Abstract
Mortality data by geographic area and trend-based surveillance are particularly relevant in orienting public health decisions targeting specific populations. We analyzed overall and site-specific cancer mortality between 1988 and 2009 in the metropolitan area of Naples and Caserta in southern Italy. Age-standardized mortality rates (SMR) were computed for each 5-y age group, by gender, primitive cancer site and specific Province in the overall population and age-defined subgroups. Cancer mortality trends were quantified by annual percent change (APC) and 95% confidence interval (CI). From Naples and Caserta, the reduction observed between 1988 and 2009 in SMR in males, but not in females, was significantly lower compared with the decrease reported at a national level (−11.4% and −28.4%, respectively). In elderly men, differences between local and national SMR were more pronounced (+13.6% compared with −2.7%). In males, the joinpoint regression analysis showed the following APC and 95% CI: −0.9%/year (−1.2; −0.7) and −0.6%/year (−1.0; −0.2) for Naples and Caserta, respectively. In females, estimates were −0.6%/year (−0.8; −0.5) and −0.7%/year (−1.2; −0.3). The overall orientation toward declining cancer mortality trends appeared in antithesis with the slight, but significant, increase for some tumors (e.g., pancreatic cancer in both genders). A complex mixture of heterogeneous factors concurs to explain the evidence observed including lifestyle, access to screening procedures, advancements in cancer diagnosis and treatment. Further details might eventually derive from biomonitoring studies for ascertaining the causal link between exposure to potential contaminants in air, water, and soil and cancer-related outcomes in the area of interest.
Keywords: cancer mortality, time trends, Naples, metropolitan area, southern Italy, joinpoint, analysis
Introduction
Cancer mortality data, along with cancer incidence and population based survival, are a cornerstone of epidemiology research, health monitoring and resource allocation for interventions aimed at cancer prevention and control. In recent years, a number of reports on cancer mortality trends have been published in Italy and other countries. Consistent with other European countries and the United States, Italy has shown declining trends in overall cancer mortality since the end of the 20th century, with mortality rates decreasing by 1.8 percent (%) and 1.1% yearly in men and women, respectively.1,2
Data availability by geographic area might render trend-based surveillance particularly relevant in public health decision making for specific target populations. Historically, in Italy, the comparison of mortality and morbidity indicators by broad geographical areas has highlighted discrepancies, with southern Italy having a lower cancer burden compared with the north and center.3,4 However, recent evidence suggests that southern Italy is progressively losing its advantage in mortality from cancer.5-8 Moreover, recent data from a geographical study have shown significant cancer mortality excess in 7 out of 23 southern provinces, with the highest standardized mortality rates in the provinces of Naples and Caserta.9 These two provinces converge in a metropolitan area whose distinctive features are the high population density (9.026 person/km2), one of the highest in Europe, and widespread industrialization process and related activities.10
In the present study, we aimed to analyze overall and site-specific cancer mortality between 1988 and 2009 in the metropolitan area of Naples and Caserta, southern Italy. Cancer mortality related to the area of interest was also presented in a frame including cancer estimates from Italy overall. The independent effect of aging on the evolution of cancer mortality trends was evaluated in men and women aged 65 and older at the time of death. Potential explanatory factors for observed mortality trends were then discussed, along with the role of health service interventions.
Results
Between 1988 and 2009, the population of the metropolitan area of Naples and Caserta accounted for a total number of 3 912 068 inhabitants out of 5 701 931 over the entire Campania region. Certified deaths from the metropolitan area of interest during the whole study period were 94 010 and 63 995 for males and females, respectively (data available upon request).
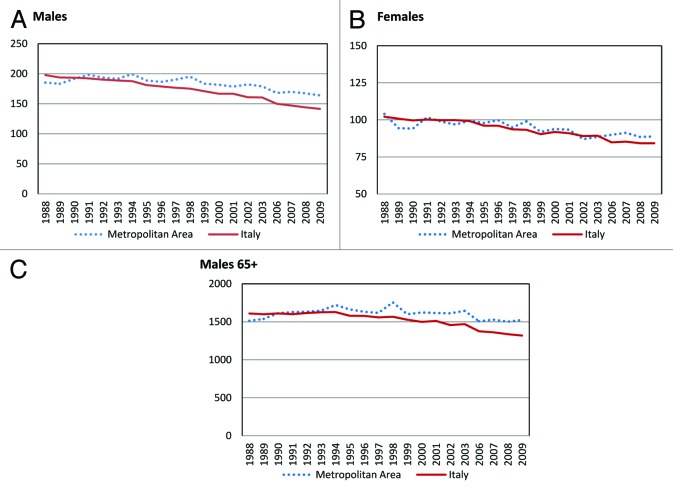
Word standardized mortality rates (SMR) for the metropolitan area of Naples and Caserta and Italy overall between 1988 and 2009 appear in Figure 1. Estimates are reported by gender and referred to all ages. In males, SMR for the metropolitan area of interest decreased from 185.3/100 000 in 1988 to 164.1/100 000 in 2009, with an overall 11.4% reduction. The decrease in SMR was more evident at a national level, with rates dropping from 197.9/100 000 to 141.6/100 000, with an overall 28.4% reduction (Fig. 1A). Conversely, in females, the decrease in SMR observed for the metropolitan area of Naples and Caserta did not substantially differ from the same estimates for Italy overall (Fig. 1B).
Figure 1. (A and B) Word standardized mortality rates × 100 000 inhabitants (dotted line, metropolitan area; solid line, Italy) from 1988 to 2009 for all cancer site by gender. (C) Word standardized mortality rates × 100 000 inhabitants in males aged 65 and older (dotted line, metropolitan area; solid line, Italy) from 1988 to 2009 for all cancer sites.
When confining the analysis to men aged 65 y and older, percent variations in standardized mortality for Naples and Caserta tended to diverge from those for Italy overall. We observed a gradual and steady decline in the national SMR from 1609.7/100 000 in 1988 to 1319.2/100 000 in 2009, with an overall 18.05% decrease. Conversely, standardized mortality for the metropolitan area of Naples and Caserta showed a 0.7% increase, with cancer deaths rising from 1513.6/100 000 in 1988 to 1524.6/100 000 in 2009 (Fig. 1C). In an additional analysis conducted in this same subgroup (i.e., males aged 65 and older) and restricted to the time frame between 2006 and 2009, the differences in mortality emerged more clearly, with national estimates decreasing from 1372 to 1319.1 (i.e., 3.8% decrease) and estimates for the metropolitan area increasing from 1507.6 to 1524. (i.e., 1.2% increase) (Fig. 2).
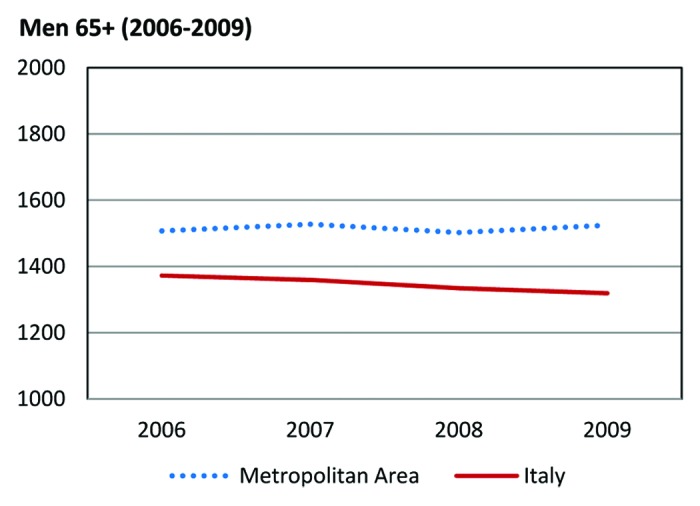
Figure 2. Word standardized mortality rates × 100 000 inhabitants in males aged 65 y and older (dotted line, metropolitan area; solid line, Italy) from 2006 to 2009 for all cancer sites.
For women aged 65 y and older, there was a substantial overlap in SMR for the metropolitan area and Italy overall, both in the 1988–2009 time frame and between 2006 and 2009 (Fig. S1).
Results from the joinpoint regression analysis are shown in Tables 1 and 2. Estimated annual percent changes (APC) and related 95% confidence intervals (CI) are given by gender and cancer site relatively to each of the two provinces converging in the metropolitan area and referred to the time frame spanning from 1998 to 2009.
Table 1. Joinpoint analysis by cancer site in the provinces of Naples and Caserta, southern Italy. Cancer deaths in males, 1988–2009.
| Metropolitan area | Italy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Naples | Caserta | ||||||||||
| Site (ICD X) | No. of deaths | % of all deaths | SMR** | APC | (95% CI) | No. of deaths | % of all deaths | SMR** | APC | (95% CI) | SMR** |
| All sites C00–C99 | 73623 | - | 171.5 | −0.9* | (−1.2; −0.7) | 20387 | - | 163.2 | −0.6* | (−1.0; −0.2) | 144.1 |
| Head and neck C01–C06, C10–C13 | 1112 | 1.5 | 2.4 | −1 | (−2.4; +0.3) | 384 | 1.9 | 2.9 | 0.3 | (−1.7; +2.3) | 2.5 |
| Esophagus C15 | 647 | 0.9 | 1.3 | −2.9* | (−4.1; −1.7) | 221 | 1.1 | 1.7 | −2.4* | (−4.4; −0.2) | 2.2 |
| Stomach C16 | 4206 | 5.7 | 8.7 | −2.5* | (−3.1; −1.8) | 1524 | 7.5 | 11.1 | −2.0* | (−2.8; −1.2) | 8.7 |
| Intestines C17–C21, C26 | 6607 | 9.0 | 16.4 | 0.08 | (−0.5; +0.6) | 1980 | 9.7 | 16.7 | +0.9* | (+0.1; +1.6) | 16.4 |
| Liver C22 | 7559 | 10.3 | 8.8 | −0.6 | (−1.5; +0.3) | 1867 | 9.2 | 7.5 | −1.3 | (−2.3; −0.3) | 5.0 |
| Pancreas C25 | 2246 | 3.1 | 7.0 | +1.9* | (+1.0; +2.9) | 606 | 3.0 | 6.3 | +1.7* | (+0.06; +3.4) | 7.5 |
| Larynx C32 | 1888 | 2.6 | 3.6 | −3.7* | (−4.3; −3.2) | 553 | 2.7 | 4.3 | −1.4 | (−3.1; +0.3) | 2.4 |
| Lung C33 | 24380 | 33.1 | 54.6 | −1.4* | (−1.7; −1.1) | 6342 | 31.1 | 49.9 | −1.0* | (−1.4; −0.5) | 37.9 |
| Skin melanoma C43 | 786 | 1.1 | 2.1 | 0.3 | (−1.1; +1.7) | 194 | 1.0 | 2.0 | 2.7 | (−0.5; +6.1) | 2.1 |
| Prostate C61 | 4544 | 6.2 | 10.0 | −0.1 | (−0.1; +0.6) | 1446 | 7.1 | 10.9 | −0.3 | (−1.4; +0.7) | 8.7 |
| Bladder C67 | 4565 | 6.2 | 9.0 | −2.8* | (−3.3; −2.3) | 1118 | 5.5 | 7.7 | −1.6* | (−2.6; −0.6) | 5.5 |
| Brain C71 | 2738 | 3.7 | 4.2 | −4.2* | (−6.1; −2.2) | 733 | 3.6 | 4.4 | −2.3* | (−4.3; −0.3) | 3.8 |
| Hodgkin disease C81 | 252 | 0.3 | 0.5 | −3.5* | (−5.1; −1.7) | 83 | 0.4 | 0.7 | −0.1 | (−5.0; +5.0) | 0.5 |
| Non-Hodgkin disease C83–C85 | 1603 | 2.2 | 4.0 | 0.01 | (−1.0; +1.2) | 394 | 1.9 | 3.1 | 1.3 | (−1.3; +3.9) | 3.7 |
| Multiple myeloma C90 | 737 | 1.0 | 2.2 | 1.2 | (−0.3; +2.7) | 210 | 1.0 | 1.8 | 0.2 | (−2.2; +2.7) | 2.2 |
| Leukemias C91–C95 | 2247 | 3.1 | 5.4 | −1.2* | (−2.04; −0.3) | 699 | 3.4 | 5.4 | −0.5 | (−1.8; +0.7) | 5.1 |
| Other sites | 7506 | 10.2 | - | - | - | 2033 | 10.0 | - | - | - | - |
*Only significant joinpoint (P < 0.05) were retained in final models for each site. **Standardized mortality rate (world standard rate × 100 000) distribution of cancer types in the provinces of Naples and Caserta and Italy between 2006 and 2009.
Table 2. Joinpoint analysis by primitive cancer site in the provinces of Naples and Caserta, southern Italy. Cancer deaths in females, 1988–2009.
| Metropolitan area | Italy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Naples | Caserta | ||||||||||
| Site (ICD X) | No. of deaths | % of all deaths | SMR** | APC | (95% CI) | No. of deaths | % of all deaths | SMR** | APC | (95% CI) | SMR** |
| All sites C00–C99 | 50887 | - | 93.8 | −0.6* | (−0.8; −0.5) | 13108 | - | 85.5 | −0.7* | (−1.2; −0.3) | 84.2 |
| Head and neck C01–C06, C10–C13 | 429 | 0.8 | 0.9 | 0.9 | (−1.3; +3.2) | 104 | 0.8 | 0.7 | 0.9 | (−1.3; +3.2) | 0.7 |
| Esophagus C15 | 301 | 0.6 | 0.6 | −1.3 | (−3.6; +1.1) | 65 | 0.5 | 0.3 | −2.9 | (−6.2; +0.4) | 0.5 |
| Stomach C16 | 2048 | 4.0 | 4.8 | −1.9* | (−2.7; −1.0) | 729 | 5.6 | 5.1 | −2.3* | (−3.5; −1.06) | 4.3 |
| Intestines C17–C21, C26 | 6569 | 12.9 | 10.5 | −1.1* | (−1.6; −0.6) | 1930 | 14.7 | 11.9 | −0.3 | (−1.3; +0.6) | 10.1 |
| Liver C22 | 4836 | 9.5 | 3.6 | −2.5* | (−3.1; −1.8) | 1060 | 8.1 | 3.0 | −2.7* | (−4.1; −1.4) | 1.6 |
| Pancreas C25 | 2186 | 4.3 | 4.5 | +2.0* | (+1.2; +2.7) | 543 | 4.1 | 4.5 | +2.5* | (+0.7; +4.4) | 5.2 |
| Larynx C32 | 166 | 0.3 | 0.3 | −0.8 | (−3.3; +1.7) | 31 | 0.2 | 0.3 | 2.6 | (−1.3; +6.5) | 0.2 |
| Lung C33 | 4700 | 9.2 | 11.5 | +2.6* | (+2.0; +3.3) | 947 | 7.2 | 7.5 | +1.3* | (+0.1; +2.5) | 9.5 |
| Skin melanoma C43 | 631 | 1.2 | 1.3 | −0.8 | (−2.5; +1.0) | 176 | 1.3 | 1.3 | −2.5 | (−5.4; +0.4) | 1.2 |
| Breast C50 | 8710 | 17.1 | 16.8 | −1.0* | (−1.4; −0.6) | 2159 | 16.5 | 15.4 | −0.4 | (−1.3; +0.4) | 15.9 |
| Uterus C53–C55 | 2715 | 5.3 | 4.1 | −3.6* | (−4.4; −2.7) | 720 | 5.5 | 4.1 | −3.8* | (−5.2; −2.4) | 3.5 |
| Ovary C56 | 1757 | 3.5 | 3.7 | −0.6 | (−1.5; +0.4) | 490 | 3.7 | 3.5 | 0.5 | (−1.3; +2.3) | 4.4 |
| Bladder C67 | 917 | 1.8 | 1.3 | −2.1* | (−3.4; −0.8) | 207 | 1.6 | 1.3 | −2.1 | (−4.8; +0.6) | 0.9 |
| Brain C71 | 2286 | 4.5 | 3.7 | −0.8 | (−2.2; +0.5) | 632 | 4.8 | 3.5 | −0.6 | (−2.9; +1.7) | 2.5 |
| Hodgkin disease C81 | 215 | 0.4 | 0.4 | −3.4* | (−5.77; −0.86) | 63 | 0.5 | 0.5 | −3.6 | (−7.3; +0.2) | 0.3 |
| Non-Hodgkin disease C83–C85 | 1428 | 2.8 | 2.7 | +2.0* | (0.2; +3.7) | 321 | 2.4 | 2.2 | 0.5 | (−1.6; +2.6) | 2.2 |
| Multiple myeloma C90 | 850 | 1.7 | 1.8 | 0.7 | (−1.1; +2.7) | 178 | 1.4 | 1.2 | −0.4 | (−3.6; +2.8) | 1.5 |
| Leukemias C91–C95 | 1946 | 3.8 | 3.2 | −6.9 | (−13.7; +0.4) | 526 | 4.0 | 3.0 | −1.7 | (−4.0; +0.5) | 3.1 |
| Other sites | 8197 | 16.1 | - | - | - | 2227 | 17.0 | - | - | - | - |
*P < 0.05. **Standardized mortality rate (world standard rate × 100 000) distribution of cancer types in the provinces of Naples and Caserta and Italy between 2006 and 2009.
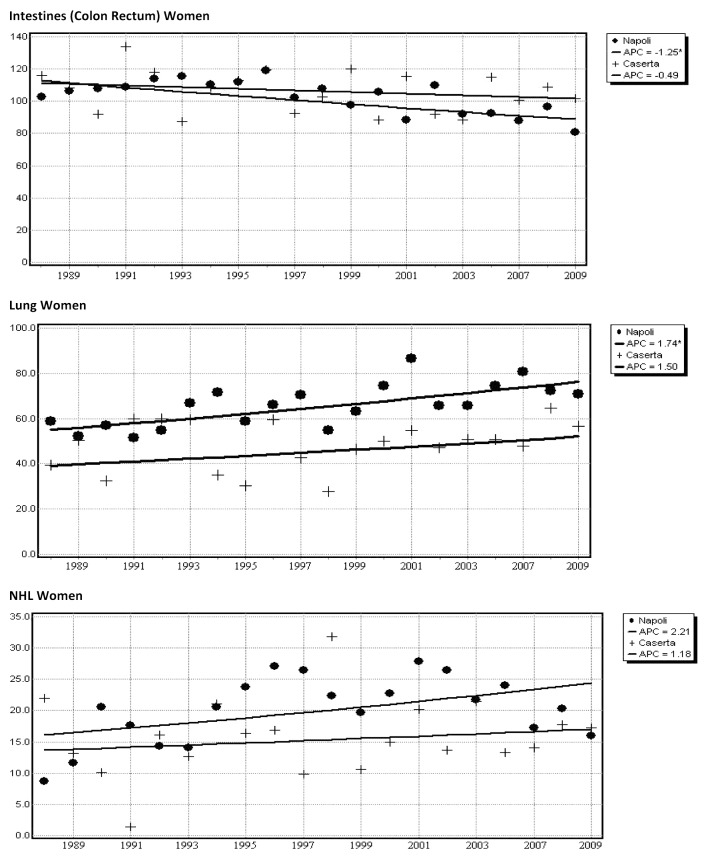
In males, the APC for all cancer sites declined slightly, though significantly, during the whole period 1988–2009 (−0.9%/year [−1.2; −0.7] and −0.6%/year [−1.0; −0.2] for Naples and Caserta, respectively). Significant reductions in APC were consistent across the two provinces for the following primitive cancer sites: esophagus (−2.9%/year [−4.1; −1.7] in Naples and −2.4%/year [−4.4; −0.2] in Caserta), stomach (−2.5%/year [−3.1; −1.8] and −2.0%/year [−2.8; −1.2]), lung (−1.4%/year [−1.7; −1.1) and −1.0%/year [−1.4; −0.5]), bladder (−2.8%/year [−3.3; −2.3]0 and −1.6%/year [−2.6; −0.6]) and brain (−4.2%/year [−6.1; −2.2] and −2.3%/year [−4.3; −0.3]). A significant increase in cancer mortality with positive APC was found regarding intestines (+0.9%/year [+0.1;+0.6]) in the province of Caserta and pancreas (+1.9%/year [+1.0;+2.9] and +1.7%/year [+0.06;+3.4]) in Naples and Caserta, respectively (Table 1). In females, cancer related mortality showed similar trends for all cancer sites across the two provinces, with APC of −0.6%/year [−0.8; −0.5) and −0.7%/year (−1.2; −0.3) in Naples and Caserta, respectively. A significant reduction in APC was observed in both provinces for the following cancer sites: stomach (−1.9%/year [−2.7; −1.0] in Naples and −2.3%/year [−3.5; −1.06] in Caserta), liver (−2.5%/year [−3.1; −1.8) and −2.7%/year [−4.1; −1.4]), and uterus (−3.6%/year [−4.4; −2.7] and −3.8%/year [−5.2; −2.4]). APC for deaths related to cancers originating from intestines, breast, bladder, and Hodgkin disease were significantly reduced in Naples only (−1.1%/year [−1.6; −0.6), −1.0%/year [−1.4; −0.6], −2.1%/year [−3.4; −0.8], and −3.4%/year [−5.8; −0.9], respectively). Significant increase in cancer mortality with positive APC was found for pancreas in both provinces (+2.0%/year [+1.2; +2.7] and +2.5%/year [+0.7; +4.4], for Naples and Caserta, respectively) and for non-Hodgkin lymphoma in Naples only (+2.0%/year [+0.2; +3.7]).
Results from a joinpoint analysis restricted to elderly people from the metropolitan area are shown in Table 3. In men, there was a suggestion for a decrease in mortality for all cancer sites, even though estimates did not reach the statistical significance (−0.3 [−0.6; 0.0]). In women, overall APC showed a slight, significant decrease (−0.4 [−0.6; −0.2]). Estimates by cancer site confirmed previous results, with significantly decreased APC for stomach and lung (−1.7 [−2.4; −0.9), and −0.4 [−0.7, −0.01], respectively) and increased APC for non-Hodgkin lymphoma in males (+1.4 [+0.3; +2.6]). In females, data confirmed decreased APC for intestines, liver, and breast (−1.0 [−1.5; −0.6], −1.6 [−2.2; −1.0], and −0.6 [−1.1; −0.08]), while an increased APC was confirmed for lung (+1.6 [+0.7; +2.5]). Mortality trends for pancreatic cancer showed a significant increase in both genders (+1.7 [+0.9; +2.5] and +1.8 [+0.9; +2.8], in males and females, respectively). APC for selected cancer sites by gender and province are graphically displayed in Figure 3.
Table 3. Joinpoint analysis by gender and primitive cancer site in the provinces of Naples and Caserta, southern Italy. Cancer deaths in elderly (65+), 1988–2009.
| Elderly (65+) | |||||||
|---|---|---|---|---|---|---|---|
| Metropolitan area | SMR** | ||||||
| Cancer site | No. of deaths | % of all deaths | APC | 95% CI | Naples | Caserta | Italy |
| Men | |||||||
| All cancers | 62004 | - | −0.3 | (−0.6; +0.0) | 1527.8 | 1475.0 | 1337.5 |
| Stomach | 3781 | 6.1 | −1.7* | (−2.4; −0.9) | 75.7 | 107.2 | 82.9 |
| Intestines | 6148 | 9.9 | 0.4 | (−0.2; +0.9) | 155.1 | 164.7 | 159.6 |
| Liver | 6331 | 10.2 | −0.1 | (−0.6; +0.4) | 66.0 | 62.6 | 44.6 |
| Pancreas | 1690 | 2.7 | +1.7* | (+0.9; +2.5) | 50.3 | 47.0 | 65.06 |
| Lung | 19718 | 31.8 | −0.4* | (−0.7; −0.01) | 492.8 | 452.3 | 354.3 |
| Non-Hodgkin disease | 1000 | 1.6 | +1.4* | (+0.3; +2.6) | 25.3 | 24.5 | 30.6 |
| Women | |||||||
| All cancers | 42434 | - | −0.4* | (−0.6; −0.2) | 696.5 | 674.8 | 661.1 |
| Stomach | 2777 | 6.5 | −2.2 | (−4.5; +0.1) | 38.4 | 50.9 | 38.1 |
| Intestines | 6348 | 15.0 | −1.0* | (−1.5; −0.6) | 89.5 | 106.4 | 90.3 |
| Liver | 4517 | 10.6 | −1.6* | (−2.2; −1.0) | 21.6 | 20.8 | 15.8 |
| Pancreas | 2045 | 4.8 | +1.8* | (+0.9; +2.8) | 40.2 | 40.7 | 48.7 |
| Lung | 3606 | 8.5 | +1.6* | (+0.7; +2.51) | 74.5 | 54.8 | 70.1 |
| Breast | 3530 | 8.3 | −0.6* | (−1.1; −0.08) | 96.3 | 92.5 | 97.1 |
| Non-Hodgkin disease | 1130 | 2.7 | 1.5 | (−0.3; +3.3) | 19.4 | 15.6 | 19.5 |
*P < 0.05. **Standardized mortality rate (world standard rate × 100 000) distribution of cancer types in the provinces of Naples and Caserta and Italy between 2006 and 2009.
Figure 3. Joinpoint analysis of age-standardized death rates for cancer in Naples and Caserta (metropolitan area) from 1988 to 2009, according to gender and selected cancer site. Naples data points: circle, 65+; solid line, truncated age group 65+ joinpoint model; Caserta data points: cross, 65+; solid line, truncated age group 65+ joinpoint model.
Discussion
We analyzed overall and site-specific cancer mortality between 1988 and 2009 in the metropolitan area of Naples and Caserta, southern Italy. Over the considered time frame, the reduction in standardized cancer mortality rates observed in males from this area was paralleled by a more pronounced decrease in the Italian population as a whole. In a subgroup analysis including men aged 65 and older, the decline in cancer mortality found at a national level diverged from the slight, but significant, increase emerging from the metropolitan area of interest. Results from joinpoint analyses showed slight, but significant, downward mortality trends for all cancer sites, independently of gender and specific province. However, in the metropolitan area of Naples and Caserta the overall orientation toward a reduction in cancer mortality appeared to be in antithesis with the slight, but significant, increase in mortality trends for some tumors, i.e., cancers originating from pancreas in both genders, cancers from lung and non-Hodgkin lymphomas in females and colorectal cancers in males.
The general tendency toward decreased cancer mortality observed in the metropolitan area of Naples and Caserta between 1998 and 2009 appeared to be in key with a more favorable trend for Italy overall. Cancer mortality trends for the whole of Italy referred to the time interval between 1998 and 2005 were reported in the 2009 report from the AIRTUM group. Overall, cancer rates decreased significantly in both genders. Percent reductions in mortality rates were −12% and −6% in Italian males and females, respectively. When analyzed by primitive cancer site, deaths by Hodgkin disease, rectum, stomach, and liver cancer were significantly decreased in both genders. Reductions in mortality were also observed for esophagus, lung, and bladder cancer in males, while deaths by colon, breast, uterus, and bone declined in women. Conversely, cancer mortality showed incremental trends in melanoma for males and lung for women. Geographic differences in cancer mortality were reported by macro-areas (i.e., northern, central, and southern Italy), with no specific focus on disparities among provinces.11
Cancer mortality in southern Italy between 1999 and 2003 was recently addressed by Bidoli and colleagues in a geographical study specifically focusing on 23 provinces including Naples and Caserta. Despite lower cancer rates observed in southern Italy compared with Italy overall, significant mortality excesses were observed in 7 out of 23 provinces examined, with the highest frequency excesses being reported for liver cancer in males (SMR: +50%, +35%, +30%, and +27% for Bari, Naples, Taranto, and Caserta, respectively) and breast cancer in females (SMR: +17, +16, and +9% for Taranto, Naples, and Bari, respectively).9
Over the 1988–1998 time window, standardized mortality rates for all cancer sites in men aged 65 and older from the metropolitan area of interest showed a 13.6% increase, which appeared in contrast with the −2.7% decrease observed for Italy overall. A comprehensive description of cancer mortality among Italian elderly between 1970 and 2008 has been recently performed by Bidoli and colleagues. Mortality data were grouped in quinquennia from 1970–74 through 1995–99, and in 2000–03 and 2006–08 groups. Although the time interval considered is noticeably longer than ours and no referral to macro-areas or specific provinces is made, consistently with our results, the highest peaks in cancer mortality rates among elderly were observed during the quinquennia 1985–89 and 1990–94 in both genders, and declined thereafter. The authors further describe increases in mortality rates between 1995 and 2008 for lung cancer (APC = +0.6%) in females and pancreatic cancer in both genders (+0.6% and 0.9% in men and women, respectively), which are in line with our results.2
The overall favorable cancer mortality trends observed in males from the metropolitan area over the past two decades were mainly, though not exclusively, driven by the decline in deaths from tobacco-related cancers. A relevant role was also played by the diminished deaths from esophageal and gastric cancers, tumors from the urinary apparatus (i.e., bladder), and nervous central system (i.e., brain). In females, major determinants of decreasing cancer mortality trends were the drop in death rates from cancers originating from uterus, breast, stomach, liver, and urinary apparatus, as well as by the fall in Hodgkin disease-related deaths. Screening and early diagnosis have profoundly, and almost exclusively, impacted mortality from uterine cancer. Conversely, the decrease in breast cancer deaths observed in Naples and confirmed in women aged 65 y and older must be also interpreted in light of the recent achievements in breast cancer characterization and treatment. Indeed, the remarkably improved knowledge of the molecular features of breast cancer has contributed significantly to orientate modern oncology toward targeted and better tolerated therapies specifically tailored to patient individual needs. In women from the province of Naples, the slight decrease in breast cancer mortality might also reflect improvements in terms of diagnostic delay and socio-economic conditions.12,13
Targeted therapy and personalized medicine, although with somewhat less brilliant results in terms of treatment outcomes, might also largely explain the slight decreasing trends in mortality from colorectal cancer, particularly in women from the area of interest.14,15 Adoption of standard therapeutic protocols and integrated treatments administered in full accordance with an evidence based approach and current guidelines might eventually explain reduced mortality from Hodgkin lymphoma.16
Mortality trends related to liver cancer appeared favorable in women from both the provinces of interest. Liver cancer incidence rates recorded in southern Italy are among the highest all over Europe and are mostly attributable to HBV and/or HCV infections.9,17 A more punctual and widespread knowledge of the most common risk factors (e.g., unscreened blood transfusions, re-use of needles and syringes, and unprotected sexual intercourses), along with the availability of testing recommendations and vaccine for HBV have largely contributed to reduce mortality from liver cancer in several western countries, including southern Italy.9,17-20
Cancer mortality from colorectal cancer showed a slightly significant increase in men from Caserta (+0.9, +0.1 to +1.6), while only a suggestion for an increasing trend was found in Naples. These data are inconsistent with those referred to the national level and, with respect to the Umbria region, regional panorama.1,2,21 Differences in lifestyle, particularly concerning a high-fat diet, and prevalence of obesity might at least partly account for the observed discrepancies.22,23
We observed increasing mortality trends for pancreatic cancers, which were consistent across the two genders and provinces of interest. Over the past two decades, pancreatic cancer mortality had generally been leveling off worldwide, although slight increases have been observed in southern and central/eastern Europe in recent years. European cancer mortality predictions for the year 2013 preannounce no improvement in mortality rates for pancreatic cancer in both genders, and, if anything, a slight increase is expected. Tobacco smoking, overweight/obesity, and, at a lesser extent, pancreatitis and heavy alcohol drinking are among the better characterized risk factors for this neoplasm. Improved accuracy in the diagnosis and certification of the disease might partly account for the observed trends. Unfortunately, the achievements in diagnostics have not been paralleled by progresses in treatment, with 5-y survival rates currently lower than 5%.24-27
In women, mortality for lung cancer showed increasing trends in both the provinces examined. This is consistent with results from previous studies focusing on cancer mortality in Italy.1,2,11 Bosetti and co-authors have recently addressed the issue of lung cancer mortality in European women between 1970 and 2009 for 33 European countries, including Italy. Their analysis indicates a steady increase in lung cancer mortality trends for European women up to most recent calendar years. Based on their projections to 2015, a further increase in mortality is expected in major European countries. These trends might be largely explained by different patterns of smoking prevalence in subsequent cohorts of women across different European countries.28,29
We observed significantly increased cancer mortality trends from non-Hodgkin lymphoma in women from the province of Naples, which were somewhat consistent with the non-significant increases reported for females from Caserta and elderly women aged 65 and older. The available evidence on the association between viral hepatitis and non-Hodgkin lymphoma in the metropolitan area of interest might provide some degree of explanation for this finding. Indeed, in a previous hospital-based case-control study including participants from Naples, HCV infection was associated with a significantly increased risk of non-Hodgkin lymphoma, with the fraction of cases attributable to HCV being 12.4%. These results were consistent with those from a previous study including people from Campania and assessing the association of HCV with a number of tumors correlated with the immune system. HCV infection was associated with greater risk not only for B-cell non-Hodgkin lymphoma, but also for multiple myeloma (odds ratios [OR] and 95% CI: 3.7, 1.9–7.4 and 4.5, 1.9–10.7).30,31 Nevertheless, existing literature suggests that exposure to polychlorinated biphenyls (PCBs) contributes to non-Hodgkin lymphoma risk.32,33
In recent years, the metropolitan area of Naples has become sadly renowned worldwide for the illegal practices of waste dumping. A remarkably high number of uncontrolled sites of urban, toxic, and industrial waste disposal, including land filling and unauthorized incineration, have been traced in this area. Though suggestive of a contributory role of waste exposure in determining health outcomes in the identified areas, including cancer related outcomes, the current available evidence is far from being conclusive.34,35
The present study has several strengths. Data presented and discussed relate to a quite wide time frame, namely, the time window between 1998 and 2009. In addition, the appropriateness of the statistical methods used to depict cancer mortality trends and the adjunct of analysis stratified by age, gender and province has further increased the level of detail reached in the analyses performed.
Mortality data were made available by the Italian National Institute of Statistics (ISTAT).36 Previous studies on the degree of agreement between mortality statistics and clinical diagnoses used for assessment by cancer registries have shown a high degree of reciprocal completeness (over 90%) for both systems and a satisfactory level of concordance.37 This increases our confidence in the quality of our study results. Future studies might orientate toward the integrative use of secondary databases as potential tools to enhance the detection of cancer related deaths. In these respects, encouraging results have come from previous work focusing on the potentials of a nationwide hospital discharge file in capturing incident breast cancer cases in Italy.38
Our study suffers also from some limitations. Data for the years 2004 and 2005 were not available. This somehow interrupts the continuum in the temporal spectrum of our evaluation, which remains however quite wide, since it spans from 1998 to 2009. The use of cancer statistics for mortality evaluation does not allow us to distinguish between endometrial and cervical cancers, which indeed differ significantly by etiopathogenetic aspects and therapeutic management. The limited coverage of the area of interest during the considered time frame refrained us from relying even partially on data from cancer registries.39 As previously suggested, the use of multiple data sources might have offered the chance to integrate missing data, thus potentially attenuating these limitations and improving the precision of the estimates herein provided. Moreover, the adopted study design is unable to provide any kind of support to the assessment of causal links between a given neoplasm and its risk factors.
In summary, in men from the metropolitan area of Naples and Caserta, the decline observed in SMR between 1998 and 2009 was less marked compared with males from Italy overall. When focusing on men aged 65 and older, the decline in cancer mortality found for Italy overall diverged from the slight increase emerged from the metropolitan area of interest. The overall tendency toward a slight reduction in cancer mortality coexisted with the slight, but significant, increase in mortality trends for some tumors (NHL, multiple myeloma, pancreas, colon-rectum, among men and lung among women). Factors contributing explanations to the depicted scenario might widely vary by their nature and include lifestyle (e.g., secular trends in smoking, alcohol assumption, diet), overweight and obesity, use of screening procedures, advancements in cancer diagnosis and treatment. By its nature, our study cannot add to the current evidence on the potential causes of the observed cancer mortality trends. However, our findings might help orient public health decision toward specific target populations throughout interventions potential spanning from implementation of screening programs to biomonitoring studies for ascertaining the causal link between exposure to chemical substances in the air, water, soil, food, other potential contaminants, and cancer-related outcomes in the area of interest.
Materials and Methods
Data sources
Population data
Population data are based on the official census of 2001 and referred to the inhabitants of the metropolitan area of Naples and Caserta.40
Death certification data
Mortality data were extracted from national death certificates by age (5-y) groups, gender, residence and cause of death. These records were made available by ISTAT for the two provinces of interest and Italy overall. The time window considered spanned from 1988 to 2009. As the International Classification of Diseases (ICD-9 and ICD-10) changed twice from 1998 to 2008, records related to cancer deaths were re-coded according to the Tenth Revision of ICD.41,42
Data analysis
From the matrices of certified deaths and resident population, we extracted mortality data related to 5-y age-groups for every calendar year between 1988 and 2009. Data for the years 2004 and 2005 were not available.
Statistical analysis
Age-standardized mortality rates were computed for each 5-y age group, by gender, primitive cancer site and province applying the direct method and using the world standard population. To quantify the recent direction of temporal trends in older populations over time, truncated age-adjusted mortality rates were calculated for people aged 65 y and older. Cancer mortality trends between 1988 and 2009 were analyzed by joinpoint regression, using the program provided by the United States Surveillance Epidemiology and End Results (US SEER). Cancer mortality trends were quantified by estimated APC and related 95% CI. This model is based on linear segments connected at joinpoints that best fit the observed data, that is, the segments that minimize the sum of the square of the differences between estimated and observed data. The time trend is divided into segments: the number of segments depends on the number of joinpoints, fixed arbitrarily a-priori. For k join-points we can have a maximum of k+1 segments with different slopes. The joinpoint year is the point in time when we estimate the trend variation.43
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/26425
References
- 1.Arfè A, Malvezzi M, Bertuccio P, Decarli A, La Vecchia C, Negri E. Cancer mortality trend analysis in Italy, 1970-2007. Eur J Cancer Prev. 2011;20:364–74. doi: 10.1097/CEJ.0b013e328345f99e. [DOI] [PubMed] [Google Scholar]
- 2.Bidoli E, Fratino L, Bruzzone S, Pappagallo M, De Paoli P, Tirelli U, Serraino D. Time trends of cancer mortality among elderly in Italy, 1970-2008: an observational study. BMC Cancer. 2012;12:443. doi: 10.1186/1471-2407-12-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Facchini U, Camnasio M, Cantaboni A, Decarli A, La Vecchia C. Geographical variation of cancer mortality in Italy. Int J Epidemiol. 1985;14:538–48. doi: 10.1093/ije/14.4.538. [DOI] [PubMed] [Google Scholar]
- 4.Malvezzi M, Bertuccio P, Chatenoud L, Negri E, La Vecchia C, Decarli A. Cancer mortality in Italy, 2003. Tumori. 2009;95:655–64. doi: 10.1177/030089160909500603. [DOI] [PubMed] [Google Scholar]
- 5.Baili P, De Angelis R, Casella I, Grande E, Inghelmann R, Francisci S, Verdecchia A, Capocaccia R, Meneghini E, Micheli A. Italian cancer burden by broad geographical area. Tumori. 2007;93:398–407. doi: 10.1177/030089160709300412. [DOI] [PubMed] [Google Scholar]
- 6.AIRTUM Working Group Italian cancer figures-report 2006. Incidence, mortality and estimates. Epidemiol Prev. 2006;30 (1-Suppl 2):8–10, 12-28, 30-101. [PubMed] [Google Scholar]
- 7.Biggeri A. Evoluzione del profilo di mortalità: l’Italia che cambia. Epidemiol Prev. 2012;2012:5–5. [Google Scholar]
- 8.Pirastu R, Zona A, Ancona C, Bruno C, Fano V, Fazzo L, Iavarone I, Minichilli F, Mitis F, Pasetto R, et al. [Mortality results in SENTIERI Project] Epidemiol Prev. 2011;35(Suppl 4):29–152. [PubMed] [Google Scholar]
- 9.Bidoli E, Montella M, Bruzzone S, De Paoli A, Fusco M, Frova L, Pace M, Pappagallo M, Serraino D. [Cancer mortality in Southern Italy, 1999-2003] Epidemiol Prev. 2011;35:200–6. [PubMed] [Google Scholar]
- 10.Regione Campania - Assessorato Ricerca Scientifica ed Informatica: Annuario Statistico Campano 1993, 1999, 2003.
- 11.AIRTUM Working Group Italian cancer figures, report 2009: Cancer trend 1998-2005. Epidemiol Prev. 2009;33(4-5 Suppl 1):1–168. [PubMed] [Google Scholar]
- 12.Montella M, Biondi E, De Marco M, Botti G, Tatangelo F, Capasso I, Marone A. Sociodemographic factors associated with the diagnostic staging of breast cancer in southern Italy. Cancer. 1995;76:1585–90. doi: 10.1002/1097-0142(19951101)76:9<1585::AID-CNCR2820760914>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 13.Montella M, Crispo A, Botti G, De Marco MR, de Bellis G, Fabbrocini G, Pizzorusso M, Tamburini M, D’Aiuto G. An assessment of delays in obtaining definitive breast cancer treatment in Southern Italy. Breast Cancer Res Treat. 2001;66:209–15. doi: 10.1023/A:1010622909643. [DOI] [PubMed] [Google Scholar]
- 14.Quaglia A, Lillini R, Crocetti E, Buzzoni C, Vercelli M, AIRTUM Working Group Incidence and mortality trends for four major cancers in the elderly and middle-aged adults: an international comparison. Surg Oncol. 2013;22:e31–8. doi: 10.1016/j.suronc.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Wils J. Adjuvant treatment of colon cancer: past, present and future. J Chemother. 2007;19:115–22. doi: 10.1179/joc.2007.19.2.115. [DOI] [PubMed] [Google Scholar]
- 16.Tsukasaki K, Tobinai K, Hotta T, Shimoyama M. Lymphoma study group of JCOG. Jpn J Clin Oncol. 2012;42:85–95. doi: 10.1093/jjco/hyr168. [DOI] [PubMed] [Google Scholar]
- 17.Franceschi S, Montella M, Polesel J, La Vecchia C, Crispo A, Dal Maso L, Casarin P, Izzo F, Tommasi LG, Chemin I, et al. Hepatitis viruses, alcohol, and tobacco in the etiology of hepatocellular carcinoma in Italy. Cancer Epidemiol Biomarkers Prev. 2006;15:683–9. doi: 10.1158/1055-9965.EPI-05-0702. [DOI] [PubMed] [Google Scholar]
- 18.Montella M, Crispo A, Serraino D, Rezza G, Franceschi S. Is the spread of HCV in Southern Italy attributable to iatrogenic transmission through unsterile injections? Eur J Cancer Prev. 2003;12:85–6. doi: 10.1097/00008469-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 19. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm
- 20. http://www.cdc.gov/vaccines/pubs/vis/downloads/vis-hep-b.pdf
- 21.Stracci F, Canosa A, Minelli L, Petrinelli AM, Cassetti T, Romagnoli C, La Rosa F. Cancer mortality trends in the Umbria region of Italy 1978-2004: a joinpoint regression analysis. BMC Cancer. 2007;7:10. doi: 10.1186/1471-2407-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo A, Franceschi S, La Vecchia C, Dal Maso L, Montella M, Conti E, Giacosa A, Falcini F, Negri E. Body size and colorectal-cancer risk. Int J Cancer. 1998;78:161–5. doi: 10.1002/(SICI)1097-0215(19981005)78:2<161::AID-IJC7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi S, La Vecchia C, Russo A, Favero A, Negri E, Conti E, Montella M, Filiberti R, Amadori D, Decarli A. Macronutrient intake and risk of colorectal cancer in Italy. Int J Cancer. 1998;76:321–4. doi: 10.1002/(SICI)1097-0215(19980504)76:3<321::AID-IJC6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Sharma C, Eltawil KM, Renfrew PD, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010. World J Gastroenterol. 2011;17:867–97. doi: 10.3748/wjg.v17.i7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosetti C, Bertuccio P, Negri E, La Vecchia C, Zeegers MP, Boffetta P. Pancreatic cancer: overview of descriptive epidemiology. Mol Carcinog. 2012;51:3–13. doi: 10.1002/mc.20785. [DOI] [PubMed] [Google Scholar]
- 26.Rosso T, Malvezzi M, Bertuccio P, Negri E, La Vecchia C, Decarli A. Cancer mortality in Italy, 2008, and predictions for 2012. Tumori. 2012;98:559–67. doi: 10.1177/030089161209800504. [DOI] [PubMed] [Google Scholar]
- 27.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24:792–800. doi: 10.1093/annonc/mdt010. [DOI] [PubMed] [Google Scholar]
- 28.Bosetti C, Malvezzi M, Rosso T, Bertuccio P, Gallus S, Chatenoud L, Levi F, Negri E, La Vecchia C. Lung cancer mortality in European women: trends and predictions. Lung Cancer. 2012;78:171–8. doi: 10.1016/j.lungcan.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Forey B, Hamling J, Lee P, Wald N, eds. International Smoking Statistics, a collection of historical data from 30 economically developed countries (2nd edition). [Google Scholar]
- 30.Talamini R, Montella M, Crovatto M, Dal Maso L, Crispo A, Negri E, Spina M, Pinto A, Carbone A, Franceschi S. Non-Hodgkin’s lymphoma and hepatitis C virus: a case-control study from northern and southern Italy. Int J Cancer. 2004;110:380–5. doi: 10.1002/ijc.20137. [DOI] [PubMed] [Google Scholar]
- 31.Montella M, Crispo A, Frigeri F, Ronga D, Tridente V, De Marco M, Fabbrocini G, Spada O, Mettivier V, Tamburini M. HCV and tumors correlated with immune system: a case-control study in an area of hyperendemicity. Leuk Res. 2001;25:775–81. doi: 10.1016/S0145-2126(01)00027-3. [DOI] [PubMed] [Google Scholar]
- 32.De Roos AJ, Hartge P, Lubin JH, Colt JS, Davis S, Cerhan JR, Severson RK, Cozen W, Patterson DG, Jr., Needham LL, et al. Persistent organochlorine chemicals in plasma and risk of non-Hodgkin’s lymphoma. Cancer Res. 2005;65:11214–26. doi: 10.1158/0008-5472.CAN-05-1755. [DOI] [PubMed] [Google Scholar]
- 33.Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benrahim-Tatlaa L, et al. Cancer Monograph Working Group IARC, Lyon France Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. The Lancent Oncology. 2013;14:287–8. doi: 10.1016/S1470-2045(13)70104-9. [DOI] [PubMed] [Google Scholar]
- 34.Altavista P, Belli S, Bianchi F, Binazzi A, Comba P, Del Giudice R, Fazzo L, Felli A, Mastrantonio M, Menegozzo M, et al. [Cause-specific mortality in an area of Campania with numerous waste disposal sites] Epidemiol Prev. 2004;28:311–21. [PubMed] [Google Scholar]
- 35.Barba M, Mazza A, Guerriero C, Di Maio M, Romeo F, Maranta P, Marino IR, Paggi MG, Giordano A. Wasting lives: the effects of toxic waste exposure on health. The case of Campania, Southern Italy. Cancer Biol Ther. 2011;12:106–11. doi: 10.4161/cbt.12.2.16910. [DOI] [PubMed] [Google Scholar]
- 36.Italian National Institute of Statistics http://demo.istat.it; http://www.istat.it
- 37.Doll R, Smith PG, Waterhouse JAH, Muir CS, Shanmugaratnam K, Powell J, et al. Comparison between registries: age standardized rates. In: Waterhouse JAH, Muir CS, Shanmugaratnam K, Powell J, Peacham D, Whelan S, editors. Cancer incidence in five continents. Lyon: IARC Press; 1982:671-675. [Google Scholar]
- 38.Piscitelli P, Barba M, Crespi M, Di Maio M, Santoriello A, D’Aiuto M, Fucito A, Losco A, Pentimalli F, Maranta P, et al. Human Health Foundation Study Group, in memory of Prof. Giovan Giacomo Giordano The burden of breast cancer in Italy: mastectomies and quadrantectomies performed between 2001 and 2008 based on nationwide hospital discharge records. J Exp Clin Cancer Res. 2012;31:96. doi: 10.1186/1756-9966-31-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crocetti E, Capocaccia R, Casella C, De Lisi V, Ferretti S, Guzzinati S, et al. Italian cancer figures report 2006. Epidemiol Prev. 2006;30:30–99. [Google Scholar]
- 40.ISTAT. XIV Censimento della popolazione e delle abitazioni. Roma, ISTAT (2003). [Google Scholar]
- 41.World Health Organization. International classification of disease, 9th revision. Geneva; World Health Organization; 1977. [Google Scholar]
- 42.World Health Organization. International statistical classification of disease and related health problems, 10th revision. Geneva; World Health Organization; 1992. [Google Scholar]
- 43.National Cancer Institute. Joinpoint Regression Program. Version 343rd edition; 2010. Available at: http//srab.cancer.gov/joinpoint
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.