Abstract
Lymphoma patients treated with autologous transplantation (ASCT) live an increasingly long life with the recent advancement in therapeutic modalities. This has resulted in an increase in the incidence of therapy-related myeloid neoplasms (t-MN), which is one of the leading causes of non-relapse mortality. Several observational studies have linked the development of t-MN after ASCT with the intensity and frequency of chemotherapy, particularly alkylating agents, use of total body irradiation (TBI), and peripheral blood progenitor cells. In addition, role of genetic factors is increasingly being identified. It is postulated that the use of chemotherapy prior to ASCT results in DNA damage of progenitor cells, mitochondrial dysfunction, and altered gene expression related to DNA repair, metabolism as well as hematopoietic regulation. Cytogenetic studies have shown the presence of abnormalities in the peripheral blood progenitor cells prior to ASCT. It is, therefore, likely that the reinfusion of peripheral blood progenitor cells, proliferative stress on infused progenitor cells during hematopoietic regeneration and associated telomere shortening ultimately result in clonal hematopoiesis and blastic transformation. Cytopenias, myelodysplasia, or cytogenetic abnormalities are common and can be transient after ASCT; therefore, only when present together, they do confirm the diagnosis of t-MN. Attempts to reduce the occurrence of t-MN should be directed toward minimizing the exposure to the identified risk factors. Although the median survival is few months to less than a year, studies have shown the promising role of allogeneic transplantation in select young t-MN patients without high-risk cytogenetics. In this review we will explain the recent findings in the field of t-MN in lymphoma patients that have implications for identifying the molecular and genetic mechanisms of leukemogenesis and discuss potential strategies to reduce the risk of t-MN in this patient population.
Keywords: therapy-related myeloid neoplasms, myelodysplasia, acute myeloid leukemia, autologous hematopoietic stem cell transplantation, Hodgkin lymphoma, non-Hodgkin lymphoma
Introduction
High-dose chemotherapy and autologous hematopoietic stem cell transplantation (ASCT) is a well-established treatment option for patients with high-risk Hodgkin lymphoma and non-Hodgkin lymphoma (NHL). With the addition of rituximab, high-dose chemotherapy, and ASCT is shown to further improve long-term outcomes.1,2 Therapy-related myeloid neoplasms (t-MN) are recognized complications of ASCT in lymphoma patients, and a leading cause of nonrelapse-related mortality (NRM)3-8 with a study showing 44% of death in remission due to t-MN.8 There is evidence that the relative risk of developing t-MN after conventional therapy with or without high-dose chemotherapy followed by ASCT for Hodgkin lymphoma is 13–19 when compared with the general population.9,10 The incidence of t-MN varies, likely as the result of exposure to the risk factors, the length of follow-up and the statistical methodology used, ranging from 1% in 30 mo4 to 11.7% in 6 y11 in different studies and continues to rise for as long as 12–15 y after ASCT8,12,13 (Table 1). Increasing use of high-dose chemotherapy and ASCT in lymphoma with improved long-term survival is likely to translate into a rising incidence of t-MN in the future.
Table 1. Characteristics of t-MN patients: Review of recent literature.
| First author | Number of patient/primary disease | Conditioning regimen | Incidence/median follow-up | Time to diagnosis from first chemotherapy regimen/diagnosis of lymphoma | Time to diagnosis from ASCT | Median survival from diagnosis of t-MN | Risk factors | Important cytogenetics | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| Waterman20 | 6/171 Follicular lymphoma 4 MDS, 2 AML |
Bu/VP-16/Cy | 3.5%, 57.9 mo | NA | NA | NA | Number of chemotherapy regimenbs, >5 d of leukapheresis required for stem cell harvest, fludarabine | Normal karyotype, complex kayotype, −7 | Incidence increases with duration |
| Kalaycio21 | 20/526 HL and NHL | Bu/VP-16/Cy | 3.8%, 69 mo | NA | NA | NA | Prior radiation therapy, ≥ 4 chemotherapy regimens , and > 5 d of apharesis | Monosomy 7, complex karyotype, 11q23 | |
| Forrest9 | 4/202 HL | CBV ± P | 1.9%, 7.8 y | 7 y (3.3–14.7 y) | 3.9 y (2.1–4.2 y) | 5.5 mo for entire cohort including patients who received conventional therapy | Age ≥ 35 y and stage III/IV at diagnosis of hodgkin’s disease | NA | ASCT does not increase the risk of t-MN compared with conventional therapy |
| Metayer18 | 19/955 HL 37/1784 NHL 46 MDS, 10 AML |
TBI + Cy; TBI + VP-16 ± Cy; VP-16 ± Cy ± other drugs | 2.0%, 21 mo (<1 mo–8 y) | NA | 2.5 y (3 mo–7 y) | 2 mo (<1 mo–2 y) after t-MN and 8 mo (<1 mo–6 y)after t-MN | Intensity of pretransplantation chemotherapy with mechlorethamine or chlorambucil compared with cyclophosphamide-based therapy and Total-body irradiation (TBI) doses of 13.2 Gy compared with ≤ 12 Gy | Del 5 and/or 7, abnormalities involving chromosomes 1, 6, 8, 10, or 11; balanced translocations 11q23 |
Peripheral blood stem cells associated with a nonsignificant increased risk compared with bone marrow grafts |
| Beauchamp-Nicoud A44 | 1/15 HL 5/104 NHL (1/48 Myeloma) |
HD-CT alone or followed by TBI or Bu | 4.2%, 7 y | 47 mo (22–182 mo) | 17 mo (3–61 mo) | 6.7 mo (2–12 mo) among 4 pt who died | NA | Complex karyotype, %q−, 7q−, +8, 20q−, t(3;21)(q26;q22) | Marked reduction in telomere length during the evolution of cytogenetic abnormalities, myelodysplasia and acute leukemia |
| Howe17 | 5/64 HL 5/166 NHL 8MDS, 2 AML |
BEAC: CBV; TBI + Cy | 4.3%, 69 (36–177) mo | 75 (36–192) mo | 40 (12–63) mo | 7 (0.5–40) mo | Male gender, prior alkylating agents (cyclophosphamide for NHL and mechlorethamine for HL) and the number of prior therapies | Complex cytogenetics including abnormalities of 5 and 7 | |
| Sevilla16 | 2/34 HL 2/43 NHL (2/22 MM) |
BEAC; Bu + Cy; Bu + Mel | 6.1%, about 2.4 y | 34 (16–104) mo | 19 (6–66) mo | Mean of 7.6 (1–15.2) mo except one patient who was alive | Number of chemotherapy cycles pre-ASCT, and total dose of mononuclear cells infused at ASCT | Normal karyotype; trisomies and monosomies | Total dose of cyclophosphamide showed trend toward increased risk (P = 0.099) |
| Hosing43 | 22/493 NHL | TBI-based regimen; chemotherapy alone | 4.5%, 3+ y | 5.9 y (6 mo-14.5 y) | 30 (3–61) mo | 7.5 (0–32) mo | TBI, especially in combination with cyclophosphamide and etoposide | Abnormalities of 5 and 7; complex karyotype | Other possible risk factors: prior fludarabine and bone marrow involvement by lymphoma by univariate analysis |
| Micallef11 | 27/230 NHL 16 MDS, 10 MDS evolving into AML and 1 AML |
TBI + Cy | 11.7%, 6 y | 9.9 y (2.7–21.6 y) after NHL diagnosis | 11 mo to 8.8 y (4.4 y) | 10 mo (0–26 mo) | Prior fludarabine therapy, older age; borderline significance, increased interval from diagnosis to ASCT, bone marrow involvement at diagnosis | Complex keyotype, monosomy 5/5q−, −7/7q−, −18/18q−, −13/13q−,−20/20q− | |
| Del Canizo4 | 6/225 HL 5/308 NHL 1/557 acute leukemia 1/189 MM |
BEAM; CBV; TBI + Cy; BEM | 1.0%, About 30 mo | 69 (29–170) mo | 28 (1.5–63) mo | 13 mo | Doses of alkylating agents | Abnormalities of 7 and 5 as well as 6, 12, 17 | The time between ASCT and t-MN was longer for patients who received bone marrow grafts vs PBPC, whereas the time from the beginning of the treatment of underlying disease was similar |
| Park90 | 3/96 HL 8/299 NHL |
CBV; BEAM; BEAC; TBI + Cy + VP-16 | 2.8%, 3 y | 71 mo | 32 mo | 12 (1–72+) mo | NA | Normal in 2, one complex with t(8;16) and another t(8;21) | More frequent in heavily pretreated patients (relapse > 1), refractory disease and with TBI (unclear if statistically significant or not) |
| Krishnan36 | 11/218 HL 11/394 NHL 16 MDS, 6 AML |
TBI + Cy + VP-16; CBV | 3.6%, 1.6+ y | 3.8 y | 1.9 y | Actuarial survival 6 (1–24) mo | Stem-cell priming with VP-16 and pre-ASCT radiotherapy among patients who did not receive VP-16 priming | Abnormalities of 5 and 7; 11q23 or 21q22 |
ASCT, autologous stem cell transplantation; BEAC, BCNU, VP-16, cytarabine, cyclophosphamide; Mel, melphalan; BEAM, BCNU, VP-16, cytarabine, melphalan; BEM, BCNU, VP-16, melphalan; Bu, busulphan; CBV ± P, cyclophosphamide, BCNU, VP-16 ± cisplatin; Cy, cyclophosphamide; HD-CT, high-dose chemotherapy; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; TBI, total body irradiation; t-MN, therapy-related myeloid neoplasms.
Risk Factors
Several studies have attempted to identify the risk factors associated with t-MN after ASCT in lymphoma patients; however, these studies are limited by their observational design and relatively small number of t-MN patients (Table 1). In general, therapies given prior to ASCT, transplantation conditioning regimens, and factors related to the stem cell harvesting are considered the major risk factors.14,15
Pre-transplantation therapy
As a part of the combination chemotherapy regimens, lymphoma patients are exposed to multiple cycles of alkylating agents as well as topoisomerase II inhibitors before ASCT, both of which are well-established to cause t-MN. Studies among transplanted lymphoma patients have shown that the intensity and the frequency of chemotherapy16-20 as well as the use of alkylating agents such as mechlorethamine, chlorambucil, cyclophosphamide, lomustine,4,5,14,17-19 or MOPP regimen19 are important risk factors. In one study, the use of 4 or more chemotherapy regimens was shown to be an independent risk factor.21 In another study, the number of cycles of mechlorethamine and cyclophosphamide, but not of procarbazine, cisplatin, or etoposide, was shown to have significant association with t-MN.17 Exposure to fluadarabine has also been identified to be a risk factor for t-MN in general22,23 as well as in lymphoma patients after ASCT.11,20,24 In follicular lymphoma, fluadarabine at any dose increases the risk for t-MN, the greatest risk being among patients exposed to a dose >500 mg/m2 whereas a cumulative dose of >150 mg/m2 also increases the risk for poor stem cell harvests.20 There have been cases of t-MN reported in association with bendamustine as well, however, the role of bendamustine in causation of t-MN is unclear and t-MN developed in a non-transplant setting.25
In an attempt to determine the extent to which prior chemotherapy vs. ASCT contributes to t-MN, a British cooperative study compared the risk of t-MN among transplanted (n = 595) vs. non-transplanted (n = 3981) Hodgkin lymphoma patients. The risk of t-MN was predominately affected by the quantity of prior therapy (P < 0.0001), exposure to MOPP (P = 0.0009) or lomustine chemotherapy (P = 0.001). After adjusting for these factors, there was no increased risk associated with transplantation (P = 0.25). Therefore, they concluded that BEAM (BCNU, etoposide, Ara-C, and melphalan) therapy and ASCT in Hodgkin lymphoma did not significantly increase the risk of t-MN.19 This study indicates that the majority of t-MN following high-dose chemotherapy and ASCT are likely the result of previous chemotherapy and most likely prior to ASCT; and the ASCT procedure itself plays a minor role. Importantly; however, in this study, the overall incidence of t-MN was less than some other studies.3,26 The difference may be explained by the fact that the majority of the patients received conditioning with BEAM chemotherapy and very few TBI (0.3%), which is one of the recognized risk factors for development of t-MN.19 Furthermore, the relatively low incidence may reflect the low leukemogenic potential of the BEAM regimen.27 The result, therefore, may not be generalizable to t-MN developing after conditioning with other high dose chemotherapy regimens such as cyclophosphamide/busulfan and TBI. A different study comparing the incidence of t-MN between transplanted and non-transplanted patients reached the same conclusion showing no difference in the incidence of t-MN. However, this study had the same limitations.28 A third study also concluded that ASCT does not increase the risk of t-MN compared with conventional therapy. In this study, although the details of are not mentioned specifically for patients who developed t-MN, only 1 out of 202 Hodgkin lymphoma patients received TBI-based or BEAM conditioning regimen. The majority received cyclophosphamide, carmustine, etoposide with or without cisplatin (n = 197) as conditioning regimen.9 These studies suggest that prior chemotherapy may play important role in the development of t-MN.
There are a number of indirect evidence supporting the role of prior chemotherapy in the development of t-MN. The number of relapses, which is considered to be reflective of the number of the cycles of chemotherapy administered, is associated with an increased risk. There is evidence supporting the correlation that ASCT without much chemotherapy or without the use of alkylating agents is associated with little-to-no development of secondary malignancies.5 The total risk has been significantly decreased since reducing the use of mechlorethamine and procarbazine.29 Allogeneic hematopoietic stem cell transplantation results in much lesser development of t-MN,4,30 which, could be because the donor progenitor cells have not been treated with chemotherapy or alkylating agents.4 Latency period for the development of t-MN after stem cell transplant, although variable, can be as brief as 1.5–6 mo (Table 1). Short latency period and presumed low leukemogenic potential of high-dose BEAM chemotherapy has led to the consideration that t-MN arising after BEAM and ASCT can originate from the pre-ASCT damage to the hematopoietic progenitor cells utilized in ASCT and hence pre-ASCT therapy play important role.27 In addition there are reports showing that cytogenetic abnormalities31-33 and dysplastic morphological changes34 have been noted even before transplantation, although not consistently,35,36 thus suggesting the role of pre-ASCT therapy in causation of t-MN. In a case series, patients with Hodgkin lymphoma were found to have clonal abnormality before bone marrow harvest, thus supporting the notion that antecedent chemotherapy prior to ASCT plays an important role in the development of t-MN.33 In fact, the cytogenetic abnormalities identified at the time of t-MN have been shown to be present before harvesting the stem cells for ASCT.32 Furthermore, abnormalities of chromosomes 5 and 7, characteristics of t-MN developing after the use of alkylating agents, are quite common among patients who develop t-MN post-ASCT (Table 1). These findings together support the idea that the prior use of intensive chemotherapy and alkylating agents play an important role in leukemogenesis.
Few studies have shown an increased risk of t-MN associated with local, subtotal radiation therapy prior to ASCT,8,21,36,37 pelvic irradiation,37 total lymph node irradiation, and low-dose TBI,38,39 particularly in combination with alkylating agents.40 However, the role of prior local radiation therapy in the causation of t-MN has been a matter of controversy and is generally considered small.41
Conditioning regimens
Several studies have identified TBI as a risk factor for t-MN.26,42,43 In St Bartholomew’s Hospital in London, the United Kingdom, high incidence of t-MN (12% at a median follow-up of 6 y) was reported with the use of TBI as a part of the conditioning regimen, however, no patient developed t-MN after the discontinuation of TBI in 1996.11 On the other hand, one study did not show any association with TBI,36 whereas another study showed that the risk might be dose-related; TBI at doses 12 Gy or less did not pose a risk whereas increased risk was seen for doses of 13.2 Gy.18 In general, TBI is accepted as a risk factor for t-MN occurring after ASCT.5,14,41
Stem cell harvesting
Several studies have shown that ASCT using peripheral blood progenitor cells have an increased risk of t-MN compared with ASCT using bone marrow cells.3,4,6,44,45 It is postulated that peripheral blood progenitor cells are harvested before the repair of chemotherapy-induced DNA damage or they are contaminated by “preleukemic” cells with DNA damage,5 which results in increased risk. The process of ASCT in general can also presumably result in the damage to the microenvironment or the stem cells during the phases of mobilization, collection, and storage,46 thus contributing to an increased risk. The rarity of t-MN after allogeneic SCT, although often attributed to the exposure of prior therapy, could also be reflective of these differences. Additionally, it has been shown that myelodysplasia34 as well as cytogenetic abnormalities47 after ASCT can be transient. Furthermore, new cytogenetic abnormalities developed after ASCT do not necessarily confer a poor prognosis for the development of t-MN for as long as 30 mo.48 On the same note, it seems reasonable to presume that the cytogenetic abnormalities and myelodysplasia seen pre-ASCT could be transient and not necessarily predictive of future t-MN.
It has been shown in a study that the cytogenetic abnormalities identified at the time of t-MN were detected in the stem cells prior to high dose chemotherapy, which supports the hypothesis that the reinfusion of previously damaged stem cells could account for t-MN.32 In a different study, a subgroup of patients received two courses of high dose chemotherapy, each course followed by peripheral blood progenitor cells harvesting. It was shown that the use of the second harvest of peripheral blood progenitor cells was a significant risk factor for t-MN. It was postulated that the second HDCT likely caused additional damage to the harvested cells.13 The second harvest has been previously shown to correlate with the shortening of telomere in PBPC.49 These findings might suggest that telomere shortening of peripheral blood progenitor cells, shown to increase the risk and precede the development of t-MN,50 is the underlying mechanism for increased risk of t-MN.
Several other factors related to stem cell harvesting have been shown to be associated with increased risk. In different studies, difficulty in harvesting stem cells defined as more than 5 d of apheresis required for stem cell harvest,20,21 as well as the dose of mononuclear cells infused at the time of ASCT16 were shown to correlate with an increased risk of t-MN. In a recent study, the high cumulative incidence of t-MN (n = 5, 17% at 42 mo follow-up) was seen among patients with hematological malignancies who underwent stem cell mobilization using plerixafor and granulocyte colony-stimulating factor because of one or more previous failed attempts at mobilization, thus questioning the contribution of plerixafor in development of t-MN.51 However, these factors indicative of marrow damage likely reflect the extent of chemotherapy administered before ASCT.
Role of genetic factors
The roles of genetic factors in the development of t-MN are increasingly being identified. X-ray repair cross-complementing 1 (XRCC1) gene is critically important in DNA repair especially the repair of single-strand breaks,52 and it displays single nucleotide polymorphisms (SNP) with the mutant A allele (AA/GA), which is associated with reduced ability to DNA repair.53,54 In a recent study, XRCC1 genotype was shown to predict the risk of t-MN after ASCT with a 4.5-fold increased likelihood of developing t-MN among carriers of the mutant A allele compared with patients with wild-type genotype. Furthermore, XRCC1 genotype also correlated with the overall survival among lymphoma patients undergoing ASCT, which might be partly related to an increased risk of t-MN.55 In another study among lymphoma patients treated with conventional therapy and ASCT (n = 49), researchers genotyped 29 single nucleotide polymorphisms (SNP) and 2 deletion polymorphisms for 23 candidate genes. Patients with deletion of glutathione S-transferase mu 1 (GSTM1), the Pro allele of proline 72 arginine (P72R) in TP53, the T allele of CYP1A1*2A and the T allele of rs6030469 of protein tyrosine phosphatase, receptor type (PTPRT) were found to have increased risk of t-MN. Furthermore, a synergistic effect between TP53 and methylenetetrahydrofolate reductase (MTHFR) was also noted. These findings were confirmed in expression analyses for GSTM1, TP53, and MTHFR. These genes are responsible for drug metabolism, DNA repair, and apoptosis, thus supporting the role of genetic susceptibility to t-MN.56 Recent studies have also identified mitochondrial dysfunction (based on gene-expression or metabolomic analysis) in peripheral blood stem cells obtained pre-ASCT among patients developed t-MN months to years later. Mitochondrial dysfunction results in reduced ability to detoxify reactive oxygen species, thus promoting DNA mutation.57,58 In addition, microarray analysis of gene expression profiles of CD34+ stem cells isolated from peripheral blood stem cells have also demonstrated altered gene expression related to metabolism and hematopoietic regulation. All these changes may be related to the therapeutic interventions prior to ASCT and/or may represent predisposing factors for t-MN. Furthermore, an optimal 38-gene peripheral blood stem cell classifier has been shown to distinguish patients who do or do not develop t-MN.58
Miscellaneous factors
In addition to above, age at ASCT,5,8,11,42,59 the length between the primary diagnosis and ASCT,5,42,59 male gender,13,17 number of transplants,42 disease status at ASCT,24 age ≥35 y, and stage III/IV at diagnosis of Hodgkin lymphoma9 are other risk factors. Although not very clear, men may be more affected due to the hormonal effects, such as a hormonal-dependent activation of the telomerase reverse transcriptase (TERT) gene and consequent changes in telomerase activity. This is suggested by the findings from a recent study that showed both androgens and estrogens can stimulate telomerase activity; however, androgens likely get converted to estrogens and acts via estrogen-receptor to mediate telomerase activation.60
A proportion of t-MN may occur independent of the therapy. In a study of AML occurring as a second malignancy, 41 out of 127 cases occurred in patients who were treated with surgery alone; the rest of the patients received chemotherapy (n = 34), radiation (n = 20), or chemoradiotherapy (n = 32). Furthermore, first-degree relatives of the patients with AML as second malignancy were more likely to have a malignancy in their lifetime compared with patients with de novo AML (36.9% vs. 27.2%, P < 0.005).61 This study indicates that AML as a second malignancy is not always a result of prior therapy, rather the genetic predisposition in conjunction with environmental factors might also play a role.
Pathobiology of the Disease
The development of the t-MN among lymphoma patients after high dose chemotherapy and ASCT is a complex phenomenon and presumably a multi-step process (Fig. 1). As discussed in the section on risk factors, exposure to chemotherapy and radiation results in DNA damage, mitochondrial dysfunction, and alteration in cellular metabolism and dysregulation of normal hematopoietic progenitor cells. High dose chemotherapy and TBI can cause similar cellular and genetic damages as triggered by the pre-transplant therapy and provides another opportunity for causing damage to the hematopoietic progenitor cells. Reinfusion of peripheral blood progenitor cells with previously damaged DNA results in clonal abnormal hematopoiesis. Proliferative stress on infused peripheral blood progenitor cells induces telomere shortening, genetic instability, and blastic evolution to AML.
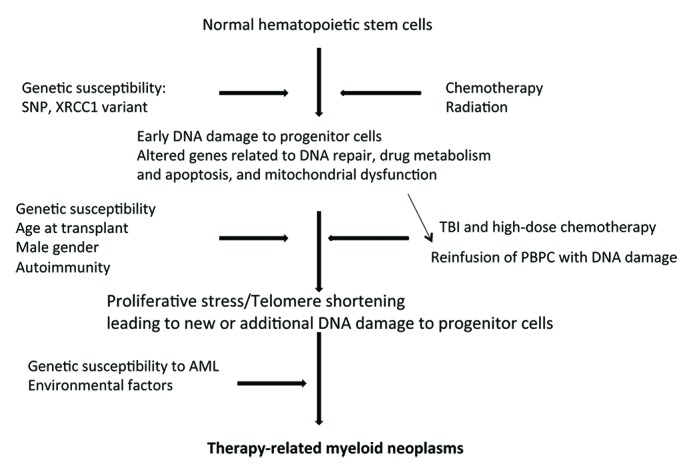
Figure 1. Pathobiology of therapy-related myeloid neoplasms. SNP, single nucleotide polymorphism; PBPC, peripheral blood progenitor cells; TBI, total body irradiation; XRCC1, X-ray repair cross-complementing 1.
Telomere shortening has been associated with the development of t-MN. The extent of telomere shortening correlates with the number of the somatic divisions.62 There is evidence that a significant reduction in hematopoietic primitive and committed progenitor cells can be seen prior to ASCT with further profound and persistent reduction in primitive progenitor cells after ASCT. Thus, increased proliferation and differentiation of primitive progenitor cells is required for hematopoietic regeneration after ASCT. Additionally, as one may expect, a shortening of telomere length during early post-transplant period with subsequent recovery to the pre-transplant level is seen. However, the recovery of committed progenitor cells and telomere length is poorer in patients who develop t-MN.46 In other studies, telomere shortening has been shown to occur before ASCT because of prior chemotherapy63,64 and older age63 as well as after ASCT.65 Previous studies have also shown the role of telomere shortening in limiting the number of cell divisions, as well as in senescence, genetic instability, and clonal disorders.65-67 The above-mentioned associations have been shown in a study which found a marked reduction in telomere length in post-ASCT lymphoma patients during the evolution of cytogenetic abnormalities, myelodysplasia, and acute leukemia.44 Thus, it can be concluded that the proliferative stress during hematopoietic regeneration leads to telomere shortening with poor recovery in certain patients, which can contribute to genetic instability, cytogenetic abnormalities, and ultimately the development of t-MN. Cytogenetic abnormalities may be present in stem cells harvested for ASCT.31-33 Infusion of peripheral blood progenitor cells with cytogenetic abnormalities can be presumed to undergo faster telomere shortening and blastic evolution when forced to rapidly proliferate after engraftment and hematopoietic regeneration.
A possible role of autoimmunity has also been suggested in the pathogenesis of t-MN after ASCT. Occurring around the time of engraftment, engraftment syndrome is characterized by the presence of fever, skin rash, capillary leak, and/or pulmonary infiltrate.68 Graft-vs.-host-disease (GVHD)-like skin rash has also been described after ASCT, which is indistinguishable from GVHD rash post-allogeneic transplantation.69 In a study, occurrence of engraftment syndrome/autologous GVHD was shown to be an independent risk factor for t-MN. The authors hypothesize that immune disturbance from prior chemotherapy or conditioning regimen might play a role in the development of t-MN post-ASCT in the elderly patients.59 The possible role of immune disturbance in t-MN is supported by previous studies showing a potential role of autoimmune mechanisms in the pathogenesis of MDS in general,70,71 the association of MDS with autoimmune conditions72 and the role of immunosuppressive therapy in the management of MDS.73,74
Morphological and Cytogenetics Features
Therapy-related myeloid neoplasms after ASCT present a unique diagnostic challenge. Post-transplant lymphoma patients can often have hypoplastic, fibrotic or dysplastic bone marrow even in the absence of t-MN.5 In a study, concurrent cytopenia and dysplastic morphological changes were noted in 37% of patients at 6 mo after ASCT and 25% at 12 mo, thus highlighting their transient nature and the limitations of morphological diagnostic criteria for MDS.34 Similar findings were noted in another study.75 On the other hand, patients can also develop clonal karyotypic abnormalities in bone marrow without clinical MDS.11,59,76 Furthermore, these cytogenetic abnormalities can be transient.47 Interestingly, in a study among de novo AML patients undergoing ASCT, the patients were detected to have new cytogenetic abnormalities after transplantation which did not confer a poor prognosis for the development of t-MN until 30 mo after the detection of cytogenetic abnormalities.48 Therefore, cytopenia and clonal chromosome abnormalities, in addition to refractory cytopenia and dysplastic morphological changes, are needed to establish the diagnosis of t-MN after ASCT.
In a review of published literature, the incidence of t-MDS (n = 132) was more common than t-AML after ASCT (n = 36). Monosomy of chromosomes 5 or 7 as well as deletion of 5q or 7q were the most common cytogenetic abnormalities noted.5 These features, characteristics of t-MN seen after the use of alkylating agents,77 confirm the role of prior exposure to alkylating agents. Approximately 14% of patients had translocations to chromosome bands 11q23 or 21q22 with the majority being exposed to topoisomerase II inhibitors. A larger portion of the patients had complex karyotype whereas only a small number of patients had normal karyotype.5 Our review showed similar findings (Table 1). Additionally, in other studies, abnormalities in chromosome 12 have also been noted3,4,26,31,43,76 including breakpoint on 12p13 in one study.4 12p region is remarkable for the location of genes which are related to hematological malignancies including MDS and AML.78 Recently, two cases of t-MN with derivative (1;7)(q10;p10) have been described after ASCT who displayed a stable disease with supportive therapy only at 36 and 48 mo after the diagnosis, thus indicating the possibility of indolent course with this subtype of t-MN.79 Also, balanced translocations involving 1p36, as a part of complex cytogenetics,31 and that involving 3q2644 have also been noted. The balanced translocation involving 1p36 and 3q26 occurring as the sole anomaly has its own unique features including a poor prognosis.80
Risk Reduction
Although there is a paucity of literature examining through which strategies we can prevent the occurrence of t-MN, based on the identified risk factors, certain suggestions can be made. Multiple cycles of chemotherapy especially alkylating agents are one of the most important risk factors. Therefore, upfront ASCT in first complete remission can be considered a better option for high-risk patients to reduce the amount of chemotherapy administered.33 In certain cases, this may also improve the outcome of the primary disease. Second, where possible, reducing the exposure to alkylating agents and fludarabine may be beneficial. Since exposure to TBI is another risk factor, avoiding TBI in conditioning regimen can reduce the probability of t-MN.41 BEAM conditioning regimen is likely of low leukemogenic potential, so it might be prudent to consider its use instead of cyclophosphamide/TBI conditioning regimen.19,27,81 Few studies have indicated that there might be cytogenetic abnormalities in the peripheral blood progenitor cells; hence standard cytogenetics and fluorescent in situ hybridization (FISH) on the infusion product before ASCT particularly in heavily pretreated patients might help detect these malignant clones.4,33 Therefore, a pre-transplant bone marrow aspiration and biopsy in order to have a morphological and cytogenetic examination in heavily treated lymphoma patients can potentially play an important role in reducing risk of t-MN.33
A study among 66 patients undergoing peripheral blood progenitor cells ASCT followed for a median of 25 mo found no cases of t-MN. It is interesting to observe that these patients did not have exposure to certain risk factors (Table 2). The conditioning regimen included chemotherapy without TBI; patients were treated with ≤2 chemotherapy lines with local radiotherapy as indicated; patients with Hodgkin lymphoma did not receive MOPP regimen routinely; patients were transplanted in complete or partial remission; and there were no cytogenetic abnormalities and myelodysplasia at the time of transplantation analysis.47 Although the absence of t-MN could be the result of small sample size or relatively short duration of follow-up, this study indicates that avoidance of the risk factors might help reduce risk of t-MN.
Table 2. Major risk factors of t-MN: Review of recent literature.
| Variables | Associated risk | Type of Analysis | Source |
|---|---|---|---|
| Pre-transplantation characteristics | RR of 2.8 with age ≥35 y | Multivariate analysis | Forrest9 |
| HR of 1.06 with older age | Multivariate analysis | Micallef11 | |
| RR of 2.6 with Stage III/IV lymphoma | Multivariate analysis | Forrest9 | |
| Pre-transplantation chemotherapy | HR of 1.7 per increase in 1 chemotherapy regimen | Univariate analysis | Waterman20 |
| HR of 13.1 with fludarabine exposure | Univariate analysis | Waterman20 | |
| HR of 3.2 with prior fludarabine therapy | Multivariate analysis | Micallef11 | |
| HR of 5.3 with prior chemotherapy regimens >4 | Multivariate analysis | Kalaycio21 | |
| RR of 2 and 4.3 respectively with <50 mg/m2 and ≥50 mg/m2 of mechlorethamine | Multivariate analysis | Metayer18 | |
| RR of 3.8 and 8.4 respectively with <10 mo and >10 mo of chlorambucil | Multivariate analysis | Metayer18 | |
| Pre-transplantation radiation | HR of 3.5 with prior radiation | Multivariate analysis | Kalaycio21 |
| RR of 3.2 with pre-transplantation radiotherapy among patients who did not receive VP-16 priming | Multivariate analysis | Krishnan36 | |
| Conditioning regimen/ASCT | HR of 18.1 with leukapheresis >5 d | Univariate analysis | Waterman20 |
| HR of 5.3 with leukapheresis >5 d | Multivariate analysis | Kalaycio21 | |
| RR of 4.6 with TBI at doses of 13.2 Gy | Multivariate analysis | Metayer18 | |
| RR of 7.7 with stem-cell priming with VP-16 | Multivariate analysis | Krishnan36 |
HR, hazard ratio; RR, relative risk; TBI, total body irradiation; t-MN, therapy-related myeloid neoplasms.
Prediction
Antecedent chemotherapy prior to ASCT plays an important role in the development of t-MN. Performing conventional karyotyping and FISH analysis prior to ASCT, therefore, may help detecting t-MN related to prior chemotherapy as well as potentially predict high risk patients.4,33,81 Given the significant association of telomere length with the development of t-MN, the measurement of telomere length after ASCT may help predicting the impending clonal abnormality and blastic transformation;44,65 however, in many patients, there might be recovery of telomere length following initial shortening during early post-ASCT period.46 X-chromosome inactivation pattern at the human androgen receptor locus has also been utilized to predict the development of t-MN;82,83 however, it can only be performed in women and is further limited by technical variability and constitutional excessive lyonization or acquired skewing in the hematopoietic system.83 Several other genetic testing as well as expression level studies have shown promising results in predicting the susceptibility to the development of t-MN in ASCT patients; these include microarray analysis of gene expression profiles of CD34+ stem cells using 38-gene peripheral blood stem cell classifier,58 single nucleotide polymorphism studies,56 XRCC1 genotyping55 and 1H-nuclear magnetic resonance (NMR)-based metabolomic analysis of cellular metabolic pathways.57 These methods require further confirmatory studies.
Treatment and Prognosis
Patients with t-MN occurring after ASCT have often received several chemotherapies and have complex karyotype. Therefore, in general, they have poor response to conventional antileukemic chemotherapy, hence allogeneic SCT is often considered the only potential curative modality for appropriate patients. In a study among predominantly young (median age 40 y) t-MN patients (n = 868) with 151 patients having received ASCT in the past, allogeneic SCT resulted in a 5-y treatment-related mortality and overall survival of 48% and 22% respectively. Age >35 y, poor-risk cytogenetics, AML not in remission at the time of transplantation, and donor type (non-sibling-related donor or mismatched unrelated donor) were found to be independent poor prognostic factors with additive adverse effect on survival. Patients with no risk factors, 1, 2, 3, and 4 risk factors showed a 5-y survival of 50%, 26%, 21%, 10%, and 4%, respectively.84 Similar risk score based on three factors: age >40 y, poor-risk cytogenetics, and AML not in remission at the time of transplantation correlated with prognosis after allogeneic transplantation in a different study among predominantly young (median age 40 y) t-MN patients (n = 461) with 7% patients having undergone ASCT previously.85 Another study showed high-risk cytogenetics, Karnofsky performance status ≤80% and disease after chemotherapy to have worse event-free survival among t-MN patients (n = 29), out of which 7 patients had undergone prior ASCT. Furthermore, non-relapse mortality was higher among patients who had received >2 lines of therapy prior to the diagnosis of t-MN and those who were transplanted >6 mo after the diagnosis of t-MN. Although transplant delay could be related to many factors such as lack of induction, therapy-induced complications, or lack of donor availability, it was also observed that patients who underwent consolidation had a trend toward higher non-relapse mortality.86 Previously, one study showed that consolidation therapy with cytarabine in de novo AML in complete remission does not offer any benefit over directly proceeding to allogeneic transplantation.87 Therefore, it can be concluded that young t-MN patients with none of the above-mentioned risk factors can significantly benefit from the allogeneic transplantation and should be offered transplantation on a timely fashion, perhaps even without waiting for consolidation. In a small study (n = 12) among patients developing relapse or secondary malignancy (lymphoma or leukemia) after ASCT for primary cancer (lymphoma, leukemia, or breast cancer), nonmyeloablative allogeneic transplantation resulted in engraftment in all recipients, reduced transplant-related toxicity, and an actuarial survival of 56% at 34 mo.88 Further studies should be conducted to evaluate this promising approach.
Patients with t-MN after ASCT have a poor prognosis with a median survival of few months to less than a year (Table 1). In a study, it was found that some t-MN patients have a shorter survival time whereas other can live for a prolonged period of time. Aggressive t-MN patients were found to have significantly more bone marrow trilineage dysplasia pre-ASCT and greater dyserythropoiesis and dysmegakaryopoiesis post-ASCT.89 As describe in previous paragraphs, risk factors such as age, cytogenetics, status of t-MN and performance status have prognostic significance.
Future Perspectives
We found only two studies registered as of March 2013 (http://clinicaltrials.gov, NCT00774046 and NCT00525746) that intend to investigate the pathogenesis and the management of t-MN occurring after chemotherapy and/or radiation. The relative rarity and the variable latency period of t-MN after ASCT make it difficult to accrue patients in any trials on t-MN after ASCT. Unfortunately, there is a lack of national and international registry to conduct large observational studies. High-quality research will be possible only by the coordinated efforts of large-volume cancer care centers within and outside the Unites States. In our institution at University of Nebraska Medical Center, we are performing exome sequencing on blood and skin samples of patients with t-MN after autologous ASCT for lymphoma to identify somatic mutations; and we have also started a retrospective analysis of the outcomes of t-MN patients who underwent allogeneic transplantation. We are hopeful that those observations would provide a better understanding of the pathobiology and management of t-MN.
Conclusions
Although observational studies have provided valuable information about several epidemiological aspects of t-MN occurring after ASCT, these studies are limited by their observational design and relatively small sample size. There is a gap in our knowledge about the extent of the contribution of pre-transplant therapies, conditioning regimens, and genetic as well as other factors in the causation of t-MN with short vs. long latency period. The presumed hypothesis about the pathogenesis requires further confirmation. The better understanding of the pathobiology can help build better strategies to reduce the development of t-MN after ASCT. Karyotyping and FISH to detect cytogenetic abnormalities, X-chromosome inactivation pattern studies at the human androgen receptor locus in women, telomere length monitoring, SNP studies, microarray analysis of gene expression profiles of stem cells, and metabolomic analysis of cellular pathways hold promising role in identifying high-risk patients and need confirmatory studies. Studies have identified poor prognostic factors and potential of good outcomes with allogeneic transplantation in select young patients; these findings when confirmed can improve the current dismal outcomes associated with the disease. It is only through the collaboration of leading cancer centers at national and international levels that a large randomized clinical trial can be conducted to answer these questions.
Acknowledgments
This work was supported in part by grants from the Department of Internal Medicine, University of Nebraska Medical Center, and Eppley Cancer Center. The authors wish to thank Carol D Cornelison for her technical assistance.
Glossary
Abbreviations:
- AML
acute myeloid leukemia
- ASCT
autologous hematopoietic stem cell transplantation
- t-MN
therapy-related myeloid neoplasm
- PBPC
peripheral blood progenitor cells
- TBI
total body irradiation
- XRCC1
X-ray repair cross-complementing 1
- FISH
fluorescent in-situ hybridization, GVHD, graft-versus-host-disease
- MDS
myelodysplastic syndrome
- MTHFR
methylenetetrahydrofolate reductase, GSTM1, glutathione S-transferase mu 1
- PTPRT
protein tyrosine phosphatase, receptor type
- P72R
proline 72 arginine
- BEAM
BCNU, etoposide, Ara-C and melphalan
- NHL
non-Hodgkin lymphoma
- MOPP
mechlorethamine, chlorambucil, cyclophosphamide, and lomustine
- HL
Hodgkin lymphoma
- NA
not available
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/26342
References
- 1.Vitolo U, Chiappella A, Angelucci E, Rossi G, Liberati AM, Cabras MG, Botto B, Ciccone G, Gaidano G, Falchi L, et al. Gruppo Italiano Multiregionale Linfomi e Leucemie (GIMURELL) Dose-dense and high-dose chemotherapy plus rituximab with autologous stem cell transplantation for primary treatment of diffuse large B-cell lymphoma with a poor prognosis: a phase II multicenter study. Haematologica. 2009;94:1250–8. doi: 10.3324/haematol.2009.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarella C, Zanni M, Magni M, Benedetti F, Patti C, Barbui T, Pileri A, Boccadoro M, Ciceri F, Gallamini A, et al. Rituximab improves the efficacy of high-dose chemotherapy with autograft for high-risk follicular and diffuse large B-cell lymphoma: a multicenter Gruppo Italiano Terapie Innnovative nei linfomi survey. J Clin Oncol. 2008;26:3166–75. doi: 10.1200/JCO.2007.14.4204. [DOI] [PubMed] [Google Scholar]
- 3.Miller JS, Arthur DC, Litz CE, Neglia JP, Miller WJ, Weisdorf DJ. Myelodysplastic syndrome after autologous bone marrow transplantation: an additional late complication of curative cancer therapy. Blood. 1994;83:3780–6. [PubMed] [Google Scholar]
- 4.Del Cañizo Mf, Amigo Mf, Hernández JM, Sanz G, Núñez R, Carreras E, Alegre A, Cuesta B, Mataix R. Incidence and characterization of secondary myelodysplastic syndromes following autologous transplantation. Haematologica. 2000;85:403–9. [PubMed] [Google Scholar]
- 5.Pedersen-Bjergaard J, Andersen MK, Christiansen DH. Therapy-related acute myeloid leukemia and myelodysplasia after high-dose chemotherapy and autologous stem cell transplantation. Blood. 2000;95:3273–9. [PubMed] [Google Scholar]
- 6.Bhatia S, Ramsay NK, Steinbuch M, Dusenbery KE, Shapiro RS, Weisdorf DJ, Robison LL, Miller JS, Neglia JP. Malignant neoplasms following bone marrow transplantation. Blood. 1996;87:3633–9. [PubMed] [Google Scholar]
- 7.Friedberg JW, Neuberg D, Stone RM, Alyea E, Jallow H, LaCasce A, Mauch PM, Gribben JG, Ritz J, Nadler LM, et al. Outcome in patients with myelodysplastic syndrome after autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17:3128–35. doi: 10.1200/JCO.1999.17.10.3128. [DOI] [PubMed] [Google Scholar]
- 8.Brown JR, Yeckes H, Friedberg JW, Neuberg D, Kim H, Nadler LM, Freedman AS. Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total-body irradiation and autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:2208–14. doi: 10.1200/JCO.2005.05.158. [DOI] [PubMed] [Google Scholar]
- 9.Forrest DL, Hogge DE, Nevill TJ, Nantel SH, Barnett MJ, Shepherd JD, Sutherland HJ, Toze CL, Smith CA, Lavoie JC, et al. High-dose therapy and autologous hematopoietic stem-cell transplantation does not increase the risk of second neoplasms for patients with Hodgkin’s lymphoma: a comparison of conventional therapy alone versus conventional therapy followed by autologous hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23:7994–8002. doi: 10.1200/JCO.2005.01.9083. [DOI] [PubMed] [Google Scholar]
- 10.Seshadri T, Pintilie M, Kuruvilla J, Keating A, Tsang R, Zadeh S, Crump M. Incidence and risk factors for second cancers after autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Leuk Lymphoma. 2009;50:380–6. doi: 10.1080/10428190902756578. [DOI] [PubMed] [Google Scholar]
- 11.Micallef IN, Lillington DM, Apostolidis J, Amess JA, Neat M, Matthews J, Clark T, Foran JM, Salam A, Lister TA, et al. Therapy-related myelodysplasia and secondary acute myelogenous leukemia after high-dose therapy with autologous hematopoietic progenitor-cell support for lymphoid malignancies. J Clin Oncol. 2000;18:947–55. doi: 10.1200/JCO.2000.18.5.947. [DOI] [PubMed] [Google Scholar]
- 12.Akpek G, Ambinder RF, Piantadosi S, Abrams RA, Brodsky RA, Vogelsang GB, Zahurak ML, Fuller D, Miller CB, Noga SJ, et al. Long-term results of blood and marrow transplantation for Hodgkin’s lymphoma. J Clin Oncol. 2001;19:4314–21. doi: 10.1200/JCO.2001.19.23.4314. [DOI] [PubMed] [Google Scholar]
- 13.Tarella C, Passera R, Magni M, Benedetti F, Rossi A, Gueli A, Patti C, Parvis G, Ciceri F, Gallamini A, et al. Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: a 20-year retrospective follow-up study in patients with lymphoma. J Clin Oncol. 2011;29:814–24. doi: 10.1200/JCO.2010.28.9777. [DOI] [PubMed] [Google Scholar]
- 14.Gilliland DG, Gribben JG. Evaluation of the risk of therapy-related MDS/AML after autologous stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:9–16. doi: 10.1053/bbmt.2002.v8.pm11846355. [DOI] [PubMed] [Google Scholar]
- 15.Roziakova L, Bojtarova E, Mistrik M, Mladosievicova B. Secondary malignancies after hematopoietic stem cell transplantation. Neoplasma. 2011;58:1–8. doi: 10.4149/neo_2011_01_1. [DOI] [PubMed] [Google Scholar]
- 16.Sevilla J, Rodríguez A, Hernández-Maraver D, de Bustos G, Aguado J, Ojeda E, Arrieta R, Hernández-Navarro F. Secondary acute myeloid leukemia and myelodysplasia after autologous peripheral blood progenitor cell transplantation. Ann Hematol. 2002;81:11–5. doi: 10.1007/s00277-001-0400-0. [DOI] [PubMed] [Google Scholar]
- 17.Howe R, Micallef IN, Inwards DJ, Ansell SM, Dewald GW, Dispenzieri A, Gastineau DA, Gertz MA, Geyer SM, Hanson CA, et al. Secondary myelodysplastic syndrome and acute myelogenous leukemia are significant complications following autologous stem cell transplantation for lymphoma. Bone Marrow Transplant. 2003;32:317–24. doi: 10.1038/sj.bmt.1704124. [DOI] [PubMed] [Google Scholar]
- 18.Metayer C, Curtis RE, Vose J, Sobocinski KA, Horowitz MM, Bhatia S, Fay JW, Freytes CO, Goldstein SC, Herzig RH, et al. Myelodysplastic syndrome and acute myeloid leukemia after autotransplantation for lymphoma: a multicenter case-control study. Blood. 2003;101:2015–23. doi: 10.1182/blood-2002-04-1261. [DOI] [PubMed] [Google Scholar]
- 19.Harrison CN, Gregory W, Hudson GV, Devereux S, Goldstone AH, Hancock B, Winfield D, MacMillan AK, Hoskin P, Newland AC, et al. High-dose BEAM chemotherapy with autologous haemopoietic stem cell transplantation for Hodgkin’s disease is unlikely to be associated with a major increased risk of secondary MDS/AML. Br J Cancer. 1999;81:476–83. doi: 10.1038/sj.bjc.6690718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterman J, Rybicki L, Bolwell B, Copelan E, Pohlman B, Sweetenham J, Dean R, Sobecks R, Andresen S, Kalaycio M. Fludarabine as a risk factor for poor stem cell harvest, treatment-related MDS and AML in follicular lymphoma patients after autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:488–93. doi: 10.1038/bmt.2011.109. [DOI] [PubMed] [Google Scholar]
- 21.Kalaycio M, Rybicki L, Pohlman B, Sobecks R, Andresen S, Kuczkowski E, Bolwell B. Risk factors before autologous stem-cell transplantation for lymphoma predict for secondary myelodysplasia and acute myelogenous leukemia. J Clin Oncol. 2006;24:3604–10. doi: 10.1200/JCO.2006.06.0673. [DOI] [PubMed] [Google Scholar]
- 22.Tam CS, Seymour JF, Prince HM, Kenealy M, Wolf M, Januszewicz EH, Westerman D. Treatment-related myelodysplasia following fludarabine combination chemotherapy. Haematologica. 2006;91:1546–50. [PubMed] [Google Scholar]
- 23.Bowcock SJ, Rassam SM, Lim Z, Ward SM, Ryali MM, Mufti GJ. High incidence of therapy-related myelodysplasia and acute leukaemia in general haematology clinic patients treated with fludarabine and cyclophosphamide for indolent lymphoproliferative disorders. Br J Haematol. 2006;134:242–3. doi: 10.1111/j.1365-2141.2006.06158.x. [DOI] [PubMed] [Google Scholar]
- 24.Laurenti L, Tarnani M, Chiusolo P, La Torre G, Garzia M, Zollino M, Zini G, Balducci M, Leone G, Sica S. Low incidence of secondary neoplasia after autotransplantation for lymphoproliferative disease: the role of pre-transplant therapy. Clin Transplant. 2008;22:191–9. doi: 10.1111/j.1399-0012.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Friedberg JW, Kahl BS, Van der Jagt RH, Tremmel L. Bendamustine produces durable responses with an acceptable safety profile in patients with rituximab-refractory indolent non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2010;10:452–7. doi: 10.3816/CLML.2010.n.079. [DOI] [PubMed] [Google Scholar]
- 26.Darrington DL, Vose JM, Anderson JR, Bierman PJ, Bishop MR, Chan WC, Morris ME, Reed EC, Sanger WG, Tarantolo SR, et al. Incidence and characterization of secondary myelodysplastic syndrome and acute myelogenous leukemia following high-dose chemoradiotherapy and autologous stem-cell transplantation for lymphoid malignancies. J Clin Oncol. 1994;12:2527–34. doi: 10.1200/JCO.1994.12.12.2527. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen-Bjergaard J, Pedersen M, Myhre J, Geisler C. High risk of therapy-related leukemia after BEAM chemotherapy and autologous stem cell transplantation for previously treated lymphomas is mainly related to primary chemotherapy and not to the BEAM-transplantation procedure. Leukemia. 1997;11:1654–60. doi: 10.1038/sj.leu.2400809. [DOI] [PubMed] [Google Scholar]
- 28.André M, Henry-Amar M, Blaise D, Colombat P, Fleury J, Milpied N, Cahn JY, Pico JL, Bastion Y, Kuentz M, et al. Treatment-related deaths and second cancer risk after autologous stem-cell transplantation for Hodgkin’s disease. Blood. 1998;92:1933–40. [PubMed] [Google Scholar]
- 29.Brusamolino E, Gotti M, Fiaccadori V. The Risk of Therapy-Related Myelodysplasia/Acute Myeloid Leukemia in Hodgkin Lymphoma has Substantially Decreased in the ABVD Era Abolishing Mechlorethamine and Procarbazine and Limiting Volumes and Doses of Radiotherapy. Mediterr J Hematol Infect Dis. 2012;4:e2012022. doi: 10.4084/mjhid.2012.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majhail NS. Old and new cancers after hematopoietic-cell transplantation. Hematology Am Soc Hematol Educ Program. 2008;•••:142–9. doi: 10.1182/asheducation-2008.1.142. [DOI] [PubMed] [Google Scholar]
- 31.Lillington DM, Micallef IN, Carpenter E, Neat MJ, Amess JA, Matthews J, Foot NJ, Young BD, Lister TA, Rohatiner AZ. Detection of chromosome abnormalities pre-high-dose treatment in patients developing therapy-related myelodysplasia and secondary acute myelogenous leukemia after treatment for non-Hodgkin’s lymphoma. J Clin Oncol. 2001;19:2472–81. doi: 10.1200/JCO.2001.19.9.2472. [DOI] [PubMed] [Google Scholar]
- 32.Abruzzese E, Radford JE, Miller JS, Vredenburgh JJ, Rao PN, Pettenati MJ, Cruz JM, Perry JJ, Amadori S, Hurd DD. Detection of abnormal pretransplant clones in progenitor cells of patients who developed myelodysplasia after autologous transplantation. Blood. 1999;94:1814–9. [PubMed] [Google Scholar]
- 33.Chao NJ, Nademanee AP, Long GD, Schmidt GM, Donlon TA, Parker P, Slovak ML, Nagasawa LS, Blume KG, Forman SJ. Importance of bone marrow cytogenetic evaluation before autologous bone marrow transplantation for Hodgkin’s disease. J Clin Oncol. 1991;9:1575–9. doi: 10.1200/JCO.1991.9.9.1575. [DOI] [PubMed] [Google Scholar]
- 34.Amigo ML, del Cañizo MC, Rios A, Garcia MA, Caballero MD, Martin A, Bermejo N, Vilches P, San Miguel JF. Diagnosis of secondary myelodysplastic syndromes (MDS) following autologous transplantation should not be based only on morphological criteria used for diagnosis of de novo MDS. Bone Marrow Transplant. 1999;23:997–1002. doi: 10.1038/sj.bmt.1701757. [DOI] [PubMed] [Google Scholar]
- 35.Weber MH, Wenzel U, Thiel E, Knauf WU. Chromosomal aberrations characteristic for sAML/sMDS are not detectable by random screening using FISH in peripheral blood-derived grafts used for autologous transplantation. J Hematother Stem Cell Res. 2000;9:861–5. doi: 10.1089/152581600750062291. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan A, Bhatia S, Slovak ML, Arber DA, Niland JC, Nademanee A, Fung H, Bhatia R, Kashyap A, Molina A, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000;95:1588–93. [PubMed] [Google Scholar]
- 37.Stone RM, Neuberg D, Soiffer R, Takvorian T, Whelan M, Rabinowe SN, Aster JC, Leavitt P, Mauch P, Freedman AS, et al. Myelodysplastic syndrome as a late complication following autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 1994;12:2535–42. doi: 10.1200/JCO.1994.12.12.2535. [DOI] [PubMed] [Google Scholar]
- 38.Mendenhall NP, Noyes WD, Million RR. Total body irradiation for stage II-IV non-Hodgkin’s lymphoma: ten-year follow-up. J Clin Oncol. 1989;7:67–74. doi: 10.1200/JCO.1989.7.1.67. [DOI] [PubMed] [Google Scholar]
- 39.Gomez GA, Aggarwal KK, Han T. Post-therapeutic acute malignant myeloproliferative syndrome and acute nonlymphocytic leukemia in non-Hodgkin’s lymphoma. Cancer. 1982;50:2285–8. doi: 10.1002/1097-0142(19821201)50:11<2285::AID-CNCR2820501111>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 40.Travis LB, Weeks J, Curtis RE, Chaffey JT, Stovall M, Banks PM, Boice JD., Jr. Leukemia following low-dose total body irradiation and chemotherapy for non-Hodgkin’s lymphoma. J Clin Oncol. 1996;14:565–71. doi: 10.1200/JCO.1996.14.2.565. [DOI] [PubMed] [Google Scholar]
- 41.Armitage JO, Carbone PP, Connors JM, Levine A, Bennett JM, Kroll S. Treatment-related myelodysplasia and acute leukemia in non-Hodgkin’s lymphoma patients. J Clin Oncol. 2003;21:897–906. doi: 10.1200/JCO.2003.07.113. [DOI] [PubMed] [Google Scholar]
- 42.Milligan DW, Ruiz De Elvira MC, Kolb HJ, Goldstone AH, Meloni G, Rohatiner AZ, Colombat P, Schmitz N, European Group for Blood and Marrow Transplantation Secondary leukaemia and myelodysplasia after autografting for lymphoma: results from the EBMT. EBMT Lymphoma and Late Effects Working Parties. Br J Haematol. 1999;106:1020–6. doi: 10.1046/j.1365-2141.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 43.Hosing C, Munsell M, Yazji S, Andersson B, Couriel D, de Lima M, Donato M, Gajewski J, Giralt S, Körbling M, et al. Risk of therapy-related myelodysplastic syndrome/acute leukemia following high-dose therapy and autologous bone marrow transplantation for non-Hodgkin’s lymphoma. Ann Oncol. 2002;13:450–9. doi: 10.1093/annonc/mdf109. [DOI] [PubMed] [Google Scholar]
- 44.Beauchamp-Nicoud A, Feneux D, Bayle C, Bernheim A, Léonard C, Koscielny S, Tchernia G, Bourhis JH. Therapy-related myelodysplasia and/or acute myeloid leukaemia after autologous haematopoietic progenitor cell transplantation in a prospective single centre cohort of 221 patients. Br J Haematol. 2003;122:109–17. doi: 10.1046/j.1365-2141.2003.04388.x. [DOI] [PubMed] [Google Scholar]
- 45.Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–8. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 46.Bhatia R, Van Heijzen K, Palmer A, Komiya A, Slovak ML, Chang KL, Fung H, Krishnan A, Molina A, Nademanee A, et al. Longitudinal assessment of hematopoietic abnormalities after autologous hematopoietic cell transplantation for lymphoma. J Clin Oncol. 2005;23:6699–711. doi: 10.1200/JCO.2005.10.330. [DOI] [PubMed] [Google Scholar]
- 47.Laurenti L, Chiusolo P, Garzia MG, Zini G, Sorà F, Piccirillo N, Piccioni P, Zollino M, Leone G, Sica S. Periodic morphologic, cytogenetic and clonality evaluation after autologous peripheral blood progenitor cell transplantation in patients with lymphoproliferative malignancies. Haematologica. 2002;87:59–66. [PubMed] [Google Scholar]
- 48.Imrie KR, Dubé I, Prince HM, Girouard C, Crump M, Keating A. New clonal karyotypic abnormalities acquired following autologous bone marrow transplantation for acute myeloid leukemia do not appear to confer an adverse prognosis. Bone Marrow Transplant. 1998;21:395–9. doi: 10.1038/sj.bmt.1701105. [DOI] [PubMed] [Google Scholar]
- 49.Ricca I, Compagno M, Ladetto M, Rocci A, Dell’Aquila M, Omedè P, De Marco F, D’Antico S, Caracciolo D, Ferrero D, et al. Marked telomere shortening in mobilized peripheral blood progenitor cells (PBPC) following two tightly spaced high-dose chemotherapy courses with G-CSF. Leukemia. 2005;19:644–51. doi: 10.1038/sj.leu.2403652. [DOI] [PubMed] [Google Scholar]
- 50.Chakraborty S, Sun CL, Francisco L, Sabado M, Li L, Chang KL, Forman S, Bhatia S, Bhatia R. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. J Clin Oncol. 2009;27:791–8. doi: 10.1200/JCO.2008.17.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deol A, Abrams J, Masood A, Al-Kadhimi Z, Abidi MH, Ayash L, Lum LG, Ratanatharathorn V, Uberti JP. Long-term follow up of patients proceeding to transplant using plerixafor mobilized stem cells and incidence of secondary myelodysplastic syndrome/AML. Bone Marrow Transplant. 2013;48:1112–6. doi: 10.1038/bmt.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brem R, Hall J. XRCC1 is required for DNA single-strand break repair in human cells. Nucleic Acids Res. 2005;33:2512–20. doi: 10.1093/nar/gki543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qu T, Morii E, Oboki K, Lu Y, Morimoto K. Micronuclei in EM9 cells expressing polymorphic forms of human XRCC1. Cancer Lett. 2005;221:91–5. doi: 10.1016/j.canlet.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 54.Leng S, Cheng J, Pan Z, Huang C, Niu Y, Dai Y, Li B, He F, Zheng Y. Associations between XRCC1 and ERCC2 polymorphisms and DNA damage in peripheral blood lymphocyte among coke oven workers. Biomarkers. 2004;9:395–406. doi: 10.1080/13547500400015618. [DOI] [PubMed] [Google Scholar]
- 55.Guillem VM, Arbona C, Hernández-Boluda JC, Terol MJ, Goterris R, Solano C, Tormo M. An XRCC1 polymorphism is associated with the outcome of patients with lymphoma undergoing autologous stem cell transplant. Leuk Lymphoma. 2011;52:1249–54. doi: 10.3109/10428194.2011.564694. [DOI] [PubMed] [Google Scholar]
- 56.Ding Y, Sun CL, Li L, Li M, Francisco L, Sabado M, Hahn B, Gyorffy J, Noe J, Larson GP, et al. Genetic susceptibility to therapy-related leukemia after Hodgkin lymphoma or non-Hodgkin lymphoma: role of drug metabolism, apoptosis and DNA repair. Blood Cancer J. 2012;2:e58. doi: 10.1038/bcj.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cano KE, Li L, Bhatia S, Bhatia R, Forman SJ, Chen Y. NMR-based metabolomic analysis of the molecular pathogenesis of therapy-related myelodysplasia/acute myeloid leukemia. J Proteome Res. 2011;10:2873–81. doi: 10.1021/pr200200y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L, Li M, Sun C, Francisco L, Chakraborty S, Sabado M, McDonald T, Gyorffy J, Chang K, Wang S, et al. Altered hematopoietic cell gene expression precedes development of therapy-related myelodysplasia/acute myeloid leukemia and identifies patients at risk. Cancer Cell. 2011;20:591–605. doi: 10.1016/j.ccr.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keung YK, Beaty MW, Pettenati M, Levitan D, Hurd DD. Possible role of engraftment syndrome and autologous graft-versus-host disease in myelodysplastic syndrome after autologous stem cell transplantations: retrospective analysis and review of the literature. Clin Lymphoma Myeloma Leuk. 2010;10:129–33. doi: 10.3816/CLML.2010.n.018. [DOI] [PubMed] [Google Scholar]
- 60.Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, Young NS. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114:2236–43. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pagana L, Pulsoni A, Tosti ME, Avvisati G, Mele L, Mele M, Martino B, Visani G, Cerri R, Di Bona E, et al. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto Clinical and biological features of acute myeloid leukaemia occurring as second malignancy: GIMEMA archive of adult acute leukaemia. Br J Haematol. 2001;112:109–17. doi: 10.1046/j.1365-2141.2001.02527.x. [DOI] [PubMed] [Google Scholar]
- 62.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 63.Akiyama M, Asai O, Kuraishi Y, Urashima M, Hoshi Y, Sakamaki H, Yabe H, Furukawa T, Yamada O, Mizoguchi H, et al. Shortening of telomeres in recipients of both autologous and allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2000;25:441–7. doi: 10.1038/sj.bmt.1702144. [DOI] [PubMed] [Google Scholar]
- 64.Fern L, Pallis M, Ian Carter G, Seedhouse C, Russell N, Byrne J. Clonal haemopoiesis may occur after conventional chemotherapy and is associated with accelerated telomere shortening and defects in the NQO1 pathway; possible mechanisms leading to an increased risk of t-AML/MDS. Br J Haematol. 2004;126:63–71. doi: 10.1111/j.1365-2141.2004.05006.x. [DOI] [PubMed] [Google Scholar]
- 65.Lee J, Kook H, Chung I, Kim H, Park M, Kim C, Nah J, Hwang T. Telomere length changes in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24:411–5. doi: 10.1038/sj.bmt.1701923. [DOI] [PubMed] [Google Scholar]
- 66.Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004;113:160–8. doi: 10.1172/JCI20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shay JW. Accelerated telomere shortening in bone-marrow recipients. Lancet. 1998;351:153–4. doi: 10.1016/S0140-6736(05)78218-0. [DOI] [PubMed] [Google Scholar]
- 68.Lee CK, Gingrich RD, Hohl RJ, Ajram KA. Engraftment syndrome in autologous bone marrow and peripheral stem cell transplantation. Bone Marrow Transplant. 1995;16:175–82. [PubMed] [Google Scholar]
- 69.Esteban JM, Somlo G. Skin biopsy in allogeneic and autologous bone marrow transplant patients: a histologic and immunohistochemical study and review of the literature. Mod Pathol. 1995;8:59–64. [PubMed] [Google Scholar]
- 70.Chamuleau ME, Westers TM, van Dreunen L, Groenland J, Zevenbergen A, Eeltink CM, Ossenkoppele GJ, van de Loosdrecht AA. Immune mediated autologous cytotoxicity against hematopoietic precursor cells in patients with myelodysplastic syndrome. Haematologica. 2009;94:496–506. doi: 10.3324/haematol.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voulgarelis M, Giannouli S, Ritis K, Tzioufas AG. Myelodysplasia-associated autoimmunity: clinical and pathophysiologic concepts. Eur J Clin Invest. 2004;34:690–700. doi: 10.1111/j.1365-2362.2004.01417.x. [DOI] [PubMed] [Google Scholar]
- 72.Giannouli S, Tzoanopoulos D, Ritis K, Kartalis G, Moutsopoulos HM, Voulgarelis M. Autoimmune manifestations in human myelodysplasia: a positive correlation with interferon regulatory factor-1 (IRF-1) expression. Ann Rheum Dis. 2004;63:578–82. doi: 10.1136/ard.2003.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biesma DH, van den Tweel JG, Verdonck LF. Immunosuppressive therapy for hypoplastic myelodysplastic syndrome. Cancer. 1997;79:1548–51. doi: 10.1002/(SICI)1097-0142(19970415)79:8<1548::AID-CNCR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 74.Yazji S, Giles FJ, Tsimberidou AM, Estey EH, Kantarjian HM, O’Brien SA, Kurzrock R. Antithymocyte globulin (ATG)-based therapy in patients with myelodysplastic syndromes. Leukemia. 2003;17:2101–6. doi: 10.1038/sj.leu.2403124. [DOI] [PubMed] [Google Scholar]
- 75.Laurenti L, d’Onofrio G, Sica S, Chiusolo P, Zini G, Piccirillo N, Zollino M, Garzia M, Sora F, Leone G. Secondary myelodysplastic syndromes following peripheral blood stem cell transplantation: morphological, cytogenetic and clonality evaluation and the limitation of FAB criteria. Bone Marrow Transplant. 2000;26:241–2. doi: 10.1038/sj.bmt.1702502. [DOI] [PubMed] [Google Scholar]
- 76.Traweek ST, Slovak ML, Nademanee AP, Brynes RK, Niland JC, Forman SJ. Clonal karyotypic hematopoietic cell abnormalities occurring after autologous bone marrow transplantation for Hodgkin’s disease and non-Hodgkin’s lymphoma. Blood. 1994;84:957–63. [PubMed] [Google Scholar]
- 77.Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35:418–29. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andreasson P, Johansson B, Arheden K, Billström R, Mitelman F, Höglund M. Deletions of CDKN1B and ETV6 in acute myeloid leukemia and myelodysplastic syndromes without cytogenetic evidence of 12p abnormalities. Genes Chromosomes Cancer. 1997;19:77–83. doi: 10.1002/(SICI)1098-2264(199706)19:2<77::AID-GCC2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 79.Papaioannou G, Batsis I, Iordanidis F, Vadikoliou C, Kaloyannidis P, Anagnostopoulos A, Athanasiadou A. Derivative (1;7)(q10;p10) in two patients with myelodysplastic syndrome after autologous haematopoietic cell transplantation. Hematol Oncol. 2011;29:161–3. doi: 10.1002/hon.979. [DOI] [PubMed] [Google Scholar]
- 80.Olney HJ, Mitelman F, Johansson B, Mrózek K, Berger R, Rowley JD. Unique balanced chromosome abnormalities in treatment-related myelodysplastic syndromes and acute myeloid leukemia: report from an international workshop. Genes Chromosomes Cancer. 2002;33:413–23. doi: 10.1002/gcc.10045. [DOI] [PubMed] [Google Scholar]
- 81.Lillington DM, Micallef IN, Carpenter E, Neat MJ, Amess JA, Matthews J, Foot NJ, Lister TA, Young BD, Rohatiner AZ. Genetic susceptibility to Hodgkin’s disease and secondary neoplasias: FISH analysis reveals patients at high risk of developing secondary neoplasia. Ann Oncol. 2002;13(Suppl 1):40–3. doi: 10.1093/annonc/13.S1.40. [DOI] [PubMed] [Google Scholar]
- 82.Gale RE, Bunch C, Moir DJ, Patterson KG, Goldstone AH, Linch DC. Demonstration of developing myelodysplasia/acute myeloid leukaemia in haematologically normal patients after high-dose chemotherapy and autologous bone marrow transplantation using X-chromosome inactivation patterns. Br J Haematol. 1996;93:53–8. doi: 10.1046/j.1365-2141.1996.4751014.x. [DOI] [PubMed] [Google Scholar]
- 83.Mach-Pascual S, Legare RD, Lu D, Kroon M, Neuberg D, Tantravahi R, Stone RM, Freedman AS, Nadler LM, Gribben JG, et al. Predictive value of clonality assays in patients with non-Hodgkin’s lymphoma undergoing autologous bone marrow transplant: a single institution study. Blood. 1998;91:4496–503. [PubMed] [Google Scholar]
- 84.Litzow MR, Tarima S, Pérez WS, Bolwell BJ, Cairo MS, Camitta BM, Cutler CS, de Lima M, Dipersio JF, Gale RP, et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010;115:1850–7. doi: 10.1182/blood-2009-10-249128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kröger N, Brand R, van Biezen A, Zander A, Dierlamm J, Niederwieser D, Devergie A, Ruutu T, Cornish J, Ljungman P, et al. Myelodysplastic Syndromes Subcommittee of The Chronic Leukaemia Working Party of European Group for Blood and Marrow Transplantation (EBMT) Risk factors for therapy-related myelodysplastic syndrome and acute myeloid leukemia treated with allogeneic stem cell transplantation. Haematologica. 2009;94:542–9. doi: 10.3324/haematol.2008.000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spina F, Alessandrino PE, Milani R, Bonifazi F, Bernardi M, Luksch R, Fagioli F, Formica C, Farina L. Allogeneic stem cell transplantation in therapy-related acute myeloid leukemia and myelodysplastic syndromes: impact of patient characteristics and timing of transplant. Leuk Lymphoma. 2012;53:96–102. doi: 10.3109/10428194.2011.603445. [DOI] [PubMed] [Google Scholar]
- 87.Tallman MS, Rowlings PA, Milone G, Zhang MJ, Perez WS, Weisdorf D, Keating A, Gale RP, Geller RB, Laughlin MJ, et al. Effect of postremission chemotherapy before human leukocyte antigen-identical sibling transplantation for acute myelogenous leukemia in first complete remission. Blood. 2000;96:1254–8. [PubMed] [Google Scholar]
- 88.Nagler A, Or R, Naparstek E, Varadi G, Slavin S. Second allogeneic stem cell transplantation using nonmyeloablative conditioning for patients who relapsed or developed secondary malignancies following autologous transplantation. Exp Hematol. 2000;28:1096–104. doi: 10.1016/S0301-472X(00)00511-7. [DOI] [PubMed] [Google Scholar]
- 89.Wilson CS, Traweek ST, Slovak ML, Niland JC, Forman SJ, Brynes RK. Myelodysplastic syndrome occurring after autologous bone marrow transplantation for lymphoma. Morphologic features. Am J Clin Pathol. 1997;108:369–77. doi: 10.1093/ajcp/108.4.369. [DOI] [PubMed] [Google Scholar]
- 90.Park S, Brice P, Noguerra ME, Simon D, Rousselot P, Kerneis Y, Morel P, Marolleau JP, Gisselbrecht C. Myelodysplasias and leukemias after autologous stem cell transplantation for lymphoid malignancies. Bone Marrow Transplant. 2000;26:321–6. doi: 10.1038/sj.bmt.1702510. [DOI] [PubMed] [Google Scholar]


