Abstract
Background and Aims
Variant in glucokinase regulatory protein (GCKR), associated with lipid and glucose traits, has been suggested to affect fatty liver infiltration. We aimed to assess whether GCKR rs780094 C→T SNP influences the expression of steatosis, lobular inflammation and fibrosis in NAFLD patients, after correction for PNPLA3 genotype.
Methods
In 366 consecutive NAFLD patients (197 from Sicily, and 169 from center/northern Italy), we assessed anthropometric, biochemical and metabolic features; liver biopsy was scored according to Kleiner. PNPLA3 rs738409 C>G and GCKR rs780094 C>T single nucleotide polymorphisms were also assessed.
Results
At multivariate logistic regression analysis in the entire NAFLD cohort, the presence of significant liver fibrosis (>F1) was independently linked to high HOMA (OR 1.12, 95% CI 1.01–1.23, p = 0.02), NAFLD activity score ≥5 (OR 4.09, 95% CI 2.45–6.81, p<0.001), and GCKR C>T SNP (OR 2.06, 95% CI 1.43–2.98, p<0.001). Similar results were observed considering separately the two different NAFLD cohorts. GCKR C>T SNP was also associated with higher serum triglycerides (ANOVA, p = 0.02) in the entire cohort.
Conclusions
In patients with NAFLD, GCKR rs780094 C>T is associated with the severity of liver fibrosis and with higher serum triglyceride levels.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a frequent and growing cause of chronic liver disease [1], [2], affecting about 20%–30% of the general population worldwide [3]. Patients with NAFLD, and especially those with non-alcoholic steatohepatitis (NASH), are at risk of progression to cirrhosis and its complications [1], [4], presenting also a high rate of cancer and cardiovascular events [5] compared to subjects without fatty liver. Classical risk factors for liver disease severity and its progression are obesity, insulin resistance (IR) and necroinflammation [6]–[9].
The above-mentioned conventional risk factors do not entirely explain the occurrence and severity of NAFLD, suggesting that a genetic background might also modulate the spectrum of liver disease and its progression. Accordingly, the severity of disease has been variably associated with different single nucleotide polymorphisms (SNPs) in genes involved in metabolic homeostasis, inflammation, oxidative stress and fibrogenesis [10]. In this context, the patatin-like phospholipase-3 (PNPLA3)/adiponutrin, rs738409 C>G SNP remains the most validated risk gene [11]–[18].
Besides the classical PNPLA3, a recent genome wide study identified other genetic variants associated with computerized tomography (TC)-proven hepatic steatosis in individuals of European ancestry, and validated the results in 592 subjects with biopsy-proven NAFLD from the NASH Clinical Research Network (NASH CRN) database [19]. Among these gene variants, the glucokinase regulatory protein (GCKR) has been further confirmed as linked with steatosis (identified either by ultrasonography, by magnetic resonance, or by computed tomography) in children, in obese patients, and in populations of different ethnicity [20]–[22]. GCKR seems to interfere with glucose and lipid homeostasis by regulating glucose storage/disposal and by providing substrates for de novo lipogenesis via inhibition of glucokinase, but its potential association with the severity of liver damage has never been tested
Having this in mind, the main outcome of this study was to assess whether GCKR rs780094 was associated with the histological features of liver damage in patients with biopsy-proven NAFLD, after correction for PNPLA3 genotype.
Patients and Methods
Patients
We analyzed data from 366 prospectively recruited Italian patients with a clinical and histological diagnosis of NAFLD, and with blood samples available for genetic analyses. The study cohort included 197 patients from the Gastrointestinal & Liver Unit of the Palermo University Hospital, and 169 central/northern Italy patients from the Department of Internal Medicine Catholic University of the Sacred Heart Rome (n = 114), and from the Gastro-hepatology Division of the University Hospital Torino (n = 55). Other causes of liver disease were ruled out, including alcohol intake (>20 g/day) evaluated by a questionnaire, viral and autoimmune hepatitis, hereditary hemochromatosis and alpha1-antitrypsin deficiency. Patients with advanced cirrhosis, hepatocellular carcinoma and current use of steatosis inducing drugs were excluded.
The study was carried out in accordance with the principles of the Helsinki Declaration, and with local and national laws. Approval was obtained from the hospital Internal Review Boards and their Ethics Committees (UOC Gastroenterologia, AOUP Policlinico of Palermo, Institute of Internal Medicine, Catholic University of the Sacred Heart, Rome, and Division of Gastro-Hepatology, San Giovanni Battista Hospital, University of Torino), and written informed consent for the study was obtained from all patients.
Clinical and Laboratory Assessment
Clinical and anthropometric data were collected at the time of liver biopsy. Body mass index (BMI) was calculated on the basis of weight in kilograms and height in meters. The diagnosis of arterial hypertension was based on the following criteria: systolic blood pressure ≥135 mm Hg and/or diastolic blood pressure ≥85 mm Hg (measured three times within 30 minutes, in the sitting position and using a brachial sphygmomanometer), or use of blood-pressure-lowering agents. The diagnosis of type 2 diabetes was based on the revised criteria of the American Diabetes Association, using a value of fasting cut-off value of blood glucose ≥126 mg/dl on at least two occasions [23]. In patients with a previous diagnosis of type 2 diabetes, current therapy with insulin or oral hypoglycemic agents was documented.
A 12-hour overnight fasting blood sample was drawn at the time of biopsy to determine serum levels of ALT, total cholesterol, HDL-cholesterol, triglycerides, plasma glucose and insulin concentrations. IR was assessed by homeostasis model assessment (HOMA), using the following equation [24]: Insulin resistance (HOMA-IR) = Fasting insulin (µU/mL)×Fasting glucose (mmol/L)/22.5. [25]. HOMAbeta was also assessed as expression of pancreatic beta-cell function [26]
Genetic Analyses
DNA was purified using the QIAmp blood Mini Kit (Qiagen, Mainz, Germany) and DNA samples were quantified using spectrophotometric determination. Genotyping for PNPLA3 (rs738409), and GCKR (rs780094) was carried out using the TaqMan SNP genotyping allelic discrimination method (Applied Biosystems, Foster City, CA, USA). Commercial genotyping assays were available for the following SNPs: rs738409 (cat. C_7241_10), rs780094 (cat. C_2862873_10).
The genotyping call was done with SDS software v.1.3.0 (ABI Prism 7500, Foster City, CA, USA). Genotyping was conducted in a blinded fashion relative to patient characteristics.
Assessment of Histology
Slides were coded and read at each clinical center by one expert pathologist, who was unaware of patients' identity and history. A minimum 15 mm-length of the biopsy specimen or the presence of at least 10 complete portal tracts was required [27]. Steatosis was assessed as the percentage of hepatocytes containing fat droplets (minimum 5%) and evaluated as a continuous variable. Kleiner classification [28] was used to compute steatosis, balloning and lobular inflammation, and to stage fibrosis from 0 to 4. NASH was considered to be present when the NAFLD activity score (NAS) was ≥5 [28].
Statistics
Continuous variables were summarized as mean ± standard deviation, and categorical variables as frequency and percentage. The t-test, ANOVA test, and chi-square test were used when appropriate.
Multiple logistic regression models were used to assess the factors independently associated with severe steatosis, NAS ≥5, and significant fibrosis. In the first model, the dependent variable was steatosis, coded as 0 = mild-moderate (steatosis grade 1–2), 1 = severe (steatosis grade 3). In the second model, the dependent variable was NAS ≥5, coded as 0 = NAS<5 or 1 = NAS ≥5. In the third model, the dependent variable was fibrosis, coded as 0 = no significant fibrosis (F0–F1) or 1 = significant fibrosis (>F1).
As candidate risk factors, we selected age, gender, BMI, the baseline levels of ALT, triglycerides, total and HDL cholesterol, blood glucose, insulin, HOMA score, the presence of diabetes, arterial hypertension, PNPLA3 rs738409, GCKR rs780094, steatosis, lobular inflammation and fibrosis.
In all models, in agreement with literature data, we compared patients homozygous for PNPLA3 G risk allele to all other variants [14], while an additive model was used GCKR C>T SNP [19].
To avoid the effect of colinearity, diabetes, HOMA score, blood glucose and insulin levels, or ALT levels and lobular inflammation were not included in the same multivariate model. Variables associated with the dependent variable at univariate analysis (probability threshold, p≤0.10) were included in the multivariate regression models; PNPLA3 and GCKR SNPs, were forced into the models when not significant associated to the tested dependent variable. Regression analyses were performed using PROC LOGISTIC, PROC REG, and subroutines in SAS [29].
Results
Patient Features and Histology
The baseline characteristics of the 197 Sicilian and the 169 Center/Northern Italian NAFLD patients are shown in Table 1. Patients from Sicily were slightly older and with a moderately lower prevalence of males, were more likely to be obese and to have more severe steatosis and lobular inflammation compared to Center/Northern Italian patients. Nevertheless, histological staging was similar in the two cohorts and significant fibrosis (>F1) was present in approximately half of the patients.
Table 1. Baseline Demographic, Laboratory, Metabolic, and Histological Features of 366 Italian Patients with Non-alcoholic Fatty Liver Disease.
| Variable | Non-alcoholic Fatty Liver Disease (Sicily n = 197) | Non-alcoholic Fatty Liver Disease (Center/Northern Italy n = 169) | P value |
| Mean Age – years | 45.0±13.3 | 42.2±11.2 | 0.03 |
| Male Gender | 131 (66.5) | 130 (76.9) | 0.03 |
| Mean Body Mass Index – kg/m2 | 29.7±4.6 | 27.8±4.1 | <0.001 |
| Alanine Aminotransferase – IU/L | 79.4±55.1 | 77.8±53.3 | 0.78 |
| Arterial Hypertension | 46 (23.3) | 47 (27.8) | 0.21 |
| Type 2 Diabetes | 28 (14.2) | 22 (11.2) | 0.39 |
| Cholesterol – mg/dL | 205.8±44.8 | 203.4±45.2 | 0.74 |
| HDL Cholesterol – mg/dL | 49.0±15.4 | 47.7±11.3 | 0.38 |
| Triglycerides – mg/dL | 147.6±78.7 | 142.7±86.9 | 0.57 |
| Blood Glucose – mg/dL | 96.1±25.2 | 94.7±21.5 | 0.56 |
| Insulin – µU/mL | 16.5±9.6 | 14.5±10.6 | 0.08 |
| HOMA Score | 4.03±2.96 | 3.57±3.41 | 0.18 |
| PNPLA3 rs738409 polymorphism | |||
| C/C | 62 (31.5) | 54 (31.9) | |
| C/G | 94 (47.7) | 79 (46.7) | |
| G/G | 41 (20.8) | 36 (21.4) | 0.98 |
| GCKR rs780094 polymorphism | |||
| C/C | 32 (16.2) | 28 (16.6) | |
| C/T | 99 (50.3) | 80 (47.3) | |
| T/T | 66 (33.5) | 61 (36.1) | 0.98 |
| Histology | |||
| Lobular inflammation | |||
| 2–3 | |||
| Balloning | 83 (42.1) | 20 (11.8) | <0.001 |
| 1–2 | 181 (91.8) | 122 (71.7) | <0.001 |
| Steatosis grade | |||
| 1 (5%–33%) | 72 (36.6) | 76 (44.9) | |
| 2 (>33%–66%) | 62 (31.5) | 62 (36.7) | |
| 3 (>66%) | 63 (31.9) | 31 (18.4) | 0.01 |
| NAS≥5 | 116 (58.8) | 41 (24.2) | <0.001 |
| Stage of Fibrosis | |||
| 2–4 | 86 (43.6) | 72 (42.6) | 0.84 |
Abbreviation: IU, international units; HOMA, homeostasis model assessment; HDL, high density lipoprotein. Data are given as mean ± standard deviation, or as number of cases (%).
The prevalence of GCKR rs780094 CC, CT and TT genotypes was 16.4%, 48.9% and 34.7% in the entire cohort. When split according to center (Sicily vs. Center/Northern Italy) the prevalence of the single SNPs was similar (16.2%, 50.3% and 33.5% in the Sicilian cohort, vs. 16.6%, 47.3% and 36.1%; p = 0.98). Similarly, the prevalence of PNPLA3 rs738409 CC, CG and GG genotypes was 31.5%, 47.7% and 20.8% in the Sicilian cohort, and 31.9%, 46.7% and 21.4% in the Center/Northern Italy cohort (p = 0.98).
Genetic frequencies of the two polymorphisms fit with Hardy–Weinberg equilibrium.
Influence of GCKR Genotype on Metabolic and Biochemical Parameters
The association of the rs780094 GCKR C→T genotype with anthropometric, metabolic and biochemical parameters in the entire cohort is shown in Table 2. The only significant association was between the TT genotype and higher triglyceride levels, although independent of age, gender distribution, and BMI. When split according to center, broadly similar data were observed in the Center/Northern Italian cohort, where an association between GCKR TT genotype and higher blood glucose levels was also observed (85.5±11.6 for CC, 96.7±24.4 for CT, 96.4±20 for TT; p = 0.03). By contrast, in the Sicilian cohort the rs780094 genotype was not associated with abnormal triglyceride levels.
Table 2. Association of the rs780094 C→T GCKR SNP with anthropometric, biochemical, metabolic and histological features in 366 Patients with Non-alcoholic Fatty Liver Disease.
| Variable | GCKR CC N = 60 | GCKR CT n = 179 | GCKR TT n = 127 | Univariate Analysis p value° |
| Mean age – years | 42.2±13.0 | 43.3±12.6 | 44.9±11.8 | 0.33 |
| Males | 48 | 127 | 86 | 0.22 |
| Mean body mass index – kg/m2 | 28.5±4.6 | 28.7±4.4 | 29.1±4.5 | 0.61 |
| Alanine aminotransferase – IU/L | 81.6±70.6 | 81.4±52.1 | 73.4±48.0 | 0.40 |
| Cholesterol – mg/dL | 198.6±47.9 | 202.4±40.9 | 209.5±48.5 | 0.23 |
| HDL cholesterol – mg/dL | 50.2±18.2 | 48.9±13.2 | 47.1±12.2 | 0.32 |
| Triglycerides – mg/dL | 139.8±83.3 | 138.0±69.8 | 158.3±96.3* | 0.09 |
| Blood glucose – mg/dL | 91.4±18.6 | 96.6±27.7 | 95.8±18.5 | 0.32 |
| Insulin – µU/mL | 15.1±9.8 | 16.0±9.1 | 15.4±11.5 | 0.78 |
| HOMA-score | 3.65±3.23 | 3.96±3.03 | 3.70±3.05 | 0.68 |
| HOMAbeta % | 144.4±63.7 | 144.6±71.5 | 131.8±61.0 | 0.25 |
| Diabetes | 8 | 21 | 18 | 0.81 |
| Arterial hypertension | 14 | 41 | 38 | 0.35 |
| Steatosis grade 3 | 13 | 44 | 37 | 0.49 |
| Lobular Inflammation Grade 2–3 | 17 | 53 | 34 | 0.86 |
| Ballooning | 46 | 151 | 105 | 0.39 |
| NAS ≥5 | 24 | 82 | 51 | 0.54 |
| Fibrosis >F1 | 17 | 71 | 70 | 0.001 |
Data are given as mean ± SD or as number of cases. HDL: high-density lipoprotein; HOMA: homeostasis model assessment.
by ANOVA;
p = 0.02 versus GCKR CC/CT.
Factors Associated with Histological Features
In the entire cohort, multivariate logistic regression analysis showed that high BMI (OR 1.10, 95% CI 1.04–1.117, p = 0.001), high HOMA considered as continuous variable (OR 1.08, 95% CI 1.00–1.17, p = 0.04), and PNPLA3 GG (OR 1.97, 95% CI 1.11–3.51, p = 0.02) were independently associated with severe steatosis (table 3 upper panel). Similar results were observed when analyses were split by center (table 3 upper panel).
Table 3. Association of the GCKR rs780094 genotype and liver damage as evaluated by unadjusted and adjusted models in 366 Patients with Non-alcoholic Fatty Liver Disease.
| Sicilian Cohort n = 197 | Center/northern Italy Cohort n = 169 | Combined n = 366 | ||||
| Variable | OR (95% C.I.) | P value | OR (95% C.I.) | P value | OR (95% C.I.) | P value |
| Steatosis (1 vs 2 vs 3) | ||||||
| Unadjusted Model | Adjusted Model | Unadjusted Model | Adjusted Model | Unadjusted Model | Adjusted Model | |
| Females | - | - | - | - | 1.94 (1.18–3.20) 0.009 | 1.43 (0.83–2.44) 0.19 |
| Mean BMI – kg/m2 | 1.09 (1.02–1.17) 0.006 | 1.08 (1.00–1.16) 0.03 | 1.15 (1.05–1.27) 0.003 | 1.12 (1.01–1.25) 0.02 | 1.13 (1.07–1.19) <0.001 | 1.10 (1.04–1.17) 0.001 |
| HOMA-score | 1.13 (1.01–1.25) 0.02 | 1.08 (0.97–1.21) 0.14 | 1.16 (1.03–1.29) 0.01 | 1.08 (0.96–1.22) 0.17 | 1.15 (1.06–1.24) <0.001 | 1.08 (1.00–1.17) 0.04 |
| GCKR CC vs CT vs TT | 1.23 (0.79–1.91) 0.35 | 1.19 (0.75–1.90) 0.44 | 1.27 (0.72–2.25) 0.40 | 1.37 (0.74–2.52) 0.31 | 1.22 (0.87–1.73) 0.24 | 1.22 (0.85–1.76) 0.27 |
| PNPLA3 CC vs CG vs GG | 2.19 (1.08–4.45) 0.02 | 2.12 (1.02–4.41) 0.04 | 2.48 (1.05–5.83) 0.03 | 2.50 (1.03–6.05) 0.04 | 2.24 (1.31–3.83) 0.003 | 1.97 (1.11–3.51) 0.02 |
| NAS≥5 | ||||||
| Mean age – years | - | - | - | - | 1.01 (1.00–1.03) 0.05 | 0.99 (0.97–1.00) 0.79 |
| Females | 2.47 (1.30–4.69) 0.006 | 1.69 (0.83–3.42) 0.14 | 3.98 (1.83–8.64) <0.001 | 3.70 (1.62–8.47) 0.002 | 3.16 (1.97–5.06) <0.001 | 2.55 (1.51–4.31) <0.001 |
| Mean BMI – kg/m2 | 1.10 (1.03–1.18) 0.003 | 1.05 (0.97–1.13) 0.21 | 1.15 (1.05–1.25) 0.002 | 1.13 (1.02–1.25) 0.01 | 1.14 (1.09–1.21) <0.001 | 1.11 (1.04–1.17) 0.004 |
| HOMA-score | 1.38 (1.17–1.64) <0.001 | 1.28 (1.07–1.53) 0.005 | 1.14 (1.02–1.27) 0.01 | 1.03 (0.91–1.17) 0.54 | 1.24 (1.12–1.36) <0.001 | 1.13 (1.03–1.25) 0.01 |
| GCKR CC vs CT vs TT | 1.14 (0.75–1.73) 0.52 | 1.10 (0.69–1.75) 0.67 | 0.76 (0.46–1.27) 0.30 | 0.75 (0.43–1.30) 0.31 | 0.96 (0.71–1.29) 0.79 | 0.91 (0.65–1.26) 0.58 |
| PNPLA3 CC/CG vs GG | 3.62 (1.57–8.35) 0.002 | 3.06 (1.26–7.41) 0.01 | 2.49 (1.13–5.51) 0.02 | 2.31 (1.00–5.31) 0.04 | 2.54 (1.52–4.27) <0.001 | 2.15 (1.22–3.77) 0.008 |
| Fibrosis (0–1 versus 2–4) | ||||||
| Mean age – years | 1.03 (1.01–1.06) 0.001 | 1.03 (1.00–1.05) 0.02 | - | - | 1.02 (1.01–1.04) 0.001 | 1.01 (0.99–1.03) 0.28 |
| Females | 2.34 (1.28–4.29) 0.006 | 1.44 (0.68–3.03) 0.33 | 2.73 (1.30–5.71) 0.007 | 2.06 (0.86–4.91) 0.10 | 2.47 (1.55–3.93) <0.001 | 1.40 (0.80–2.45) 0.23 |
| Mean BMI – kg/m2 | 1.10 (1.03–1.18) 0.002 | 1.02 (0.94–1.11) 0.52 | 1.17(1.07–1.28) <0.001 | 1.12 (1.01–1.23) 0.02 | 1.12 (1.07–1.18) <0.001 | 1.05 (0.98–1.11) 0.10 |
| HOMA-score | 1.31 (1.14–1.52)<0.001 | 1.15 (1.00–1.32) 0.04 | 1.16 (1.03–1.32) 0.01 | 1.06 (0.91–1.23) 0.43 | 1.24 (1.12–1.36) <0.001 | 1.12 (1.01–1.23) 0.02 |
| Arterial hypertension | - | - | - | - | 1.93 (1.20–3.12) 0.007 | 1.36 (0.76–2.43) 0.29 |
| NAS≥5 | 7.83 (3.93–15.5) <0.001 | 7.05 (3.22–15.4) <0.001 | 5.58 (2.55–12.2) <0.001 | 4.43 (1.80–10.9) 0.001 | 5.06 (3.23–7.93) <0.001 | 4.09 (2.45–6.81) <0.001 |
| GCKR CC vs CT vs TT | 1.82 (1.18–2.81) 0.006 | 2.07 (1.22–3.49) 0.006 | 1.75 (1.10–2.76) 0.01 | 2.23 (1.31–3.82) 0.003 | 1.77 (1.30–2.44) <0.001 | 2.06 (1.43–2.98) <0.001 |
| PNPLA3 CC/CG vs GG | 1.47 (0.73–2.93) 0.27 | 0.62 (0.26–1.49) 0.29 | 1.94 (0.92–4.09) 0.07 | 1.32 (0.54–3.18) 0.53 | 1.67 (1.01–2.67) 0.04 | 1.03 (0.56–1.87) 0.92 |
Unadjusted and adjusted OR were presented for PNPLA3 and GCKR SNPs, and only for clinical, metabolic and histological variables significant at univariate analysis.
PNPLA3 and GCKR SNPs, when not significant, were forced into the models.
In the whole NAFLD cohort, NAS score ≥5 was independently associated with female gender (OR 2.55, 95% CI 1.51–4.31, p<0.001), high BMI (OR 1.11 95% CI 1.04–1.17, p = 0.004), HOMA (OR 1.13 95% CI 1.03–1.25, p = 0.01), and PNPLA3 GG (OR 2.15, 95% CI 1.22–3.77, p = 0.008) at multivariate logistic regression analysis (table 3).
Finally, the presence of significant liver fibrosis (>F1) was independently linked to high HOMA (OR 1.12, 95% CI 1.01–1.23, p = 0.02), NAS ≥5 (OR 4.09, 95% CI 2.45–6.81, p<0.001), and GCKR C>T SNP (OR 2.06, 95% CI 1.43–2.98, p<0.001) in the whole study population (table 3 lower panel). When histological variables were removed from the model, >F1 fibrosis was independently linked to female gender (OR 1.77, 95% CI 1.05–2.99, p = 0.03), BMI (OR 1.08, 95% CI 1.02–1.14, p = 0.008), HOMA (OR 1.16, 95% CI 1.05–1.28, p = 0.004), and GCKR C>T SNP (OR 1.88, 95% CI 1.33–2.66, p<0.001).
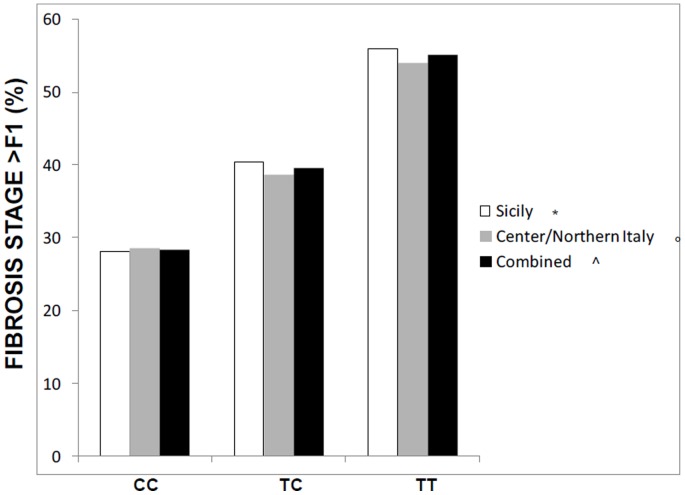
Figure 1 shows the stepwise increased prevalence of significant fibrosis (>F1) according to GCKR genotype. Similar results were observed when the NAFLD cohorts were split by center (table 3 lower panel).
Figure 1. Prevalence of significant fibrosis according to GCKR rs780094 SNP in the Sicilian cohort, in the Center/Northern Italian cohort, and in the combined cohorts of NAFLD patients.
*p = 0.02 for prevalence of F2–F4 fibrosis according to GCKR genotype; ° p = 0.04 for prevalence of F2–F4 fibrosis according to GCKR genotype; ∧p = 0.001 for prevalence of F2–F4 fibrosis according to GCKR genotype.
Discussion
The main finding of this study on a large cohort of Italian patients with NAFLD is the association between GCKR rs780094 C>T SNP and high triglyceride levels and the severity of liver fibrosis, independent of other known risk factors for liver damage
The complex interplay between genetic background and environmental factors in the development of NAFLD [26] is progressively unveiled by the recognition of the role of specific SNPs, such as PNPLA3 C>G SNPs. The GCKR C→T SNPs is also emerging as an important genetic determinant of NAFLD. This trait has been initially associated with imaging-based diagnosis of NAFLD in a population of healthy subjects [19], and the association has been further validated in children and in patients of different ethnicity [20]–[22]. In this study, besides confirming the associations between PNPLA3 C>G SNP and steatosis/lobular inflammation [11]–[15], for the first time we also highlighted the potential impact of GCKR C→T SNP on liver fibrosis in an European cohort of histologically diagnosed NAFLD patients.
Of importance, this association was independent from other well-known determinants and the severity of liver fibrosis showed a stepwise increase from patients carrying one risk allele to those carrying two risk alleles. It is noteworthy that this trend was observed in both tested cohorts. Our findings are in agreement with the evidence of a link between GCKR C→T SNP and higher ALT levels reported by Hernaez et al [22] in the US Health and Nutrition Examination Survey III, and also agree with preliminary unpublished data from FLIP cohort [30], and published data from a small Asiatic population [31] reporting the association between GCKR gene variant and severity of liver damage in NAFLD.
Although this study was not designed to clarify the pathogenic link between GCKR SNPs and liver fibrosis in NAFLD, some hypotheses can be provided. The GCKR gene product, the glucokinase regulatory protein, acts as an inhibitor of glucokinase (GCK) activity, a key liver enzyme for glucose metabolism, whose hepatic concentrations are increased in fatty liver [32], [33]. GCKR rs780094 C>T SNP might facilitate liver fibrogenesis by reducing the inhibitory effect of GCKR, thereby increasing GCK activity. GCKR rs780094 SNP is in linkage disequilibrium with the GCKR rs1260326 SNP, that is associated with an increased GCK liver activity [20], [34]. By inducing de novo hepatic lipogenesis and by suppressing hepatic fatty acid oxidation, GCK liver activity prompts the hepatic accumulation of triglycerides, causing organ damage. In addition, GCKR rs780094 SNP might favor liver fibrogenesis along two more pathways: a) by affecting the expression of nearby genes, and specifically by increasing the expression of C2orf16 mRNA in the liver [19]; b) by favoring a low grade chronic inflammation as expressed by higher serum levels of C-reactive protein [35].
In our study we also demonstrated that patient homozygous for the T allele of GCKR had higher serum triglycerides levels, in agreement with previous finding by Speliotes et al [19], and with the hypothesis that the GCKR rs780094 SNP could lead to a higher activity of liver glcokinase. In addition we also observed higher triglycerides levels in Sicilian compared with Center/Northern Italy cohort, probably attributable to the more unhealthy lifestyle characterizing the southern population. By contrast we did not identify any association between severity of steatosis and GCKR genotype. Our data are not in contrast with the study of Speliotes et al [19] and more recent studies showing a link between GCKR SNP and the presence of steatosis [20]–[22]. We did not test subjects without steatosis of the same geographic area, and therefore we were not able to discriminate between presence/absence of fatty liver infiltration. Obviously, the lack of these data could partially affect the interpretation of our results, and in particular of the significance of the real effect of GCKR SNP on steatosis.
Finally, we did not confirm the reported association between PNPLA3 genotype and severity of liver fibrosis [14]. Differences in the characteristics of the individual study cohorts could explain the lack of association in our cohort.
The main limitation of this study lies in its cross-sectional nature, making it impossible to dissect the temporal relation between genetic background and progression of liver disease over time. This issue should be tested in longitudinal analyses. A further methodological question is the potentially limited external validity of the results for different populations and settings. Our study included cohorts of Italian patients enrolled at tertiary care centers, who may be different, in terms of both metabolic features and severity of liver disease, from the majority of prevalent cases of NAFLD in the general population. However, validation of the results in two independent, largely different populations from South and Northern/Center Italy supports a general involvement of GCKR rs780094 C>T polymorphisms in NAFLD progression.
In conclusion, this study on a large cohort of patients with histological diagnosis of NAFLD, showed an independent link between GCKR SNPs and significant hepatic fibrosis. Further studies are needed to explore the pathogenic mechanisms underlying this association.
Funding Statement
This study was funded by grants from the FP7/2007–2013 (n°Health-F2-2009-241762), from PRIN 2009 (Prot. N. 2009ARYX4T_004), and from PRIN 2010–2011 (Prot. N. 2010C4JJWB_001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Petta S, Muratore C, Craxì A (2009) Non-alcoholic fatty liver disease pathogenesis: the present and the future. Dig Liver Dis 41: 615–25. [DOI] [PubMed] [Google Scholar]
- 2. Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, et al. (2003) Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37: 917–23. [DOI] [PubMed] [Google Scholar]
- 3. Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, et al. (2005) Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 42: 44–52. [DOI] [PubMed] [Google Scholar]
- 4. Petta S, Craxì A (2010) Hepatocellular carcinoma and non-alcoholic fatty liver disease: from a clinical to a molecular association. Curr Pharm Des 16: 741–52. [DOI] [PubMed] [Google Scholar]
- 5. Targher G, Marra F, Marchesini G (2008) Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia 51: 1947–53. [DOI] [PubMed] [Google Scholar]
- 6. Petta S, Tripodo C, Grimaudo S, Cabibi D, Cammà C, et al. (2011) High liver RBP4 protein content is associated with histological features in patients with genotype 1 chronic hepatitis C and with nonalcoholic steatohepatitis. Dig Liver Dis 43: 404–10. [DOI] [PubMed] [Google Scholar]
- 7. van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, et al. (2008) Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 48: 449–57. [DOI] [PubMed] [Google Scholar]
- 8. Bugianesi E, Marchesini G, Gentilcore E, Cua IH, Vanni E, et al. (2006) Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: Role of insulin resistance and hepatic steatosis. Hepatology 44: 1648–55. [DOI] [PubMed] [Google Scholar]
- 9. Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH (2009) Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 51: 371–9. [DOI] [PubMed] [Google Scholar]
- 10. Tilg H, Moschen A (2010) Update on nonalcoholic fatty liver disease: genes involved in nonalcoholic fatty liver disease and associated inflammation. Curr Opin Clin Nutr Metab Care 13: 391–6. [DOI] [PubMed] [Google Scholar]
- 11. Sookoian S, Castano GO, Burgueno AL, Fernandez GT, Rosselli MS, et al. (2009) A nonsynonymous gene variant in adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res 50: 2111–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ (2010) The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology 52: 894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Speliotes EK, Butler JL, Palmer CD, Voight BF, Hirschhorn JN (2010) PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. HEPATOLOGY 52: 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, et al. (2010) Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 51: 1209–1217. [DOI] [PubMed] [Google Scholar]
- 15. Sookoian S, Pirola CJ (2011) Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 53: 1883–94. [DOI] [PubMed] [Google Scholar]
- 16. Petta S, Grimaudo S, Cammà C, Cabibi D, Di Marco V, et al. (2012) IL28B and PNPLA3 polumorphisms affect histological liver damage in patients with non-alcoholic fatty liver disease. J Hepatol 56: 1356–62. [DOI] [PubMed] [Google Scholar]
- 17. Petta S, Ferraro D, Cammà C, Cabibi D, Di Cristina A, et al. (2012) Vitamin D levels and IL28B polymorphisms are related to rapid virological response to standard of care in genotype 1 chronic hepatitis C. Antiviral Therapy 17: 823–31. [DOI] [PubMed] [Google Scholar]
- 18. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, et al. (2009) Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461: 399–401. [DOI] [PubMed] [Google Scholar]
- 19. Speliotes EK, Yerges-Armstrong LM, Kim LJ, Palmer CD, Gudnason V, et al. (2011) Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet 7: e1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santoro N, Zhang CK, Zhao H, Pakstis AJ, Kim G, et al. (2012) Variant in the Glucokinase Regulatory Protein (GCKR) Gene is Associated With Fatty Liver in Obese Children and Adolescents. Hepatology 55: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palmer ND, Musani SK, Yerges-Armstrong LM, Feitosa MF, Bielak LF, et al. (2013) Characterization of European-ancestry NAFLD-associated Variants in individuals of African and Hispanic descent. Hepatology 2013 Apr 8. doi:10.1002/hep.26440 [Epub ahead of print] PubMed PMID: 23564467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hernaez R, McLean J, Lazo M, Brancati FL, Hirschhorn JN, et al. (2013) Association Between Variants in or Near PNPLA3, GCKR, and PPP1R3B With Ultrasound-Defined Steatosis Based on Data From the Third National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol 2013 Feb 13. doi:pii: S1542-3565(13)00188-2. 10.1016/j.cgh.2013.02.011 [Epub ahead of print] PubMed PMID: 23416328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Diabetes Association (2000) Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. American Diabetes Association: Clinical Practice Recommendations 2000 Committee Report, Diabetes Care 23: S4–19. [PubMed] [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 25. Ikeda Y, Suehiro T, Nakamura T, Kumon Y, Hashimoto K (2001) Clinical significance of the insulin resistance index as assessed by homeostasis model assessment. Endocr J 48: 81–86. [DOI] [PubMed] [Google Scholar]
- 26.University of Oxford website. Available at: Diabetes Trials Unit,University of Oxfor, at http://www.dtu.ox.ac.uk/homa. Accessed 2013 December 4.
- 27. Colloredo G, Guido M, Sonzogni A, Leandro G (2003) Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol 39: 239–244. [DOI] [PubMed] [Google Scholar]
- 28. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, et al. (2005) Nonalcoholic Steatohepatitis Clinical Research Network, Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 411: 313–321. [DOI] [PubMed] [Google Scholar]
- 29.SAS Technical Report (1992) SAS/STAT software: changes and enhancement, Release 6.07. Cary, NC: SAS Institute, Inc.
- 30. Anstee Q, Darlay R, Leathart J, Liu Y, Tordjman J, et al. (2013) A CANDIDATE-GENE APPROACH TO VALIDATION OF GENETIC MODIFIER ASSOCIATIONS USING A LARGE COHORT WITH HISTOLOGICALLY CHARACTERISED NON-ALCOHOLIC FATTY LIVER DISEASE. Journal of Hepatology 58: S46, 104. [Google Scholar]
- 31. Tan HL, Zain SM, Mohamed R, Rampal S, Chin KF, et al. (2013) Association of glucokinase regulatory gene polymorphisms with risk and severity of non-alcoholic fatty liver disease: an interaction study with adiponutrin gene. J Gastroenterol 2013 Jun 26. [Epub ahead of print] PubMed PMID: 23800943. [DOI] [PubMed] [Google Scholar]
- 32. Peter A, Stefan N, Cegan A, Walenta M, Wagner S, et al. (2011) Hepatic glucokinase expression is associated with lipogenesis and fatty liver in humans. JCEM 96: e1126–30. [DOI] [PubMed] [Google Scholar]
- 33. Bechmann LP, Gastaldelli A, Vetter D, Patman GL, Pascoe L, et al. (2012) Glucokinase links Krüppel-like factor 6 to the regulation of hepatic insulin sensitivity in nonalcoholic fatty liver disease. Hepatology 55: 1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PR, et al. (2011) The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Gene 18: 4081–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang Z, Wen J, Tao X, Lu B, Du Y, et al. (2011) Genetic variation in the GCKR gene is associated with nonalcoholic fatty liver disease in Chinese people. Mol Biol Rep 38: 1145–1150. [DOI] [PubMed] [Google Scholar]