Abstract
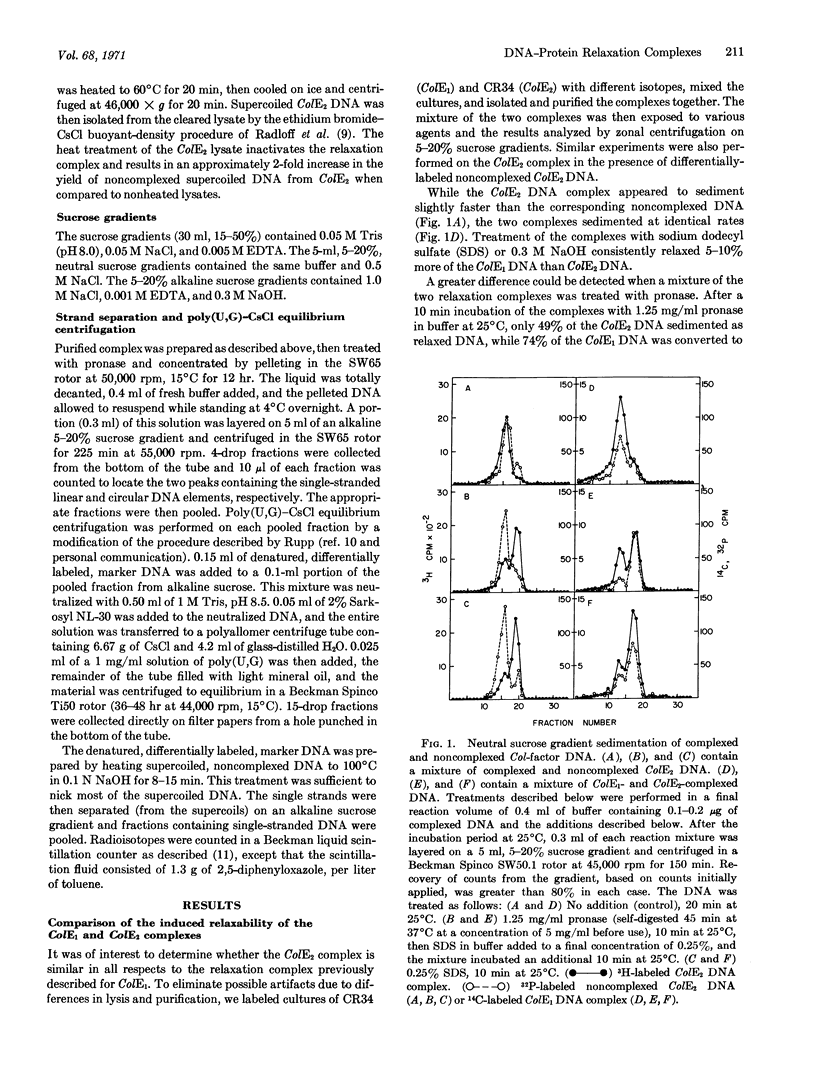
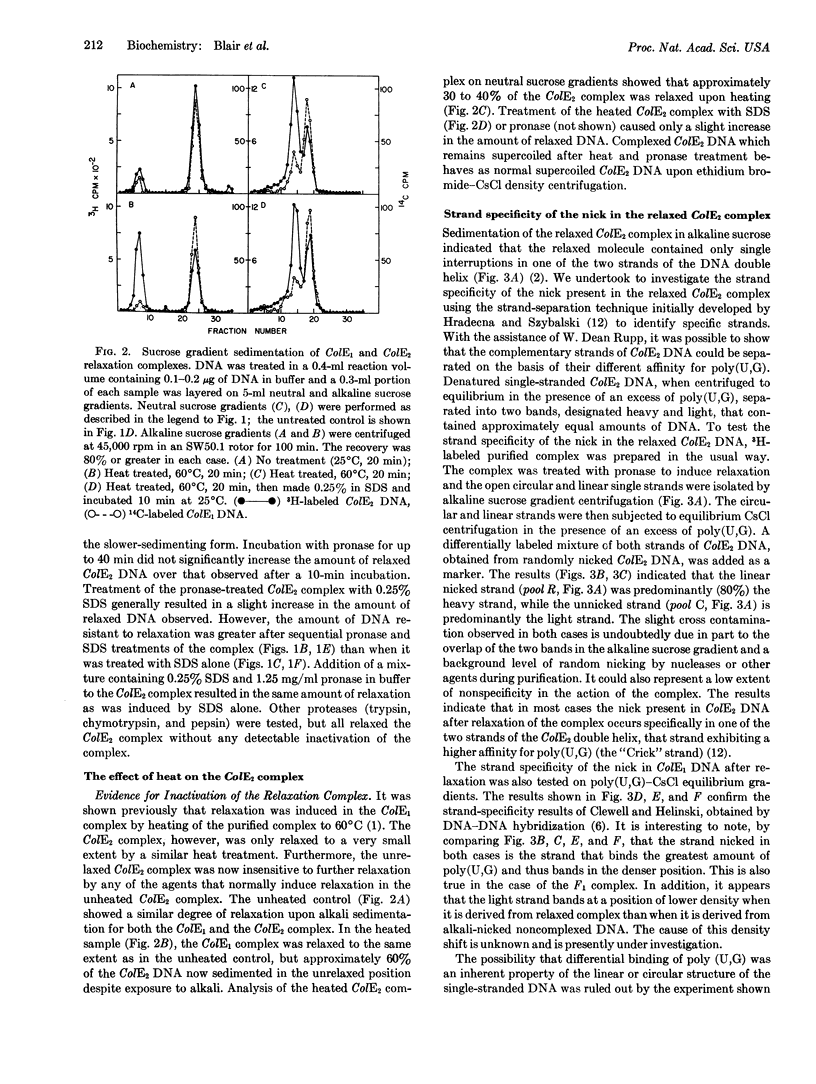
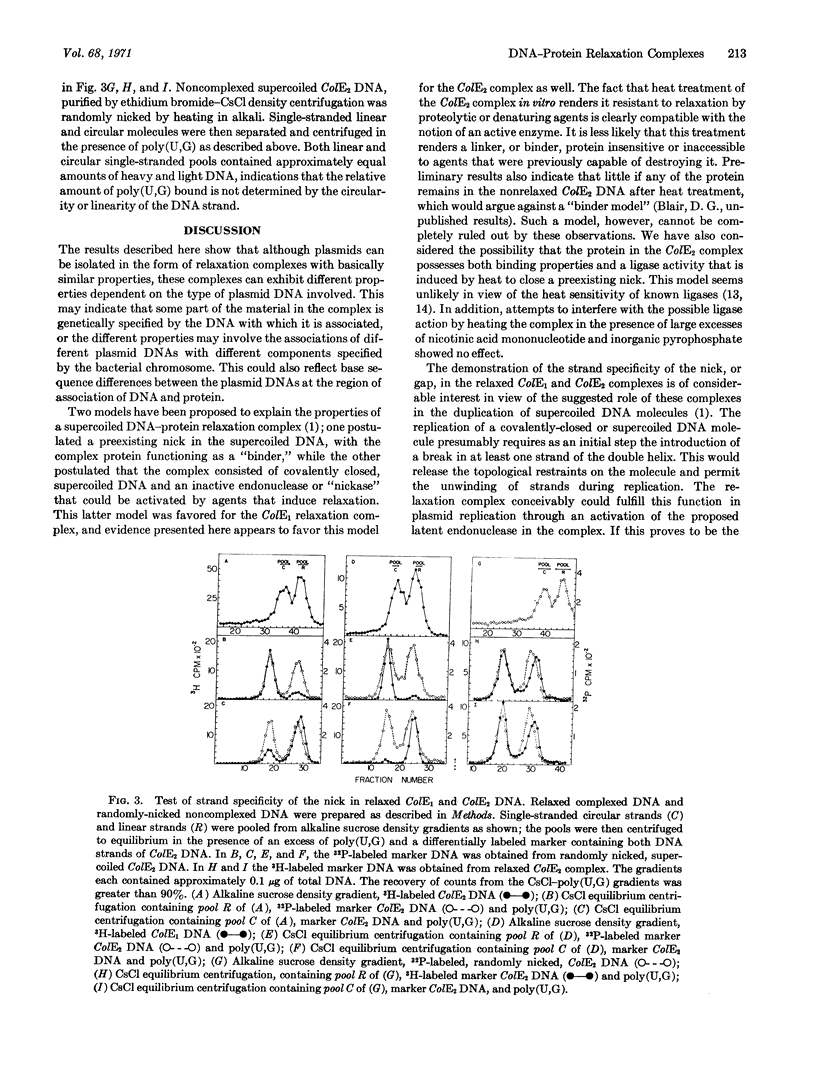
Certain bacterial plasmids can be isolated as unique complexes of supercoiled circular DNA and protein. These complexes are distinguished by the conversion of the supercoiled DNA to the relaxed or open-circular DNA form upon treatment with ionic detergents, proteases, or alkali. This report demonstrates that the open-circular DNA resulting from the pronase-induced relaxation of the complexes of colicinogenic factors E1 (ColE1) and E2 (ColE2) possesses a strand-specific break. In each case this break is found in the heavy strand of the DNA as defined by CsCl centrifugation in the presence of poly(U,G). In addition, the ColE1 and ColE2 complexes exhibit certain properties that are plasmid specific. Heat treatment, and to a lesser extent pronase treatment, inactivates the ColE2 complex, making it insensitive to agents that formerly were capable of inducing relaxation (conversion of the DNA to the open-circular form). In contrast, the ColE1 complex is not inactivated by these treatments. The potential role of these strand-specific relaxation complexes in DNA replication is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clewell D. B., Helinski D. R. Evidence for the existence of the colicinogenic factors E2 and E3 as supercoiled circular DNA-protein relaxation complexes. Biochem Biophys Res Commun. 1970 Aug 11;40(3):608–613. doi: 10.1016/0006-291x(70)90947-2. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. Formation of covalent circles of lambda DNA by E. coli extracts. Proc Natl Acad Sci U S A. 1967 Jan;57(1):148–155. doi: 10.1073/pnas.57.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Hickson F. T., Roth T. F., Helinski D. R. Circular DNA forms of a bacterial sex factor. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1731–1738. doi: 10.1073/pnas.58.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH S. M., OZEKI H., STOCKER B. A. TRANSFER OF COLE1 AND COLE2 DURING HIGH-FREQUENCY TRANSMISSION OF COLI IN SALMONELLA TYPHIMURIUM. J Gen Microbiol. 1963 Nov;33:231–242. doi: 10.1099/00221287-33-2-231. [DOI] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]


