Abstract
Nasopharyngeal carcinoma (NPC) is uncommon worldwide but often highly invasive in late stages. Due to its special location and lack of specific symptoms, NPC is hardly detected in regular medical examination at the beginning. Development of sensitive and specific biomarkers should help to save lives against this type of disease. In the present report, we investigated the value of plasma miRNAs for diagnosis and prognosis of NPC. Using candidate approach, we selected 21 miRNAs from literature to compare their expression levels in the plasma of NPC patients and controls. As a result, 5 miRNAs showed diagnostic potentials (P < 0.01). Among them, miR-16, -21, -24, and -155 had increased levels in NPC patients, whereas the level of miR-378 was decreased. There was a negative correlation between plasma miRNA expression and cancer progression, where miR-21 was statistically significant in T and N staging and miR-16 and 24 were significant in N staging only. Combination of miR-16, -21, -24, -155, and -378 gives 87.7% of sensitivity and 82.0% of specificity for NPC diagnosis. Without miR-16, combination of the rest 4 miRNAs gives the same sensitivity but a slightly reduced specificity. After treatment, all 5 miRNAs were somewhat back to normal levels in patients without cancer recurrence but the prognostic value was not statistically significant. In conclusion, plasma miRNA expression is a useful biomarker for NPC diagnosis but not for its prognosis. More importantly, it is simple, effective, and non-invasive. Combination of several plasma miRNAs can increase both NPC diagnostic sensitivity and specificity.
Keywords: nasopharyngeal carcinoma, NPC, miRNA, plasma, biomarker, diagnosis, prognosis
Introduction
Cancer can grow from any tissue of human body. Nasopharyngeal carcinoma (NPC) is not a common type of cancer that we often see in clinic worldwide. Instead, it is characterized by its confined geographical prevalence. In some area like the southern part of mainland China, as well as Hong Kong, Taiwan, and some southeast Asian countries, NPC is on the top list of deadly cancerous diseases.1 Although many studies have shown that NPC has very close association with Epstein–Barr virus (EBV) infection, the cause of this type of cancer is still not very well understood.2,3 The clinical symptoms of NPC are usually nonspecific, but its location is somewhat special and examination of nasopharynx area requires expertise, which render early detection of NPC very difficult. To date, it remains a challenge to find efficient biomarkers for early detection/diagnosis and prognosis of this type of malignant disease.
MicroRNAs (miRNAs) are a class of non-translated small nuclear RNAs (snRNAs), which play important roles in cell growth, differentiation and specific functions.4-6 Currently, close to 2000 mature miRNAs derived from over 1500 gene loci have been identified in human cells, and maybe more await discovery.7 Expression of miRNAs can be tissue-specific, and cancer cells often contain dysregulated levels of miRNAs.8,9 Studies have shown that miRNA profiling can be useful biomarkers for diagnosis, prognosis, and predicting a patient’s response to cancer treatment.10 Meanwhile, it has been known for a long time that the circulating bloodstream carries not only cells and chemicals vital for life, but also small amount of genetic materials, i.e., nucleic acids such as various types of DNAs and RNAs.11 The possible source of genetic materials found in blood varies from naturally dead cells, physiological secretion, and viral infections. Under normal conditions in a healthy human body, miRNAs are also present in circulating peripheral blood, and predicted to regulate differentiation of primary blood cells, maintain biological homeostasis, and control metabolic pathways.12,13 In cancer patients, elevated levels of miRNA in their serum have been found.14 Notably, much of these miRNAs are protected in microvesicles or in exosomes, of which the latter are a type of small membrane vesicles of endocytic origin that is often released from cancer cells into peripheral blood circulation,15 resulting in remarkable change in the miRNA profile in blood.16,17 These extra miRNAs in a cancer patient’s plasma may function in signaling events that affect the development of cancer cell’s tolerance, tumorigenesis, and organ/tissue-specific metastasis.18-21 Therefore, there are several reasons for plasma miRNA to become very promising cancer biomarkers: (1) miRNA expression is often dysregulated in cancer cells and seems to be tissue-specific; (2) miRNAs appear to be very stable in serum and/or plasma; (3) blood samples are easy to obtain and its method is non-invasive compared with tissue biopsy; (4) change of miRNA levels in the plasma are supposed to be earlier than any of their downstream effect on cancer growth. In fact, in the past few years studies on plasma miRNA profiling have been reported on breast, liver, lung, prostate, colorectal, pancreatic, gastric, head, and neck cancers.16,22,23 Although not all studies had reached satisfactory results, they opened up an exciting prospective for cancer treatment.
MicroRNA plays an important role in the development of nasopharyngeal carcinoma (NPC). In recent years, miRNA expression in NPC has been studied in small or large scales. Most of them were focused on NPC-associated EBV viral miRNAs,24 or cellular miRNAs isolated from cancer biopsy specimen.25,26 There were also several attempts to evaluate miRNA as diagnostic and prognostic biomarkers for NPC.27-29 However, studies on plasma miRNA in NPC patients have not been reported yet. In the present report, we examined expression of 21 miRNAs in the plasma of NPC patients with proper control. We performed statistical analysis for association studies on miRNA expression and NPC clinical staging, and also compared the levels of miRNA before and after treatment. Our findings indicate that plasma miRNA profiling is a simple, reliable and non-invasive biomarker for early detection of NPC and therefore will be helpful to treat this type of cancer in human.
Results
Patient characteristics
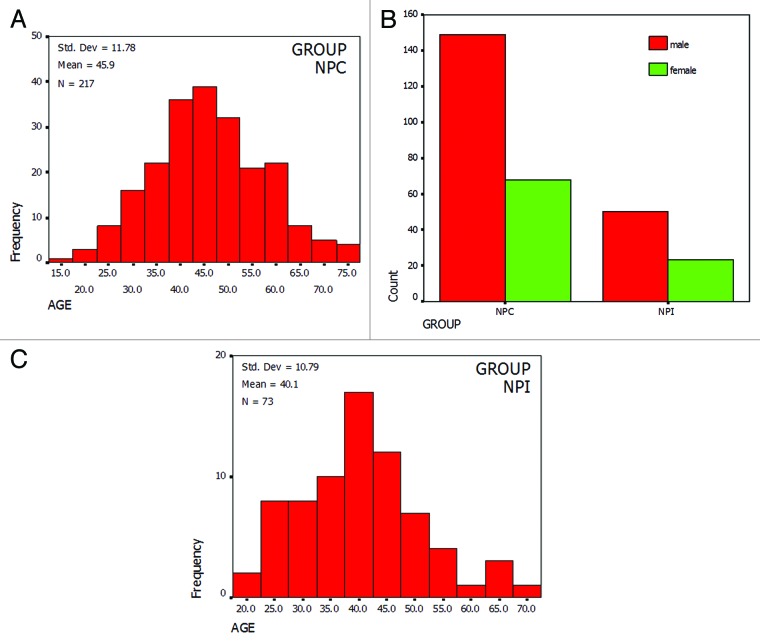
Among 217 NPC patients, there were 149 males and 68 females with age ranging from 17 to 77 y old on average of 45.93 ± 11.78, whereas in the NPI control group there were 50 males and 23 females with age ranging from 18 to 68 y old on average of 40.08 ± 10.79. The male to female ratio was about 2.19:1 among NPC patients, which was similar to that of control group. The distribution of NPC patients and NPI control individuals among age groups and between sexes was shown in Figure 1. Most incidences of NPC were first diagnosed on patients between 35 to 55 y old.
Figure 1. Distribution of NPC patients and control. (A) Among age. (B) Between sexes. NPC, nasopharyngeal carcinoma; NPI, nasopharyngitis.
NPC patients were classified using two different systems: (1) the TNM cancer staging system, which was created by the American Joint Committee on Cancer (AJCC); (2) the Chinese NPC 2008 staging system, which was created and recommended by the Chinese Committee for Clinical Staging of Nasopharyngeal Carcinoma. In the latter, the T, N, and M categories were combined and further divided into 4 groups designated as I–IV. Compared with its previous version, the newly established Chinese NPC 2008 staging system was largely based on MRI analysis, and proved to be more accurate and practical.30,31 In the present study, the number of patients in stages I, II, III and IV was 10, 52, 69, and 86, respectively.
Pilot study
A pilot study was performed to validate 21 pre-selected miRNA candidates (Table 1) in a small group (10%) of patients, which consisted of 22 NPC patients and 7 controls. Blood samples were collected and processed as described in Materials and Methods. RNA was extracted and reverse transcribed into the first strand of cDNA. Then, RT-qPCR was performed to measure the expression levels of these miRNAs in patient’s plasma. Expression levels were compared in relative to internal controls as described in Materials and Methods, and analyzed by the two-independent parametric Student t test. As shown in Table 2, 7 miRNAs had increased plasma expression levels in NPC patients compared with control. Among them, miR-16, miR-21, miR-24, and miR-155 were changed by 6- to 28-fold. The other 14 miRNAs had lower plasma expression levels in NPC patients compared with control. Most of the changes were subtle except miR-378, which was less than 10% of the control (P < 0.001). Therefore, miR-16, 21, 24, 155, and 378 were subjected to further analysis.
Table 1. Primer design (http://www.mirbase.org).
| Name | RNA sequence (5′–3′) | Primer (5′–3′) |
|---|---|---|
| Cel-miR-39 | UCACCGGGUG UAAAUCAGCU UG | TCACCGGGTG TAAATCAGCT TG |
| Let-7a | UGAGGUAGUA GGUUGUAUAG UU | TGAGGTAGTA GGTTGTATAG TT |
| miR-009 | UCUUUGGUUA UCUAGCUGUA UGA | TCTTTGGTTA TCTAGCTGTA TGA |
| miR-010b | UACCCUGUAG AACCGAAUUU GUG | TACCCTGTAG AACCGAATTT GTG |
| miR-015a | UAGCAGCACA UAAUGGUUUG UG | TAGCAGCACA TAATGGTTTG TG |
| miR-016 | UAGCAGCACG UAAAUAUUGG CG | TAGCAGCACG TAAATATTGG CG |
| miR-021 | UAGCUUAUCA GACUGAUGUU GA | TAGCTTATCA GACTGATGTT GA |
| miR-024 | UGGCUCAGUU CAGCAGGAAC AG | TGGCTCAGTT CAGCAGGAAC AG |
| miR-026a | UUCAAGUAAU CCAGGAUAGG CU | TTCAAGTAAT CCAGGATAGG CT |
| miR-029c | UAGCACCAUU UGAAAUCGGU UA | TAGCACCATT TGAAATCGGT TA |
| miR-031 | AGGCAAGAUG CUGGCAUAGC U | AGGCAAGATG CTGGCATAGC T |
| miR-034a | UGGCAGUGUC UUAGCUGGUU GU | TGGCAGTGTC TTAGCTGGTT GT |
| miR-092a | UAUUGCACUU GUCCCGGCCU GU | TATTGCACTT GTCCCGGCCT GT |
| miR-130a | CAGUGCAAUG UUAAAAGGGC AU | CAGTGCAATG TTAAAAGGGC AT |
| miR-141 | UAACACUGUC UGGUAAAGAU GG | TAACACTGTC TGGTAAAGAT GG |
| miR-143 | UGAGAUGAAG CACUGUAGCU C | TGAGATGAAG CACTGTAGCT C |
| miR-145 | GUCCAGUUUU CCCAGGAAUC CCU | GTCCAGTTTT CCCAGGAATC CCT |
| miR-155 | UUAAUGCUAA UCGUGAUAGG GGU | TTAATGCTAA TCGTGATAGG GGT |
| miR-184 | UGGACGGAGA ACUGAUAAGG GU | TGGACGGAGA ACTGATAAGG GT |
| miR-211 | UUCCCUUUGU CAUCCUUCGC CU | TTCCCTTTGT CATCCTTCGC CT |
| miR-221 | AGCUACAUUG UCUGCUGGGU UUC | AGCTACATTG TCTGCTGGGT TTC |
| miR-378 | ACUGGACUUG GAGUCAGAAG G | ACTGGACTTG GAGTCAGAAG G |
| U6 | CGCAAGGAUG ACACGCAAAU UCGU | CGCAAGGATG ACACGCAAAT TCGT |
Table 2. Pilot study on plasma miRNA expression.
| Name | ΔCt | 2-ΔΔCt | t | P | |
|---|---|---|---|---|---|
| NPC (n = 22) | NPI (n = 7) | ||||
| miR-16 | −11.102 ± 3.828 | −6.251 ± 1.489 | 28.860 | 4.893 | <0.001** |
| miR-155 | −4.061 ± 5.777 | −0.036 ± 1.986 | 16.287 | 2.791 | 0.010* |
| miR-24 | −5.807 ± 3.416 | −2.589 ± 1.808 | 9.305 | 2.369 | 0.025* |
| miR-21 | −8.120 ± 3.555 | −5.387 ± 3.803 | 6.648 | 1.744 | 0.093 |
| miR-143 | 6.810 ± 6.789 | 8.484 ± 2.613 | 3.191 | 0.631 | 0.533 |
| miR-141 | −4.425 ± 2.489 | −4.236 ± 1.291 | 1.140 | 0.191 | 0.850 |
| miR-10b | −4.474 ± 2.463 | −4.386 ± 1.838 | 1.063 | 0.087 | 0.931 |
| miR-15a | 0.207 ± 3.700 | 0.060 ± 2.162 | 0.903 | −0.099 | 0.922 |
| miR-26a | 0.616 ± 3.802 | 0.380 ± 2.903 | 0.849 | −0.150 | 0.882 |
| miR-130a | −4.345 ± 3.195 | −4.609 ± 2.001 | 0.833 | −0.204 | 0.840 |
| let-7a | 4.708 ± 3.132 | 4.350 ± 3.162 | 0.780 | −0.263 | 0.795 |
| miR-145 | −4.561 ± 2.522 | −4.940 ± 1.765 | 0.769 | −0.364 | 0.718 |
| miR-221 | −2.914 ± 2.294 | −3.333 ± 2.653 | 0.748 | −0.406 | 0.688 |
| miR-92a | 9.005 ± 3.637 | 8.297 ± 1.834 | 0.612 | −0.491 | 0.627 |
| miR-211 | −2.734 ± 2.426 | −3.733 ± 3.215 | 0.500 | −0.878 | 0.388 |
| miR-34a | −1.731 ± 2.686 | −2.790 ± 3.042 | 0.480 | −0.881 | 0.386 |
| miR-31 | −4.014 ± 3.060 | −5.137 ± 1.841 | 0.459 | −0.913 | 0.369 |
| miR-9 | −4.143 ± 3.234 | −5.316 ± 2.331 | 0.443 | −0.884 | 0.385 |
| miR-184 | −1.216 ± 2.845 | −2.939 ± 2.780 | 0.303 | −1.402 | 0.172 |
| miR-29c | −4.024 ± 3.750 | −5.967 ± 2.360 | 0.260 | −1.622 | 0.124 |
| miR-378 | −1.922 ± 3.173 | −5.970 ± 3.656 | 0.091 | −2.839 | 0.009** |
*P < 0.05; **P < 0.01
To monitor PCR conditions, graphical analysis on amplification curves and melting peaks of miR-16, 21, 24, 155, and 378 and miRNA controls are shown in Figure 2. All PCR amplification of miRNAs including internal control U6 and positive control Cel-miR-39, but not the negative control (water only), had a single melting peak, indicating that the amplification was specific. Moreover, amplification curves of miRNA covered initial phase, amplification phase, and stationary phase, which was essential for quantification analysis.
Figure 2. Illustration of amplification plots and melting curves from RT-qPCR. Representative results are shown for amplification of plasma miR-16, 21, 24, 155, and 378, while the snRNA U6 serves as internal control. A set of PCR includes synthetic miRNA Cel-miR-39 and distilled water as positive and negative controls, respectively.
Expression of miR-16, 21, 24, 155, and 378 in large scale analysis
Given the PCR condition in pilot experiment, we examined the expression of miR-16, 21, 24, 155, and 378 on the plasma specimen of 217 NPC patients and 73 NPI controls followed by covariance analysis. As shown in Table 3, expression of miR-16, 21, 24, and 155 in NPC patients had remarkable increase compared with control, which was ranging from about 5- to 10-fold. The expression of miR-378 in NPC patients remarkably decreased at similar magnitude, which was less than 25% of the control. In all cases, the difference between NPC and control groups was statistically significant (P < 0.01). To analyze the expression of miRNAs in relation to age, we used the Pearson test and found that miR-378 but not 16, 21, 24, and 155, had strong association with age (P = 0.007). In both groups, miR-378 expression decreased in elderly people. Meanwhile, we performed a two-independent parametric Student t test for sex association and found that none of these 5 miRNAs had any significant difference between males and females (data not shown).
Table 3. Expression of 5 miRNAs in the plasma of NPC patients and control.
| Name | ΔCt | 2−ΔΔCt | F | P | |
|---|---|---|---|---|---|
| NPC (n = 217) | NPI (n = 73) | ||||
| miR-16 | −6.295 ± 3.721 | −3.840 ± 3.140 | 5.483 | 25.369 | <0.001** |
| miR-21 | −6.687 ± 2.915 | −3.364 ± 3.252 | 10.001 | 72.535 | <0.001** |
| miR-24 | −3.851 ± 2.881 | −1.586 ± 2.594 | 4.808 | 34.710 | <0.001** |
| miR-155 | −2.001 ± 3.137 | 0.475 ± 2.257 | 5.561 | 40.680 | <0.001** |
| miR-378 | −1.286 ± 1.842 | −3.302 ± 3.173 | 0.247 | 37.960 | <0.001** |
**P < 0.01
Association of plasma miRNA expression with cancer progression
To examine the expression of plasma miRNAs in relation to cancer progression, we used Spearman test for non-parametric correlation analysis between each miRNA expression and cancer progression stages. As shown in Table 4, we found that the expression of all 5 miRNAs was somewhat negatively correlated with the progression of NPC disease in T stages, where in the case of miR-21 the correlation was statistically significant (P < 0.05). We observed a similar situation in N staging categories, where miR-16, 21, and 24 gave strong but negative correlation to the progression of NPC. No significant correlation was found in M stages. Based on the Chinese NPC 2008 staging system, it also appears that the expression of all these 5 miRNAs was negatively correlated with cancer progression. Among them, miR-21 had the highest level of correlation (P = 0.056), which indicated that it might be associated with the early stage of NPC.
Table 4. Statistical analysis of miRNA expression in plasma among clinical stages.
| Clinical stage | N | miR-16 | miR-21 | miR-24 | miR-155 | miR-378 |
|---|---|---|---|---|---|---|
| T1 | 41 | 107.48 | 128.84 | 115.60 | 111.88 | 112.68 |
| T2 | 55 | 128.55 | 117.69 | 125.88 | 124.28 | 120.09 |
| T3 | 66 | 96.48 | 92.91 | 87.88 | 91.72 | 99.63 |
| T4 | 55 | 105.60 | 104.83 | 112.55 | 112.31 | 106.41 |
| Spearman | r | −0.085 | −0.159 | −0.090 | −0.064 | −0.075 |
| P | 0.214 | 0.019* | 0.188 | 0.348 | 0.269 | |
| N0 | 34 | 127.50 | 133.88 | 134.57 | 121.76 | 105.37 |
| N1 | 100 | 115.61 | 108.54 | 107.60 | 112.02 | 117.02 |
| N2 | 50 | 88.51 | 98.61 | 97.53 | 94.21 | 101.16 |
| N3 | 33 | 100.95 | 100.50 | 104.27 | 109.11 | 100.32 |
| Spearman | r | −0.187 | −0.160 | −0.149 | −0.105 | −0.068 |
| P | 0.006** | 0.018* | 0.028* | 0.122 | 0.318 | |
| M0 | 200 | 107.26 | 110.36 | 108.57 | 109.83 | 110.80 |
| M1 | 17 | 129.53 | 93.06 | 114.12 | 99.24 | 87.88 |
| Spearman | r | 0.096 | −0.074 | 0.024 | −0.045 | −0.098 |
| P | 0.161 | 0.277 | 0.727 | 0.505 | 0.149 | |
| I | 10 | 115.30 | 126.35 | 135.60 | 105.20 | 94.30 |
| II | 52 | 131.70 | 122.88 | 125.90 | 123.77 | 123.90 |
| III | 69 | 92.55 | 104.41 | 90.90 | 99.59 | 105.08 |
| IV | 86 | 107.74 | 102.27 | 110.21 | 108.06 | 104.84 |
| Spearman | r | −0.106 | −0.130 | −0.086 | −0.061 | −0.076 |
| P | 0.118 | 0.056 | 0.205 | 0.369 | 0.264 |
*P < 0.05; **P < 0.01
Receiver operating characteristics (ROC)
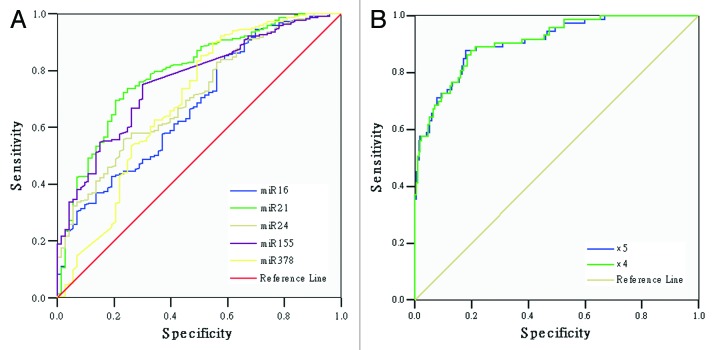
To examine the diagnostic value of these 5 miRNAs, we performed statistical analysis on their receiver operating characteristics (ROC).32 The ROC curves were calculated and shown in Figure 3, while area under ROC curve (AUR) was given in Table 5. As what we can see, miR-16, 21, 24, 155, and 378 demonstrated fairly good (>60%) specificities and sensitivities for NPC diagnosis. In particular, miR-21 and 155 yielded 79.2% and 76.3% of AUR, respectively (Fig. 3A). A combination of 5 miRNAs yielded 90.8% of AUR, in which it provided 87.7% of sensitivity and 82.0% of specificity for NPC diagnosis. In the absence of miR-16, combination of the rest 4 miRNAs had no change in diagnostic sensitivity, while the diagnostic specificity only dropped slightly from 82.0% to 80.2% (Fig. 3B).
Figure 3. Diagnostic value of plasma miRNAs. (A) ROC curve regression analysis was performed for expression of miR-16, 21, 24, 155, and 378 in the plasma of NPC patients. (B) ROC curve Binary Logistic regression analysis for combination of 4 or 5 miRNAs. ROC, receiver operative characteristic.
Table 5. Diagnostic value of 5 plasma miRNAs.
| Name | AUR | SD | 95% confidence | Sensitivity | Specificity | P | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| miR-16 | 0.677 | 0.036 | 0.606 | 0.747 | 0.687 | 0.507 | <0.001** |
| miR-21 | 0.792 | 0.030 | 0.733 | 0.852 | 0.760 | 0.699 | <0.001** |
| miR-24 | 0.707 | 0.034 | 0.641 | 0.773 | 0.668 | 0.575 | <0.001** |
| miR-155 | 0.763 | 0.031 | 0.703 | 0.823 | 0.751 | 0.699 | <0.001** |
| miR-378 | 0.688 | 0.040 | 0.609 | 0.766 | 0.671 | 0.604 | <0.001** |
| × 5 | 0.908 | 0.020 | 0.869 | 0.948 | 0.877 | 0.820 | <0.001** |
| × 4 | 0.909 | 0.020 | 0.871 | 0.948 | 0.877 | 0.802 | <0.001** |
× 5, Combination of 5 miRNAs 16, 21, 24, 155, and 378; × 4, Combination of 4 miRNAs 21, 24, 155, and 378; AUR, area under ROC curve; SD, standard deviation. **P < 0.01
Treatment and follow-ups
All patients were given treatment based on the most recent NPC treatment guidelines. A total of 40 NPC patients had complete records of follow-up check, where 31 patients were males and 10 were females. Among them, 12 patients were followed up for more than 24 mo, 9 were between 12 to 24 mo, 7 were between 3 to 12 mo, and another 7 patients were less than 3 mo. Five patients had cancer relapse and displayed metastasis. To predict the patient’s survival rate based on miRNA information, we performed regression analysis using Cox model. As a result, none of these 5 miRNAs had any association with any side effects that had been tested in the present study, and all of them failed to predict the outcome of treatment, which was given by the patient’s survival rate (data not shown). Therefore, the prognostic values of these plasma miRNAs alone or in combination are, if any, very limited. However, all 5 miRNA expressions were altered after therapeutic treatment (Table 6). The Student t test for paired samples indicated that expression levels of miR-16, 21, 24, and 155 in plasma were significantly reduced after treatment in cancer survivors without cancer relapse (P < 0.05), but they were increased in patients with cancer relapse and metastasis. On the contrary, miR-378 level in plasma increased to a certain extent in survivors without relapse but further decreased in patients with cancer relapse and metastasis (data not shown). Therefore, the changing pattern of plasma miRNA somewhat reflected the disease development tendency. For all miRNAs in test, their expression levels seemed to go back to normal after treatment in patients without cancer relapse, but deregulate when the disease became worse. Analysis by the two-independent parametric Student t test indicated that in those 5 patients with cancer relapse, expression levels of miR-16, 21, 24, and 155 were somewhat correlated with cancer clinical manifestations but not statistically significant probably due to the small size of samples (data not shown).
Table 6. Expression of 5 miRNAs in the plasma of NPC patients before and after treatment.
| Name | ΔCt | t | P | ||
|---|---|---|---|---|---|
| Before | After | Change | |||
| miR-16 | −6.746 ± 4.460 | −3.032 ± 3.182 | −3.715 ± 5.088 | −4.380 | <0.001** |
| miR-21 | −6.664 ± 3.228 | −2.404 ± 2.912 | −4.259 ± 3.993 | −6.400 | <0.001** |
| miR-24 | −4.077 ± 3.328 | −1.404 ± 2.718 | −2.674 ± 4.474 | −3.586 | 0.001** |
| miR-155 | −2.482 ± 3.209 | 0.315 ± 2.455 | −2.796 ± 3.823 | −4.389 | <0.001** |
| miR-378 | −1.508 ± 1.980 | −2.150 ± 3.173 | 0.643 ± 3.476 | 1.109 | 0.275 |
**P < 0.01
Discussion
To date, close to 2000 miRNAs have been identified that affect cell growth at certain levels in human.7 It is difficult to examine every miRNA for cancer association at one time. First, clinical specimens are limited so that we do not have enough RNA sample for high-throughput screening. Second, making microchips for a whole set of miRNAs for real-time PCR analysis is costly. In this regard, we used candidate approach to select miRNAs for detail analysis. These miRNA candidates were chosen for several reasons: First, they have been previously shown differential expression in nasopharyngeal carcinoma by others. We want to validate these results in the present study. Second, we selected genes that have been well studied in our lab. Third, these miRNAs are expressed in plasma, and some are known to associate with angiogenesis for cancer growth. In the present study, we found that miR-16, 21, 24, and 155 had elevated levels in the plasma specimen of NPC patients, whereas the level of miR-378 was significantly reduced (Tables 2 and 3). Surprisingly, the expression of these miRNAs in plasma did not persistent when cancer progressed into late stages and instead it was negatively correlated with most of the cancer staging categories (Table 4). This finding suggested that these 5 miRNAs in test may play a role in the early stage of cancer development prior to metastasis. Accordingly, it would be also interesting to examine the protein levels of some of these miRNAs’ downstream targets in the future for their associations with cancer development.
Since its discovery in 2001, miR-21 has been found associated with cancer development. In fact, it is one of the most studied miRNAs in human cancers. MiR-21 was highly expressed in solid tumors and leukemia cells,33 and targets PDCD4, PTEN, SPRY, ERCK, and Bcl-2 family proteins. It was regulated at transcription by AP-1, STAT3, and TGF-β, but the mechanism of dysregulation in cancer cells is not clear.34 Biopsy analysis indicated that miR-21 expression was sensitive and specific for diagnosis of cholangiocarcinoma, glioblastoma, and colorectal cancer. Meanwhile, miR-21 expression in serum had proved to be a useful diagnostic marker in breast, lung, gastric, and ovarian cancers, and correlated with the survival rate of B lymphoma patients. In the present study, miR-21 was highly expressed the plasma of NPC patients, but reduced in parallel to cancer progression based on traditional TNM cancer staging, as well as the Chinese 2008 NPC staging system (Table 4). The molecular mechanism is not clear, which deserves further studies.
The function of miR-24 in cells is far more obscure. In some studies, it inhibited cancer growth, while in others it had the opposite effect. Such discrepancy may be explained, in part, by the variable targets of miR-24 in cell signaling. These downstream effectors include but not limited to FAF1, p16, Bcl-2, H2AX, Net1A, and Notch1. The latter two are involved in regulation of EMT in cells, which depends on the TGF-β/Smad/Net1A signaling. In patients with oral carcinoma, miR-24 expression was increased both inside the cancer cells and in the plasma of peripheral blood.35 In consistence with these findings, we found that the expression of miR-24 was higher in the plasma of NPC patients. Statistical analysis indicated that the measurement of plasma miR-24 resulted in a diagnostic value, which was slightly lower than that of miR-21 (Table 4). Meanwhile, somewhat similar to miR-21 the expression of miR-24 was reduced when cancer progressed into higher clinical stages.
Our results have shown that a single miRNA could be useful for diagnosis purpose in human cancer patients, but a combination of several miRNA would significantly increase the sensitivity and specificity of their diagnosis value (Fig. 3; Table 5). This is also true in some studies by others. For example, a combination of miR-122, 192, 21, 223, 26a, 27a, and 801 were very useful for diagnosis of liver cancer,36 whereas combination of miR-21, 486, 375, and 200b gave 80.6% of sensitivity, and 91.7% of specificity in the diagnosis of human lung cancer.37 In the present study, miR-21 was the best among all miRNAs in test, which gave 76% of sensitivity and 70% of specificity for diagnosis of NPC. However, in combination with miR-16, 24, 155, and 378 these values can be increased to 87.7% and 82%, respectively. Without miR-16, the sensitivity remains unchanged, and the specificity only slightly decreased to 80.2%.38 Therefore, we concluded that grouping of miRNA markers is a promising strategy to yield clinically relevant information in the future study.
Early detection and new treatments have largely improved survival rates in human cancer patients. This is especially true in the case of NPC. The presence of certain cancer markers can help to predict cancer diagnosis, prognosis and therapeutic efficacy, and even help to determine whether adjuvant drug treatment should be given. In the present study, we found that miR-16, 21, 24, 155, and 378 exhibited deregulation of expression in the plasma of NPC cancer patients, and 4 or 5 of them demonstrated strong association with cancer staging. After treatment, all of them were somewhat back to normal levels, except those in patients that cancer recurrence took place (data not shown). It appears that using those 4 or 5 biomarkers that have been identified in this study, alone or in combination, should increase the chance of success in early detection, which will help in the battle against this type of cancer in human.
Materials and Methods
Patients
Between December 2007 and June 2011 a total of 217 previously untreated patients, who were initially diagnosed with nasopharyngeal carcinoma in Nanfang Hospital, were included in this study. All patients were given questionnaires about their symptoms and medical history before taking a physical exam. When tumor was found by electric nasopharyngoscope, the patient was hospitalized and tissue biopsy samples were collected and sent for pathological examination. All patients gave blood, urine, and stool samples, which were tested for infectious diseases including HIV, hepatitis B and C viruses, and syphilis as routine. Electrocardiogram examination was also performed. Diagnoses were confirmed by CT, magnetic resonance imaging (MRI), chest X-ray, emission CT (ECT), and whole abdomen ultrasound, urinary tract ultrasound, and/or full body bone scan. In some cases, patients were given whole body PET-CT exam for a complete TNM tumor staging. In addition, all patients received oral and dental care prior to radiation therapy. In the control group, there are 73 patients who suffered chronic suppurative otitis media and chronic sinusitis, which were collectively named nasopharyngitis (NPI).
All patients have been informed and signed a letter of consent for this study. The protocol was approved by Ethnical Committee of Nanfang Hospital, and registered at NIH Clinical Trials (NCT01171235) in the United States.
Blood samples
Approximately 6–8 ml whole blood sample was drawn from each patient into EDTA-containing Vacutainer® tube, mixed well and kept at room temperature. Then, blood samples were centrifuged at 1000 × g for 5 min to separate cellular fractions and plasma. The plasma-containing supernatant was transferred into micro-centrifuge tubes, followed by centrifuge again at 13 000 × g for 10 min. to completely remove cellular components. Finally, plasma was aliquoted and stored at −80 °C until use. All blood samples were processed and completed within 4 h after collection.
RT-qPCR
Total RNA was extracted from plasma specimen with Trizol® LS RNA Extraction Kit (Invitrogen) and reverse transcribed using All-in-One™ miRNA First-strand cDNA Synthesis Kit (Gene Copoeia). Quantitative real-time PCR (RT-qPCR) was performed in duplicate using LightCycler® 480 SYBR Green II real-time PCR system (Roche Applied Science) equipped with LightCycler® 480 software (Release 1.5.0 SP3) according to manufacturer’s instruction for a total of 45 cycles at an annealing temperature of 60 °C. When the reactions were finished, expression levels of miRNA were automatically calculated using the number of cycle threshold (Ct) and normalized to internal control U6 snRNA (ΔCt). The Ct value in each reaction was negatively correlated with the log value of initial copy number of the template. Fold change in miRNA expression after normalization was calculated using equation 2−ΔΔCt.38 In each experiment we included synthetic Cel-miR-39 as positive control and H2O as negative control. Forward primers are listed in Table 1.
Treatment
All patients received standard radiation therapy according to the Practice Guidelines in Oncology for Head and Neck Cancers, v.2.2008, which was written by the National Comprehensive Cancer Network (NCCN). Radiation consisted of 70 Gy in total with a once daily fractionation schedule of 2 Gy per fraction, administered five days per week over 35 treatment days. Sometimes intensity-modulated radiation therapy (IMRT) was performed, which consisted of 66–70 Gy in total with 2.2 Gy per fraction over 30 treatment days. In the case that cancer had spread to neck lymph nodes, 66 Gy in total was used with 2 Gy per fraction over 30 treatment days. Otherwise, 50–56 Gy in total was used with 2 Gy per fraction over 25−30 treatment days.
Patients in Stage I according to Chinese NPC 2008 staging system (see description in Results) received radiation therapy only, whereas those in Stages II–IV received induction chemotherapy prior to radiation or adjuvant chemotherapy after radiation.
Follow-up
All patients provided contact information including telephone and cell phone numbers. Follow-up check was scheduled and delivered to patient by direct phone call. The first return visit usually took place 3 mo after treatment, and then once every half year. The efficacy of treatment was evaluated by video laryngoscope and diagnostic imaging. About 5–6 ml blood samples were collected each time for molecular biology analysis.
Statistical analysis
Statistical analyses were performed by using SPSS 13.0 software (IBM, USA). A P value lower than 0.05 was considered statistically significant.
Disclosure of Potential Conflict of Interests
There were no conflict of interests in the present study.
Acknowledgments
We thank Mr Shengli An from the Department of Biostatistics at Southern Medical University for his technical assistance and Dr Frank Li from Canada for proofreading the manuscript. This work was supported by grants from National Science Foundation of China (81172053) and NSFC-Guangdong Joint Fund (U1132003) to XP Li.
Glossary
Abbreviations:
- CLL
chronic lymphatic leukemia
- CT
computed tomography
- Ct
cycle threshold
- EBV
Epstein–Barr virus
- ECT
emission computed tomography
- HIV
human immunodeficiency virus
- IMRT
intensity-modulated radiation therapy
- miRNA
microRNA
- MRI
magnetic resonance imaging
- NCCN
National Comprehensive Cancer Network
- NIH
National Institutes of Health
- NPC
nasopharyngeal carcinoma
- NPI
nasopharyngitis
- PCR
polymerase chain reaction
- RT-qPCR
quantitative real-time PCR
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/26170
References
- 1.Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004;5:423–8. doi: 10.1016/S1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 2.Busson P, Keryer C, Ooka T, Corbex M. EBV-associated nasopharyngeal carcinomas: from epidemiology to virus-targeting strategies. Trends Microbiol. 2004;12:356–60. doi: 10.1016/j.tim.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Abdulamir AS, Hafidh RR, Abdulmuhaimen N, Abubakar F, Abbas KA. The distinctive profile of risk factors of nasopharyngeal carcinoma in comparison with other head and neck cancer types. BMC Public Health. 2008;8:400. doi: 10.1186/1471-2458-8-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 6.Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310:1817–21. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 7.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Database issue):D152–7. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shell S, Park SM, Radjabi AR, Schickel R, Kistner EO, Jewell DA, Feig C, Lengyel E, Peter ME. Let-7 expression defines two differentiation stages of cancer. Proc Natl Acad Sci U S A. 2007;104:11400–5. doi: 10.1073/pnas.0704372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar FH, Li Y, Wang Z, Kong D, Ali S. Implication of microRNAs in drug resistance for designing novel cancer therapy. Drug Resist Updat. 2010;13:57–66. doi: 10.1016/j.drup.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–64. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsang JC, Lo YM. Circulating nucleic acids in plasma/serum. Pathology. 2007;39:197–207. doi: 10.1080/00313020701230831. [DOI] [PubMed] [Google Scholar]
- 12.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee ML, Schmittgen TD, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schöler N, Langer C, Döhner H, Buske C, Kuchenbauer F. Serum microRNAs as a novel class of biomarkers: a comprehensive review of the literature. Exp Hematol. 2010;38:1126–30. doi: 10.1016/j.exphem.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–5. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 15.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 17.Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–92. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, Mandelboim M, Mandelboim O. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol. 2008;9:1065–73. doi: 10.1038/ni.1642. [DOI] [PubMed] [Google Scholar]
- 19.Iguchi H, Kosaka N, Ochiya T. Secretory microRNAs as a versatile communication tool. Commun Integr Biol. 2010;3:478–81. doi: 10.4161/cib.3.5.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro A, Monzo M. MicroRNAs in human embryonic and cancer stem cells. Yonsei Med J. 2010;51:622–32. doi: 10.3349/ymj.2010.51.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orozco AF, Lewis DE. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A. 2010;77:502–14. doi: 10.1002/cyto.a.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiland M, Gao XH, Zhou L, Mi QS. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol. 2012;9:850–9. doi: 10.4161/rna.20378. [DOI] [PubMed] [Google Scholar]
- 23.Ma R, Jiang T, Kang X. Circulating microRNAs in cancer: origin, function and application. J Exp Clin Cancer Res. 2012;31:38. doi: 10.1186/1756-9966-31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosmopoulos K, Pegtel M, Hawkins J, Moffett H, Novina C, Middeldorp J, Thorley-Lawson DA. Comprehensive profiling of Epstein-Barr virus microRNAs in nasopharyngeal carcinoma. J Virol. 2009;83:2357–67. doi: 10.1128/JVI.02104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen HC, Chen GH, Chen YH, Liao WL, Liu CY, Chang KP, Chang YS, Chen SJ. MicroRNA deregulation and pathway alterations in nasopharyngeal carcinoma. Br J Cancer. 2009;100:1002–11. doi: 10.1038/sj.bjc.6604948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T, Chen JX, Fu XP, Yang S, Zhang Z, Chen KhH, Li Y. microRNA expression profiling of nasopharyngeal carcinoma. Oncol Rep. 2011;25:1353–63. doi: 10.3892/or.2011.1204. [DOI] [PubMed] [Google Scholar]
- 27.Wong AM, Kong KL, Tsang JW, Kwong DL, Guan XY. Profiling of Epstein-Barr virus-encoded microRNAs in nasopharyngeal carcinoma reveals potential biomarkers and oncomirs. Cancer. 2012;118:698–710. doi: 10.1002/cncr.26309. [DOI] [PubMed] [Google Scholar]
- 28.He ML, Luo MX, Lin MC, Kung HF. MicroRNAs: potential diagnostic markers and therapeutic targets for EBV-associated nasopharyngeal carcinoma. Biochim Biophys Acta. 2012;1825:1–10. doi: 10.1016/j.bbcan.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Liu N, Chen NY, Cui RX, Li WF, Li Y, Wei RR, Zhang MY, Sun Y, Huang BJ, Chen M, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol. 2012;13:633–41. doi: 10.1016/S1470-2045(12)70102-X. [DOI] [PubMed] [Google Scholar]
- 30.Mao YP, Li WF, Chen L, Sun Y, Liu LZ, Tang LL, Cao SM, Lin AH, Hong MH, Lu TX, et al. [A clinical verification of the Chinese 2008 staging system for nasopharyngeal carcinoma] Ai Zheng. 2009;28:1022–8. doi: 10.5732/cjc.009.10425. [DOI] [PubMed] [Google Scholar]
- 31.Lin ZX, Yang ZN, Zhan YZ, Xie WJ, Li GW, Feng HT. [Application study of the 2008 staging system of nasopharyngeal carcinoma] Ai Zheng. 2009;28:1029–32. doi: 10.5732/cjc.009.10431. [DOI] [PubMed] [Google Scholar]
- 32.Scott PW. Making a spectacle. Vet Rec. 1992;130:232. doi: 10.1136/vr.130.11.232. [DOI] [PubMed] [Google Scholar]
- 33.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jazbutyte V, Thum T. MicroRNA-21: from cancer to cardiovascular disease. Curr Drug Targets. 2010;11:926–35. doi: 10.2174/138945010791591403. [DOI] [PubMed] [Google Scholar]
- 35.Lin SC, Liu CJ, Lin JA, Chiang WF, Hung PS, Chang KW. miR-24 up-regulation in oral carcinoma: positive association from clinical and in vitro analysis. Oral Oncol. 2010;46:204–8. doi: 10.1016/j.oraloncology.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z, Wang JF, Zhang Z, Lu S, Huang X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29:4781–8. doi: 10.1200/JCO.2011.38.2697. [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Todd NW, Xing L, Xie Y, Zhang H, Liu Z, Fang H, Zhang J, Katz RL, Jiang F. Early detection of lung adenocarcinoma in sputum by a panel of microRNA markers. Int J Cancer. 2010;127:2870–8. doi: 10.1002/ijc.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]