Table 2 were less than 0.6. This result suggests that all gene pairs have evolved mainly under the influence of purifying selection. Based on the divergence rate of 6.1×10−9 synonymous mutations per synonymous site per year as previously proposed for soybean [60], duplications of these 41 paralogous pairs was estimated to have occurred between 6.03 to 18.39 Mya (Table 2).
Comparative analysis of the HD-Zip genes in soybean, Arabidopsis and rice
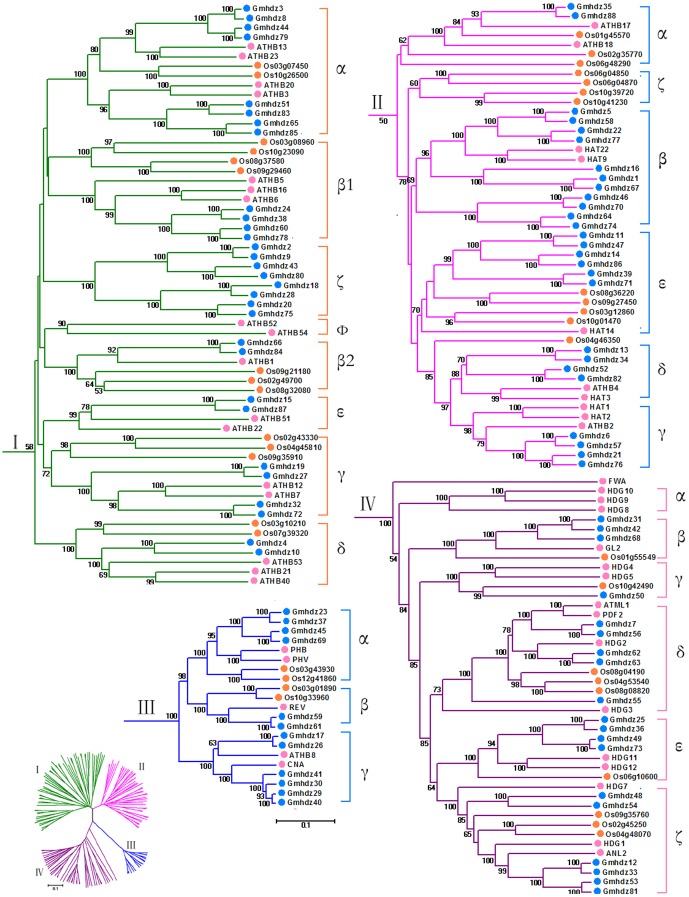
The abundance of soybean HD-Zip genes compared to that in other plant species may have been derived from multiple gene duplication events, represented by a whole-genome duplication following multiple segmental and tandem duplications. To verify this hypothesis, we first constructed a NJ phylogenetic tree with MEGA5.0 using full-length HD-Zip protein sequence alignments of soybean, rice and Arabidopsis HD-Zip proteins to reveal the evolutionary relationships among plant HD-Zip proteins. At first glance, four subfamilies (I to IV) are evident in the tree (Fig. 3), the same as described previously and with significant statistical support. The phylogenetic tree reveals that the plant HD-Zip sequence distribution predominates with species bias (Fig. 3). HD-Zip I genes generally comprise the largest subfamilies in these plant species, while HD-Zip III genes comprise the smallest number of HD-Zip members.
Figure 3. Phylogenetic trees of full-length HD-Zip domain proteins from soybean, Arabidopsis and rice.
The tree was generated with the MEGA5.0 program using the NJ method. Numbers at nodes indicate the percentage bootstrap scores, and only bootstrap values higher than 50% from 1,000 replicates are shown. Soybean, Populus and Arabidopsis HD-Zip proteins were marked with different colored dots. The scale bar corresponds to 0.1 estimated amino acid substitutions per site. The unrooted tree in the lower-left corner was constructed with the same method.
Both subfamilies contain rice, Arabidopsis and soybean HD-Zip genes, suggesting that the main characteristics of this family in plants were generated before the dicot-monocot split. To clarify the paralogous and orthologous relationships among this family, we further divided the subfamilies into subclasses. We labeled the clades in the tree using previously defined clades from studies of Arabidopsis and rice, as shown in Figure 3. Subfamily I was divided into seven subclasses, i.e., α, β, γ, δ, ε, φ and ζ. Clade ζ contains only sequences from soybean, while clade φ exclusively contains Arabidopsis genes. Clade ε contains sequences from both soybean and Arabidopsis, while no members of rice were detected in this subclades, suggesting that rice lost its members of this group during the long period of evolution. The HD-Zip II subfamily was divided into six subclasses, from α to ζ, while α to ε were designated as described by Ciarbelli et al. (2008) [33]. Clade ζ is entirely composed of HD-Zip genes from rice. The HD-Zip III subfamily was classified into three subclades (designated α, β and γ) using the definitions of the previous studies. Clade γ excludes rice genes. The HD-Zip IV subfamily was also divided into six subclades. All of the genes in Clade α originated from Arabidopsis. The bootstrap values for all of the subclades were quite high, suggesting that the genes in each subclade may share a similar origin. Only one pair of orthologous genes from soybean and rice was identified, i.e., Gmhdz50 and Os10g42490 in subfamily IV. Most genes in the HD-Zip family are contained in paralogous pairs. This lineage-specific pattern suggests that HD-ZIP genes in these subgroups may be expanded and then diversified after the monocot-eudicot division.
Expression profiling of soybean HD-Zip genes under drought and salt stress
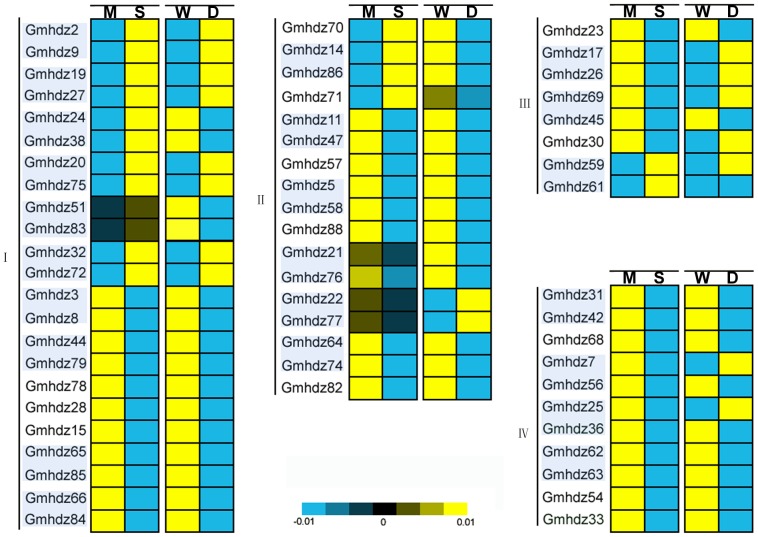
To gain more insights into the roles of soybean HD-Zip genes in salinity and drought tolerance, we reanalyzed the expression profiles of all soybean HD-Zip genes in response to drought and salinity stresses using publicly available microarray data. Fifty-nine HD-Zip genes were included on both GSE40627 and GSE41125,including class I (23 genes), class II (17 genes), class III(8 genes) and class IV(11 genes) (Table S4). The expression of most HD-Zip genes was suppressed or induced under both stresses (Fig. 4). Salt stress caused upregulation of 16 genes, including 12 genes from HD-Zip I (Gmhdz2, -9, -19, -20, -24, -27, -32, -38, -51, -72, -75 and -83), four gene from HD-Zip II (Gmhdz14, -70, -71 and -86) and two genes in the HD-Zip III subfamilies (Gmhdz59 and -61). In response to drought stress, 40 genes showed downregulation, whereas the transcripts of 18 were significantly upregulated (Fig. 4). Nine genes were upregulated under both salt and drought stress treatments, including eight genes from HD-Zip I (Gmhdz2, -9, -19, -20, -27, -32, -72 and -75) and one gene from HD-Zip III (Gmhdz59), while 33 genes were downregulated under both stresses. The responses of HD-Zip genes to stress also differed among some genes. For instance, eight genes (Gmhdz14, -24, -38, -51, -70, -71, -83 and -86) were significantly upregulated under salt stress, whereas downregulation were observed in these genes under drought stress (Fig. 4).
Figure 4. Differential expression of soybean HD-Zip genes under salinity and drought stress.
Expression is indicated as fold-change of experimental treatments relative to control samples and visualized in heatmaps. Color scale represents log2 expression values, with yellow representing low levels and blue indicating high levels of transcript abundance. To increase the contrast in this figure, the color scale values were reduced. The heatmap shows hierarchical clustering of 59 genes under salinity and drought stress. The pairs with light blue background are paralogous genes. Microarray data (under accession numbers GSE40627 and GSE41125) were obtained from the NCBI GEO database. M, mock; S, salinity stress; W, well-watered; D, drought stress. The paralogous pairs are indicated with a light blue background.
Duplicate genes may have different evolutionary fates, i.e., nonfunctionalization, neofunctionalization or subfunctionalization, which may be indicated by the divergence in their expression patterns. From the above results, we determined that there are 41 paralogous gene pairs in the soybean HD-Zip gene family. Based on in silico expression data from 23 paralogous pairs in response to salt and drought stress, expression divergence of drought stress was evident in three pairs(Gmhdz69/-45, Gmhdz7/-56, Gmhdz25/-36), which supports the notion that the expression of paralogs can diverge significantly after duplication. For example, Gmhdz45 was upregulated in response to drought stress, whereas its duplicated counterpart Gmhdz69 was downregulated. As shown in Figure 4, most pairs of paralogs in soybean share similar expression patterns and thus show functional redundancy. From the online database, 8 Gmhdz genes (2 for HD-Zip I, 5 for HD-Zip II and 1 for HD-Zip IV), were undetectable at the transcription level in all 14 tissues (Table S5 and Figure 5). Most soybean HD-Zip genes have a broad expression spectrum. The genes in different subfamilies had their primary abundant transcripts in different tissues, such as HD-Zip I and HD-Zip II in flower and root, both HD-Zip III and IV in young leaf, one com pod and pod shell of 10 days after flowering, some of HD-Zip IV genes also highly expressed in flower. There are a few genes strongly expressed in seed and node. From the expression data of the 34 detected paralogous pairs in14 soybean tissues, expression divergence was also obviously evidenced. For example, Gmhdz35 from HD-Zip II highly expressed in seed of 14 days after flowering while Gmhdz88 was undetectable. However, the paralogous pairs also have the same expression pattern, such as both Gmhdz19 and Gmhdz27 from HD-Zip I strongly expressed in flower and barely in other tissues.
Figure 5. Hierarchical clustering of expression profiles of soybean HD-Zip genes in different tissues.
The RNA-seq relative expression data of 14 tissues was used to re-construct expression patterns of soybean genes. Sources of the samples are as follows: YL (young leave), F (flower), P.1cm (one cm pod), PS.10d (pod shell 10DAF), PS.14d (pod shell 14DAF), S.10d (seed 10DAF), S.14d (seed 14 DAF), S.21d (seed 21DAF), S.25d (seed 25DAF), S.28d (seed 28DAF), S.35d (seed 35DAF), S.42d (seed 42DAF), R (root), N (nodule). The raw data was downloaded from the website http://soybase.org/soyseq/. Gene names in light green background showed paralogous pairs. The normal relative expressions of 88 HD-Zip genes were in the Table S6.
Examination of HD-Zip gene expression by qRT-PCR
The HD-Zip I genes have been shown to play major roles in abiotic stress [61]. Soil salinity, one of the most severe abiotic stresses, hinders the improvement of agricultural productivity on nearly 20% of irrigated land worldwide [46]. As water becomes limited, plants redistribute this valuable resource by restricting transpiration and growth, and they frequently flower early [62]. Therefore, there is a need to identify the master regulators and their regulatory pathways involved in stress adaptation. To screen soybean HD-Zip genes regulated by salt and drought stress, qRT-PCR was employed to validate 12 candidate genes (Gmhdz2, -9, -19, -20, -24, -27, -32, -38, -51, -72, -75 and -83) from HD-Zip I subfamily that are highly induced by salt stress based on microarray data. The 12 genes are also highly induced by drought stress (except for Gmhdz24, -38, -51 and -83, which are downregulated). In addition, the 12 genes comprise six paralogous pairs. The qRT-PCR results showed that all 12 HD-Zip genes were drought-and salinity-responsive, but some differences were observed among these genes (Fig. 6). Under NaCl treatment, only Gmhdz20 was highly expressed at a comparatively early stage (6 h after treatment), whereas the levels of Gmhdz2, -9, -19, -27, -32, -38, -61, -75 and -83 expression peaked at 12 h after treatment. Gmhdz24 was downregulated by NaCl treatment across all time points. Notably, Gmhdz51 and -83 were strongly upregulated (>400-fold) at 12 h after NaCl treatment but were dramatically downregulated thereafter (Fig. 6). Under drought stress, the highest expression levels of Gmhdz9, -27, -51, -75 and -83 were found at 6 h after treatment, while those of Gmhdz24, -32 and -38 were observed later, at 12 h after treatment.
Figure 6. Expression analysis of 12 selected HD-Zip genes under drought and salinity stress using qRT-PCR.
The relative mRNA abundance of 12 selected HD-Zip genes was normalized with respect to the reference gene CYP2 under drought and salinity stress treatment. Bars represent standard deviations (SD) of three technical replicates. The X-axes show the time courses of stress treatments for each gene.
Notably, Gmhdz72 was highly expressed at 24 h after treatment. Similar to their response to NaCl treatment, Gmhdz51 and -83 were also obviously upregulated by drought stress. Furthermore, the induced expression levels of Gmhdz51 and -83 were higher under drought treatment than under salinity treatment. By comparing the expression patterns of the 12 segmental duplicated genes, we found that three paralogous pairs, Gmhdz2 and -9, Gmhdz19 and -27, Gmhdz51 and -83, exhibited similar expression profiles under both stress treatments. In summary, the expression patterns of the soybean HD-Zip genes detected by qRT-PCR were roughly consistent with those observed with microarray analyses, even in response to two different abiotic stress (drought and salt) treatments.
Discussion
Preliminary analysis of HD-Zip gene family has been performed in the model plants Arabidopsis and rice. However, this family has not previously been studied in soybean. In the current study, we performed an overall analysis of the HD-Zip gene family in soybean, including analysis of their phylogeny, chromosomal location, gene structure, conserved motifs and expression profiles. A total of 88 full-length HD-Zip genes were identified in the soybean genome, which is 1.8 times that of Arabidopsis. However, our result was lower than a previous estimate, i.e., that members of the HD-Zip family are 2.9-times more abundant in soybean than in Arabidopsis [48]. Overall, there are two key reasons for this discrepancy. First, more and more sequences have been assembled and introduced into the soybean genome database. Second, the search methods employed in the current and previous studies differed. We used the BLASTP program (based on amino acid sequences) while the previous study used TblastN (based on nucleotide sequences). These two factors may have led to the different results obtained in the two studies.
In each subfamily, the characteristics of exon/intron structure and motif compositions were relatively conserved in recent paralogs. Indeed, previous studies have indicated that few insertions and deletions have accumulate within introns over the past 13 million years [48]. Soybean HD-Zip genes were clustered into four distinct subfamilies based on phylogenetic analysis. Compared to the Arabidopsis, Populus, rice and maize HD-Zip subfamilies, the number of soybean genes in each subfamily is much larger, implying a genome expansion of the soybean HD-Zip counterparts. The main objective of this phylogenetic study was to identify putative orthologous and paralogous HD-Zip genes. In the combined tree of the HD-Zip genes in all three species, we found only one monocot and dicot orthologous pair (Gmhdz50 and Os10g42490), suggesting that the ortholog pair originated from common ancestral genes that existed before the divergence of monocots and dicots. The fact that the genes in paralogs accounted for the most of the family members confirms that soybean has undergone two duplication events after the monocot/dicot split, and most HD-Zip genes in soybean expanded in a species-specific manner.
Gene duplication is one of the major evolutionary mechanisms for generating novel genes that help organisms adapt to different environments [63], [64]. Three principal evolutionary patterns were attributed to gene duplications, including segmental duplication, tandem duplication and transposition events such as retroposition and replicative transposition [65]. Among these, segmental duplication occurs most frequently in plants because most plants are diploidized polyploids and retain numerous duplicated chromosomal blocks within their genomes [66]. In our analysis, we found that a high proportion of HD-Zip genes are distributed preferentially in duplicated blocks, suggesting that segmental duplications contribute significantly to the expansion of the soybean HD-Zip gene family. Previous studies have shown that the soybean genome has undergone two rounds of whole genome duplication, including an ancient duplication prior to the divergence of papilionoid (58–60 Mya) and a Glycine-specific duplication that is estimated to have occurred ∼13 Mya [48]. By calculating the duplication dates of the paralogous pairs, we demonstrated that all of the segmental duplication events in the soybean HD-Zip family occurred during the recent whole genome duplication event. The two tandem duplication pairs Gmhdz29/-30 and Gmhdz40/-41 were formed 9.18 and 8.95 Mya, respectively, while the segmental duplication of Gmhdz29/-40 occurred 7.18 Mya. The results indicate that the two tandem duplication events occurred before the formation of segmental duplication.
Legumes, the third major crop worldwide, can also generate on their roots other de novo meristems, leading to the formation of lateral roots and symbiotic nitrogen-fixing nodules [67]. In the model legume Medicago truncatula, the HD-Zip I transcription factor HB1 is expressed in primary and lateral root meristems and induced by salt stress. The mutant is also affected in nodule density (nodule number per root centimeter). In silico analysis, three HD-Zip I genes(Gmhdz2, -78, -84), three HD-Zip II genes(Gmhdz16, -57, -77) and one HD-ZIP III gene(Gmhdz30) were detected higher both in the root and noodle (Figure 5). The result indicates that these genes may affect roots and symbiotic nodules. Roots sense the edaphic water deficit, send chemical signals to shoots, and maintenance of root growth despite reduced water availability can contribute to drought tolerance through water foraging [68],[69]. The genes from HD-Zip I and II expressed broadly in the root are potentially related to regulation of developmental adaptation to environmental stress conditions such as drought. In a previous study, it was reported that HAHB10, a sunflower HD-Zip transcription factor belonging to subfamily II, was able to accelerate flowering and thereby shortens the plant's life cycle when ectopically expressed in Arabidopsis plants [70]. Arabidopsis ATHB2/HAT4 induces early flowering when ectopically expressed and it has been described as a master switch in the shade avoidance response [33]. The tissues expression data analyses shows that 11 genes(Gmhdz16, -21, -22, -35, -64, -70, -71, -74, -76, 77, -88) are expressed in flowers, suggesting their putative roles in the induction of flowering. In Arabidopsis and rice, the function of HD-Zip III genes is related to apical embryo patterning, embryonic shoot meristem formation, organ polarity, vascular development, and meristem function [36], [71]. Leaves, the principal lateral organ of the shoot, develop as polar structures typically with distinct dorsoventrality. In comparison, the 12 soybean genes from HD-ZIP III subfamily identified in the present study showed preferentially high expression levels in young leaf, suggesting their putative roles in the regulation adaxial leaf fate. HD-ZIP IV genes have been identified in several plant species including Arabidopsis, maize, and rice, and a common feature of the vast majority of these genes resides in their epidermis-specific expression pattern [42], [72]. In maize, OCL1 (OUTER CELL LAYER1) is expressed in the epidermis of embryo, endosperm, meristematic tissues, and organ primordia [73]. In soybean, seven genes (Gmhdz48, -49, - 55, -56, -62, -68, -73) from HD-ZIP IV show comparatively higher transcript abundances in seed. How these soybean HD-ZIPs perform their functional roles in the seed remains to be elucidated and further functional analyses will be required to understand their biological roles in soybean. In addition, fwa(flowering late) semi-dominant mutants are late flowering [74], [75], a phenotype shared by plants overexpressing PDF2 [20]. Two orthologous genes (Gmhdz7,-56) of PDF2 were strongly expressed in the flower(Figure 5). Overexpression of Gmhdz7 and Gmhdz56 in soybean probably leads to a strong delay in flowering time. Soybean HD-Zip genes may also involve in other biological processes, such as fruit and seed development, for their abundant expression in the one cm pod and pod shell.
During their life cycles, the growth and productivity of plants are frequently threatened by environmental stresses such as drought and high salinity. Many stress-related genes are induced to help plants adapt to these environmental stresses. Compared with subfamily II, III, IV, subfamily I display a important role in particular in abiotic stress [11]. The Arabidopsis and rice have 17 and 14 subfamily 1 genes respectively. According to the expression patterns studied in Arabidopsis and rice, some HD-Zip family I genes are regulated by drought and salt stresses [76]–[82]. In the soybean, 8 of 23 genes were induced in both salt and drought stress and 7 genes were not presented (Fig. 4).
In this study, 12 soybean HD-Zip genes from subfamily I were predicted to be stress-related genes based on microarray data analysis, and their expression patterns under drought and salinity treatments were investigated. The results show that the 12 soybean HD-Zip genes are responsive to the two stressors. Phylogenetic analysis places Os04g45810 (Oshox22), Os02g43330 (Oshox24), ATHB-7 and ATHB-12 in the same subgroup (γ clade) of the HD-Zip family I, and they all regulate the salt and drought stress. Based on in silico analysis and our RT-qPCR, Gmhdz19/-27 and Gmhdz32/-72 belonging to γ clade also response to the two stressors. Gmhdz51/-83 and Gmhdz24/-38 have a close relationship with ATHB2/-20 and ATHB5/-6/-16 respectively. While ATHB2/-20 and ATHB5/-6/-16 play a role as a negative regulator under the two stress, Gmhdz51/-83 and Gmhdz24/-38 increased their expression level. In particular, Gmhdz51 and -83 were strongly induced by both stimuli. We conclude that Gmhdz51 and -83 may play essential roles in responses to abiotic stress. Gmhdz2/-9 and Gmhdz19/-27 contained to the clade ζ which is unique to the soybean are also up-regulated under the two stress. As noted above, though the closely related genes share many similar characteristics, they may have different expression patterns. Throughout evolution, they diversified and acquired new genes that may have important roles in plant development.
By comparing the six pairs of these duplicated genes, we observed that Gmhdz2/-9, Gmhdz19/-27 and Gmhdz51/-83 exhibit similar expression patterns, indicating that the responses of paralogs to stress conditions did not undergo much divergence along with the evolution of each gene after duplication and that the duplicated genes may have redundant functions in response to salinity and drought stress. By contrast, the functions of the gene pair Gmhdz24/-38 apparently diverged under salinity treatment while those of Gmhdz20/-72 diverged under drought treatment. The new information obtained in this study may aid in the selection of appropriate candidate genes for further functional characterization.
Methods
Database search and nomenclature of genes
The Glycine max genome database (release 1.0, http://www.phytozome.net/soybean.php) was searched to identify HD-Zip proteins using Basic Local Alignment Search Tool algorithms (BLASTP) with the published Arabidopsis HD-Zip protein sequences as query sequences. All obtained protein sequences were examined for the presence of the HD (PF00046, SM000389) and LZ (PF02183 SM000340) domains using the Hidden Markov Model of Pfam (http://pfam.sanger.ac.uk/search) [83] and SMART (http://smart.embl-heidelberg.de/) [84] tools. Physicochemical parameters of each gene were calculated using ExPASy (http://www.expasy.org/tools/) [85]. Information regarding cDNA sequences, genomic sequences, ORF lengths and chromosome locations was obtained from the Phytozome database.
Phylogenetic analysis
Phylogenetic trees were constructed using MEGA 5.0 [86] with the Neighbor-Joining (NJ) method, and bootstrap analysis was conducted using 1,000 replicates with the pairwise gap deletion mode, which allows the divergent domains to contribute to the topology of the NJ tree [57]. Multiple sequence alignments of the full-length protein sequences from soybean, rice and Arabidopsis were also performed with MEGA 5.0 using default parameters.
Gene structure analysis and identification of conserved motifs
Identification of the exon/intron organization of the HD-Zip genes was performed with Gene Structure Display Server (GSDS; http://gsds.cbi.pku.edu.cn/) [87] by alignment of the cDNAs with their corresponding genomic DNA sequences. Structural motif annotation was performed using the MEME program with the following parameters: number of repetitions, any; maximum number of motifs, 30 and the optimum motif widths, between six and 200 residues. In addition, structural motif annotation was performed using the Pfam (http://pfam.sanger.ac.uk/search) and SMART (http://smart.embl-heidelberg.de/) tools.
Chromosomal location and gene duplication
The segment duplication coordinates of the target genes was detected from the SoyBase browser (http://soybase.org/gb2/gbrowse/gmax1.01/) [68]. Information about the chromosome locations was obtained from the Phytozome database. The relative duplicate blocks representing homologous chromosome segments were anchored on the 20 soybean chromosomes and indicated with the same color. Genes were considered to have undergone segmental duplication if they were found to be coparalogs that were located on duplicated chromosomal blocks, as proposed by Wei et al. (2007) [88]. Paralogs were considered to be tandem duplicated genes if the two genes were separated by five or fewer genes in a 100-kb region [89].
Calculation of Ka/Ks values
Synonymous (Ks) and nonsynonymous substitution (Ka) rates were calculated according to a previous study [57]. Pairs from the segmental duplication events were first aligned by Clustal X2.0. Subsequently, the aligned sequences and the original cDNA sequences were analyzed by the PAL2NAL program (http://www.bork.embl.de/pal2nal/) [90], using the CODEML program of PAML [91] to estimate substitution rates. For each gene pair, the Ks value was translated into divergence time in millions of years based on a rate of 6.1×10−9 substitutions per site per year. The divergence time (T) was calculated as T = Ks/(2×6.1×10−9)×10−6 Mya [60].
Microarray analysis of Gmhdzs
The genome-wide microarray data for the salt and drought stresses were downloaded from the Gene Expression Omnibus (GEO) [92] database at the National Center for Biotechnology Information under accession numbers GSE41125 (from Glycine max) [93] and GSE40627 (from Glycine max) respectively. Probe sets corresponding to the putative soybean HD-Zips were identified using an online Probe Match tool available at the NetAffx Analysis Center (http://www.affymetrix.com/). For genes with more than one probe set, the highest expression value was considered. When several genes had the same probe set, they were considered to have the same transcriptional profile.
The tissue-specific transcript data of 88 HD-Zip genes were investigated based on the RNA Seq-Atlas from fourteen tissues (http://soybase.org/soyseq/), including underground tissues (root and nodule), seed development (seed 10-DAF, seed 14-DAF, seed 21-DAF, seed 25-DAF, seed 28-DAF, seed 35-DAF and seed 42-DAF) and aerial tissues (leaf, flower, pod-shell 10-DAF, pod-shell 14-DAF and one-cm pod). The expression data were gene-wise normalized and the heatmap was drawn using Cluster (version 3.0) with the average linkage program [94].
Plant materials, growth conditions and stress treatments
Seedlings of soybean (Glycine max L.) Williams 82 were used to study gene expression levels in all experiments. For expression analysis of soybean HD-Zip genes under stress, four-week-old seedlings grown in a growth chamber with a continuous 30°C temperature, a photoperiod of 12 h/12 h, 80 µmolm−2 s−1photon flux density and 50% relative humidity were used [93]. Salt stress was conducted by watering the plants with a sodium chloride (NaCl) solution at a concentration of 150 mM to saturation [95]. For drought stress, the intact roots of plants were placed onto filter paper and exposed to the air at 70–80% humidity at 25°C under dim light [57]. Seedlings without treatment were used as the control. Leaves of the stress-treated plants were collected at time intervals of 0, 1, 6, 12 and 24 h. After all of the materials were collected, they were immediately frozen in liquid nitrogen and stored at −80°C for RNA extraction. Three biological replicates were employed per sample.
RNA extraction and qRT-PCR analysis
Total RNA was extracted from frozen samples using an RNAprep Pure Plant Kit (Tiangen) according to the manufacturer's instructions. The first-strand cDNA was then synthesized using a PrimeScriptTM RT Reagent Kit (TaKaRa). Gene-specific primers were designed using Primer5.0, and their specificity was checked using information provided on the NCBI website. CYP2 (cyclophilin), a constitutively expressed soybean housekeeping gene, was used as reference for normalization [96]. RT-PCR was performed in a 25 µl volume containing 12.5 µl 2×SYBR® Premix Ex Taq™ (TaKaRa), 1 µl diluted cDNA, 0.15 µl of each gene-specific primer and 11.2 µl ddH2O. The PCR conditions were as follows: 95°C for 10 min, 40 cycles of 15 s at 95°C, 58°C for 1 min. Three biological replicates were used per sample. The relative expression level was calculated as 2−ΔΔCT [ΔCT = CT, Target−CT, CYP2. ΔΔCT = ΔCT, treatment−ΔCT, CK (0 h)].The relative expression level (2−ΔΔCT, CK (0 h)) in the control plants without treatment was normalized to 1 as described previously [97]. Statistical analyses were performed using SDS software 1.3.1 (Applied Biosystems).
Supporting Information
The information of 88 Gmhdz genes. A complete list of 88 HD-ZIP gene sequences identified in the present study. Genomic DNA sequences are obtained from Phytozome (http://www.phytozome.net/soybean, release 1.0). Amino acid sequences are deduced from the corresponding coding sequences.
(TXT)
Motifs of 88 soybean HD-Zip proteins. Thirty motifs were identified through MEME (http://meme.nbcr.net/meme/), and then motif organizations of 88 soybean HD-Zips were investigated through MAST (http://meme.nbcr.net/meme/).
(TIF)
The detailed information on conserved amino acid sequences and lengths of the 30 motifs.
(XLSX)
Recent Synteny blocks of soybean and soybean (13 Mya) genomes containing HD-ZIP genes.
(XLS)
Pairwise identities between paralogous pairs of HD-ZIP genes from Soybean.
(XLS)
The probes and microarray data of soybean HD-ZIP genes.
(XLS)
The transcriptions of soybean HD-Zip genes through RNA-seq analysis.
(XLS)
A list of primer sequences of the 12 selected HD-ZIP genes for qRT-PCR analysis.
(XLS)
Acknowledgments
We thank the members of the Laboratory of Modern Biotechnology for their assistance in this study.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (No. 31370561) and Anhui Provincial Natural Science Foundation (No. 1308085MC36). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Carretero-Paulet L, Galstyan A, Roig-Villanova I, Martínez-García JF, Bilbao-Castro JR, et al. (2010) Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, poplar, rice, moss, and algae. Plant physiology 153: 1398–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xiong Y, Liu T, Tian C, Sun S, Li J, et al. (2005) Transcription factors in rice: a genome-wide comparative analysis between monocots and eudicots. Plant molecular biology 59: 191–203. [DOI] [PubMed] [Google Scholar]
- 3. Ma J, Ptashne M (1987) A new class of yeast transcriptional activators. Cell 51: 113–119. [DOI] [PubMed] [Google Scholar]
- 4. Schena M, Davis RW (1992) HD-Zip proteins: members of an Arabidopsis homeodomain protein superfamily. Proceedings of the National Academy of Sciences 89: 3894–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGinnis W, Garber RL, Wirz J, Kuroiwa A, Gehring WJ (1984) A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell 37: 403–408. [DOI] [PubMed] [Google Scholar]
- 6. Scott MP, Weiner AJ (1984) Structural relationships among genes that control development: sequence homology between the Antennapedia, Ultrabithorax, and fushi tarazu loci of Drosophila. Proceedings of the National Academy of Sciences 81: 4115–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mukherjee K, Brocchieri L, Bürglin TR (2009) A comprehensive classification and evolutionary analysis of plant homeobox genes. Molecular biology and evolution 26: 2775–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vollbrecht E, Veit B, Sinha N, Hake S (1991) The developmental gene Knotted-1 is a member of a maize homeobox gene family. [DOI] [PubMed] [Google Scholar]
- 9. Chan RL, Gago GM, Palena CM, Gonzalez DH (1998) Homeoboxes in plant development. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression 1442: 1–19. [DOI] [PubMed] [Google Scholar]
- 10. Wen BQ, Xing MQ, Zhang H, Dai C, Xue HW (2011) Rice Homeobox Transcription Factor HOX1a Positively Regulates Gibberellin Responses by Directly Suppressing EL1F. Journal of integrative plant biology 53: 869–878. [DOI] [PubMed] [Google Scholar]
- 11. Ariel FD, Manavella PA, Dezar CA, Chan RL (2007) The true story of the HD-Zip family. Trends in plant science 12: 419–426. [DOI] [PubMed] [Google Scholar]
- 12. Baima S, Nobili F, Sessa G, Lucchetti S, Ruberti I, et al. (1995) The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development 121: 4171–4182. [DOI] [PubMed] [Google Scholar]
- 13. Cristina M, Sessa G, Dolan L, Linstead P, Baima S, et al. (1996) The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. The Plant Journal 10: 393–402. [DOI] [PubMed] [Google Scholar]
- 14. Sessa G, Carabelli M, Ruberti I, Lucchetti S, Baima S, et al. (1994) Identification of distinct families of HD-Zip proteins in Arabidopsis thaliana. NATO ASI SERIES H CELL BIOLOGY 411–411. [Google Scholar]
- 15. Sessa G, Morelli G, Ruberti I (1993) The Athb-1 and-2 HD-Zip domains homodimerize forming complexes of different DNA binding specificities. The EMBO journal 12: 3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meijer AH, Scarpella E, Dijk EL, Qin L, Taal AJ, et al. (1997) Transcriptional repression by Oshox1, a novel homeodomain leucine zipper protein from rice. The Plant Journal 11: 263–276. [DOI] [PubMed] [Google Scholar]
- 17. Frank W, Phillips J, Salamini F, Bartels D (1998) Two dehydration-inducible transcripts from the resurrection plantCraterostigma plantagineumencode interacting homeodomain-leucine zipper proteins. The Plant Journal 15: 413–421. [DOI] [PubMed] [Google Scholar]
- 18. Johannesson H, Wang Y, Engström P (2001) DNA-binding and dimerization preferences of Arabidopsis homeodomain-leucine zipper transcription factors in vitro. Plant molecular biology 45: 63–73. [DOI] [PubMed] [Google Scholar]
- 19. Sessa G, Steindler C, Morelli G, Ruberti I (1998) The Arabidopsis Athb-8,-9 and genes are members of a small gene family coding for highly related HD-ZIP proteins. Plant molecular biology 38: 609–622. [DOI] [PubMed] [Google Scholar]
- 20. Abe M, Katsumata H, Komeda Y, Takahashi T (2003) Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130: 635–643. [DOI] [PubMed] [Google Scholar]
- 21.Dolan L (2013) Expression Of Transcription Factor Encoding Genes. US Patent 20,130,081,153.
- 22. Tron AE, Bertoncini CW, Chan RL, Gonzalez DH (2002) Redox regulation of plant homeodomain transcription factors. Journal of Biological Chemistry 277: 34800–34807. [DOI] [PubMed] [Google Scholar]
- 23. Ponting CP, Aravind L (1999) START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends in biochemical sciences 24: 130–132. [DOI] [PubMed] [Google Scholar]
- 24. Schrick K, Nguyen D, Karlowski WM, Mayer KF (2004) START lipid/sterol-binding domains are amplified in plants and are predominantly associated with homeodomain transcription factors. Genome biology 5: R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mukherjee K, Bürglin TR (2006) MEKHLA, a novel domain with similarity to PAS domains, is fused to plant homeodomain-leucine zipper III proteins. Plant Physiology 140: 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valdés AE, Övernäs E, Johansson H, Rada-Iglesias A, Engström P (2012) The homeodomain-leucine zipper (HD-Zip) class I transcription factors ATHB7 and ATHB12 modulate abscisic acid signalling by regulating protein phosphatase 2C and abscisic acid receptor gene activities. Plant molecular biology 80: 405–418. [DOI] [PubMed] [Google Scholar]
- 27. Ariel F, Diet A, Verdenaud M, Gruber V, Frugier F, et al. (2010) Environmental regulation of lateral root emergence in Medicago truncatula requires the HD-Zip I transcription factor HB1. The Plant Cell Online 22: 2171–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Delarue M, Prinsen E, Va H, Caboche M, Bellini C (1998) Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. The Plant Journal 14: 603–611. [DOI] [PubMed] [Google Scholar]
- 29. Morelli G, Ruberti I (2000) Shade avoidance responses. Driving auxin along lateral routes. Plant Physiology 122: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sawa S, Ohgishi M, Goda H, Higuchi K, Shimada Y, et al. (2002) The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. The Plant Journal 32: 1011–1022. [DOI] [PubMed] [Google Scholar]
- 31. Rueda E, Dezar C, Gonzalez D, Chan R (2005) Hahb-10, a sunflower homeobox-leucine zipper gene, is involved in the response to dark/light conditions and promotes a reduction of the life cycle when expressed in Arabidopsis. Plant and Cell Physiology 46: 1954–1963. [DOI] [PubMed] [Google Scholar]
- 32. Sessa G, Carabelli M, Sassi M, Ciolfi A, Possenti M, et al. (2005) A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Genes & development 19: 2811–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ciarbelli AR, Ciolfi A, Salvucci S, Ruzza V, Possenti M, et al. (2008) The Arabidopsis homeodomain-leucine zipper II gene family: diversity and redundancy. Plant molecular biology 68: 465–478. [DOI] [PubMed] [Google Scholar]
- 34. Dezar CA, Giacomelli JI, Manavella PA, Ré DA, Alves-Ferreira M, et al. (2011) HAHB10, a sunflower HD-Zip II transcription factor, participates in the induction of flowering and in the control of phytohormone-mediated responses to biotic stress. Journal of experimental botany 62: 1061–1076. [DOI] [PubMed] [Google Scholar]
- 35. Mattsson J, Ckurshumova W, Berleth T (2003) Auxin signaling in Arabidopsis leaf vascular development. Plant Physiology 131: 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, et al. (2005) Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. The Plant Cell Online 17: 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prigge MJ, Clark SE (2006) Evolution of the class III HD-Zip gene family in land plants. Evolution & development 8: 350–361. [DOI] [PubMed] [Google Scholar]
- 38. Baima S, Possenti M, Matteucci A, Wisman E, Altamura MM, et al. (2001) The Arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiology 126: 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim J, Jung JH, Reyes JL, Kim YS, Kim SY, et al. (2005) microRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. The Plant Journal 42: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ilegems M, Douet V, Meylan-Bettex M, Uyttewaal M, Brand L, et al. (2010) Interplay of auxin, KANADI and Class III HD-ZIP transcription factors in vascular tissue formation. Development 137: 975–984. [DOI] [PubMed] [Google Scholar]
- 41. Robischon M, Du J, Miura E, Groover A (2011) The Populus class III HD ZIP, popREVOLUTA, influences cambium initiation and patterning of woody stems. Plant physiology 155: 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakamura M, Katsumata H, Abe M, Yabe N, Komeda Y, et al. (2006) Characterization of the class IV homeodomain-leucine zipper gene family in Arabidopsis. Plant physiology 141: 1363–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wei Q, Kuai B, Hu P, Ding Y (2012) Ectopic-overexpression of an HD-Zip IV transcription factor from Ammopiptanthus mongolicus (Leguminosae) promoted upward leaf curvature and non-dehiscent anthers in Arabidopsis thaliana. Plant Cell, Tissue and Organ Culture (PCTOC) 110: 299–306. [Google Scholar]
- 44. Depège-Fargeix N, Javelle M, Chambrier P, Frangne N, Gerentes D, et al. (2011) Functional characterization of the HD-ZIP IV transcription factor OCL1 from maize. Journal of experimental botany 62: 293–305. [DOI] [PubMed] [Google Scholar]
- 46. Rhoades J, Loveday J (1990) Salinity in irrigated agriculture. Agronomy 1089–1142. [Google Scholar]
- 47. Nuccio ML, Rhodest D, McNeil SD, Hanson AD (1999) Metabolic engineering of plants for osmotic stress resistance. Current opinion in plant biology 2: 128–134. [DOI] [PubMed] [Google Scholar]
- 48. Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183. [DOI] [PubMed] [Google Scholar]
- 49. Zhang G, Chen M, Chen X, Xu Z, Guan S, et al. (2008) Phylogeny, gene structures, and expression patterns of the ERF gene family in soybean (Glycine max L.). Journal of experimental botany 59: 4095–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yin G, Xu H, Xiao S, Qin Y, Li Y, et al. (2013) The large soybean (Glycine max) WRKY TF family expanded by segmental duplication events and subsequent divergent selection among subgroups. BMC plant biology 13: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu H, Li Y, Yan Y, Wang K, Gao Y, et al. (2010) Genome-scale identification of Soybean BURP domain-containing genes and their expression under stress treatments. BMC plant biology 10: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fan C-M, Wang X, Wang Y-W, Hu R-B, Zhang X-M, et al. (2013) Genome-Wide Expression Analysis of Soybean MADS Genes Showing Potential Function in the Seed Development. PloS one 8: e62288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Du H, Yang S-S, Liang Z, Feng B-R, Liu L, et al. (2012) Genome-wide analysis of the MYB transcription factor superfamily in soybean. BMC plant biology 12: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hao YJ, Wei W, Song QX, Chen HW, Zhang YQ, et al. (2011) Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. The Plant Journal 68: 302–313. [DOI] [PubMed] [Google Scholar]
- 55. Zhao Y, Zhou Y, Jiang H, Li X, Gan D, et al. (2011) Systematic analysis of sequences and expression patterns of drought-responsive members of the HD-Zip gene family in maize. PloS one 6: e28488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hu R, Chi X, Chai G, Kong Y, He G, et al. (2012) Genome-wide identification, evolutionary expansion, and expression profile of homeodomain-leucine zipper gene family in poplar (Populus trichocarpa). PloS one 7: e31149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hu R, Qi G, Kong Y, Kong D, Gao Q, et al. (2010) Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC plant biology 10: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li W-H, Gojobori T, Nei M (1981) Pseudogenes as a paradigm of neutral evolution. Nature 292: 237–239. [DOI] [PubMed] [Google Scholar]
- 59. Juretic N, Hoen DR, Huynh ML, Harrison PM, Bureau TE (2005) The evolutionary fate of MULE-mediated duplications of host gene fragments in rice. Genome research 15: 1292–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lynch M, Conery JS (2000) The evolutionary fate and consequences of duplicate genes. Science 290: 1151–1155. [DOI] [PubMed] [Google Scholar]
- 61. Harris JC, Hrmova M, Lopato S, Langridge P (2011) Modulation of plant growth by HD-Zip class I and II transcription factors in response to environmental stimuli. New Phytologist 190: 823–837. [DOI] [PubMed] [Google Scholar]
- 62. Skirycz A, Inzé D (2010) More from less: plant growth under limited water. Current Opinion in Biotechnology 21: 197–203. [DOI] [PubMed] [Google Scholar]
- 63. Bowers JE, Chapman BA, Rong J, Paterson AH (2003) Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 422: 433–438. [DOI] [PubMed] [Google Scholar]
- 64. Gu Z, Steinmetz LM, Gu X, Scharfe C, Davis RW, et al. (2003) Role of duplicate genes in genetic robustness against null mutations. Nature 421: 63–66. [DOI] [PubMed] [Google Scholar]
- 65. Kong H, Landherr LL, Frohlich MW, Leebens-Mack J, Ma H, et al. (2007) Patterns of gene duplication in the plant SKP1 gene family in angiosperms: evidence for multiple mechanisms of rapid gene birth. The Plant Journal 50: 873–885. [DOI] [PubMed] [Google Scholar]
- 66. Cannon SB, Mitra A, Baumgarten A, Young ND, May G (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC plant biology 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Crespi M, Frugier F (2008) De novo organ formation from differentiated cells: root nodule organogenesis. Science Signaling 1: re11. [DOI] [PubMed] [Google Scholar]
- 68. Grant D, Nelson RT, Cannon SB, Shoemaker RC (2010) SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic acids research 38: D843–D846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany 49: 419–432. [Google Scholar]
- 70. Rueda EC, Dezar CA, Gonzalez DH, Chan RL (2005) Hahb-10, a sunflower homeobox-leucine zipper gene, is regulated by light quality and quantity, and promotes early flowering when expressed in Arabidopsis. Plant and cell physiology 46: 1954–1963. [DOI] [PubMed] [Google Scholar]
- 71. Byrne ME (2006) Shoot meristem function and leaf polarity: the role of class III HD–ZIP genes. PLoS genetics 2: e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ito M, Sentoku N, Nishimura A, Hong S-K, Sato Y, et al. (2003) Roles of rice GL2-type homeobox genes in epidermis differentiation. Breeding science 53: 245–253. [Google Scholar]
- 73. Ingram GC, Boisnard-Lorig C, Dumas C, Rogowsky PM (2000) Expression patterns of genes encoding HD-ZipIV homeo domain proteins define specific domains in maize embryos and meristems. The Plant Journal 22: 401–414. [DOI] [PubMed] [Google Scholar]
- 74. Koornneef M, Hanhart C, Van der Veen J (1991) A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Molecular and General Genetics MGG 229: 57–66. [DOI] [PubMed] [Google Scholar]
- 75. Soppe WJ, Jacobsen SE, Alonso-Blanco C, Jackson JP, Kakutani T, et al. (2000) The Late Flowering Phenotype of fwa Mutants Is Caused by Gain-of-Function Epigenetic Alleles of a Homeodomain Gene. Molecular cell 6: 791–802. [DOI] [PubMed] [Google Scholar]
- 76. Maruyama K, Todaka D, Mizoi J, Yoshida T, Kidokoro S, et al. (2012) Identification of cis-acting promoter elements in cold-and dehydration-induced transcriptional pathways in Arabidopsis, rice, and soybean. DNA research 19: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, et al. (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. The Plant Journal 31: 279–292. [DOI] [PubMed] [Google Scholar]
- 78. Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, et al. (2003) Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant physiology 133: 1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bray EA (2004) Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. Journal of Experimental Botany 55: 2331–2341. [DOI] [PubMed] [Google Scholar]
- 80. Agalou A, Purwantomo S, Övernäs E, Johannesson H, Zhu X, et al. (2008) A genome-wide survey of HD-Zip genes in rice and analysis of drought-responsive family members. Plant molecular biology 66: 87–103. [DOI] [PubMed] [Google Scholar]
- 81. Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57: 781–803. [DOI] [PubMed] [Google Scholar]
- 82. Henriksson E, Olsson AS, Johannesson H, Johansson H, Hanson J, et al. (2005) Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiology 139: 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Finn RD, Mistry J, Schuster-Böckler B, Griffiths-Jones S, Hollich V, et al. (2006) Pfam: clans, web tools and services. Nucleic acids research 34: D247–D251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Letunic I, Copley RR, Schmidt S, Ciccarelli FD, Doerks T, et al. (2004) SMART 4.0: towards genomic data integration. Nucleic Acids Research 32: D142–D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, et al. (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic acids research 31: 3784–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guo A-Y, Zhu Q-H, Chen X, Luo J-C (2007) GSDS: a gene structure display server]. Yi chuan = Hereditas/Zhongguo yi chuan xue hui bian ji 29: 1023. [PubMed] [Google Scholar]
- 88. Wei F, Coe E, Nelson W, Bharti AK, Engler F, et al. (2007) Physical and genetic structure of the maize genome reflects its complex evolutionary history. PLoS Genetics 3: e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang L, Guo K, Li Y, Tu Y, Hu H, et al. (2010) Expression profiling and integrative analysis of the CESA/CSL superfamily in rice. BMC plant biology 10: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Suyama M, Torrents D, Bork P (2006) PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic acids research 34: W609–W612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Molecular biology and evolution 24: 1586–1591. [DOI] [PubMed] [Google Scholar]
- 92. Barrett T, Edgar R (2006) [19] Gene Expression Omnibus: Microarray Data Storage, Submission, Retrieval, and Analysis. Methods in enzymology 411: 352–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hyun TK, Eom SH, Jeun YC, Han SH, Kim J-S (2013) Identification of glutamate decarboxylases as a γ-aminobutyric acid (GABA) biosynthetic enzyme in soybean. Industrial Crops and Products 49: 864–870. [Google Scholar]
- 94. Chen Q, Yang L, Ahmad P, Wan X, Hu X (2011) Proteomic profiling and redox status alteration of recalcitrant tea (Camellia sinensis) seed in response to desiccation. Planta 233: 583–592. [DOI] [PubMed] [Google Scholar]
- 95. Du Q-L, Cui W-Z, Zhang C-H, Yu D-Y (2010) GmRFP1 encodes a previously unknown RING-type E3 ubiquitin ligase in Soybean (Glycine max). Molecular biology reports 37: 685–693. [DOI] [PubMed] [Google Scholar]
- 96. Jian B, Liu B, Bi Y, Hou W, Wu C, et al. (2008) Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC molecular biology 9: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nature protocols 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The information of 88 Gmhdz genes. A complete list of 88 HD-ZIP gene sequences identified in the present study. Genomic DNA sequences are obtained from Phytozome (http://www.phytozome.net/soybean, release 1.0). Amino acid sequences are deduced from the corresponding coding sequences.
(TXT)
Motifs of 88 soybean HD-Zip proteins. Thirty motifs were identified through MEME (http://meme.nbcr.net/meme/), and then motif organizations of 88 soybean HD-Zips were investigated through MAST (http://meme.nbcr.net/meme/).
(TIF)
The detailed information on conserved amino acid sequences and lengths of the 30 motifs.
(XLSX)
Recent Synteny blocks of soybean and soybean (13 Mya) genomes containing HD-ZIP genes.
(XLS)
Pairwise identities between paralogous pairs of HD-ZIP genes from Soybean.
(XLS)
The probes and microarray data of soybean HD-ZIP genes.
(XLS)
The transcriptions of soybean HD-Zip genes through RNA-seq analysis.
(XLS)
A list of primer sequences of the 12 selected HD-ZIP genes for qRT-PCR analysis.
(XLS)