Abstract
Background
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States when combining both genders. Circulating tumor cells (CTCs) are a prognostic marker for stage IV CRC patients. We hypothesized that CTC quantity varies among stage IV CRC populations.
Methods
Blood (7.5 ml) was prospectively collected from 90 stage IV CRC patients. EpCAM+ CTCs were analyzed with the FDA-approved CellSearch® system. CRC tumors were immunohistochemically stained for EpCAM expression. Imaging and clinicopathological data were collected. Statistical analysis was performed using correlation analysis, Kruskal–Wallis, Fisher exact, and log-rank test.
Results
CTCs were detectable in 36/90 (40%) patients. Diffuse CRC metastases were associated with the highest CTC prevalence (24/40 [60%]), in contrast to limited lung (2/19 [11%]) or liver (10/31 [32%]) metastases (P = 0.027). The overall mean CTC number was 2.0 (range 0–56.3). The mean CTC number in patients with diffuse metastases was significantly higher (3.7 [SEM ± 1.7, range 0–56.3]) than with limited lung metastases (0.1 [± 0.1; range 0–1]) or liver metastases (0.9 [± 0.3, range 0–7.0]) (P = 0.001). CRC tumors were consistently expressing EpCAM. CTC numbers did not correlate with serum CEA levels or other routine clinical parameters (P = N.S.). Patients with diffuse metastases had the poorest overall survival (P = 0.0042).
Conclusions
CRC patients with diffuse metastases have the highest number of CTCs, in contrast to limited metastases to the liver or lungs. Future studies should correlate CTCs with recurrence patterns in patients with resected CRC lung or liver metastases to investigate whether CTCs represent micrometastatic disease causing early relapses.
Keywords: colorectal cancer, circulating tumor cells, metastasis
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer mortality occurring in both genders in the United States.1 Liver and lungs are the most frequent sites of metastatic CRC spread, and patients with resectable metastases limited to the liver or lung are potentially curable by multidisciplinary management with a 5-y survival of 30%.2 However, patients with additional spread have poor outcome and usually need to undergo palliative chemotherapy. The decision process for surgery of oligometastatic disease to the lung or liver is a clinical challenge with regard to avoiding an early recurrence. Further improvements in risk stratification are needed to achieve better outcomes.
Circulating tumor cells (CTCs) are believed to detach from the tumor mass, enter the blood circulation and finally contribute to distant spread in other organs or even recurrent disease at the original site through tumor self-seeding.3 Significant improvements have been accomplished to analyze peripheral blood for the presence of CTCs using different techniques.4 The Veridex CellSearch® system is a detection method that utilizes several molecular parameters to isolate CTCs: immunomagnetic enrichment for epithelial cell adhesion molecule (EpCAM), nuclear staining with 4’,6-diamidino-2-phenylindole (DAPI), and immunofluorescence detection of cytokeratins and CD45.5 Due to its reliability and prognostic impact, CellSearch® is the only system approved by the Food and Drug Administration (FDA) for the enumeration of CTCs in metastatic colorectal, prostate, and breast cancers. More than 3 CTCs/7.5 ml of whole blood are an independent prognostic marker for stage IV CRC patients.6 Detection and characterization of CTCs has potential high-impact implications for prognostication and therapy. However, CTCs are rare and probably only a low percentage of CTCs has the potential to eventually grow to a solid metastasis.7,8
We hypothesized that the quantity of CTCs vary among different stage IV CRC populations, in particular with regard to patients with potentially resectable CRC metastases limited to the liver or lungs compared with unresectable diffuse spread. The study demonstrates that presence of CTCs in stage IV CRC patients is associated with diffuse spread.
Results
Patients’ characteristics
In a multispecialty CRC cancer clinic at a tertiary care center, 90 stage IV CRC patients were prospectively analyzed for CTCs in 7.5 ml of blood with the CellSearch® method. All patients had stage IV CRC as determined by imaging and biopsies. Patients that received cancer-directed therapies within the last 4 weeks were not tested for CTCs. If several CTC draws were accomplished, the average CTC number was calculated, and overall means ± SEM were used for further analysis.
Patients’ characteristics are listed in Table 1. The mean age of all patients was 59, with a range of 20–88. 34 (38%) patients were females, and 56 (62%) males. The location of the primary tumors were predominantly rectosigmoid (60 [67%]), followed by the descending (6 [7%]), transverse (2 [2%]), and right (cecum and ascending) (22 [24%]) colon. Forty-seven (52%) patients presented with synchronous metastases at initial CRC diagnosis, vs. 43 (48%) that had metachronous metastatic disease (>6 mo after initial diagnosis).
Table 1. Patients’ characteristics of stage IV CRC patients that were analyzed for presence of CTCs.
| Total number of patients | 90 |
| Age | |
| Mean (± SEM) | 59 (± 1.4) |
| Median (range) | 58 (20–88) |
| Gender | |
| Females | 34 (38%) |
| Males | 56 (62%) |
| Location of primary tumor | |
| Rectosigmoid | 60 (67%) |
| Descending colon | 6 (7%) |
| Transverse colon | 2 (2%) |
| Right colon/cecum | 22 (24%) |
| Synchronous vs. metachronous metastases | 47 (52%) vs. 43 (48%) |
| pT stage of primary tumor (data available in 72/90 patients) | |
| pT1 | 5 (7%) |
| pT2 | 4 (5%) |
| pT3 | 43 (60%) |
| pT4 | 20 (28%) |
| Nodal status of primary tumor N− vs. N+ (data available in 74/90 patients) | 25 (34%) vs. 49 (66%) |
| CEA serum level (ng/ml) (data available in 87/90 patients) | |
| Mean (± SEM) | 147.8 (± 65.6) |
| Median (range) | 4.9 (1–4967.7) |
| CA19.9 serum level (U/ml) (data available in 25/90 patients) | |
| Mean (± SEM) | 1150.8 (± 635) |
| Median (range) | 40.1 (1–15 022) |
| Microsatellite instability (MSI-H and MSI-L) | 6/30 (20%) |
| KRAS mutation (codon 12 or 13) | 17/64 (27%) |
| BRAF mutation (codon 600) | 6/46 (13%) |
| NRAS mutation (codon 12, 13, and 61) | 1/19 (5%) |
| PI3K mutation (codon 1047) | 1/21 (5%) |
| High EGFR expression | 24/33 (73%) |
| High ERCC1 expression | 5/33 (15%) |
| High TS expression | 11/33 (33%) |
| Metastatic organ involvement: | |
| Lung metastases | 19 (21%) |
| Liver metastases | 31 (34%) |
| Diffuse metastases | 40 (45%) |
| Lung and extrahepatic | 6 (7%) |
| Liver and extrapulmonary | 7 (8%) |
| Extrapulmonary/-hepatic | 11 (12%) |
| Lung and liver | 8 (9%) |
| Liver and lung and other | 8 (9%) |
Extrahepatic and -pulmonary metastases were mediastinal/retroperitoneal/pelvic/mesenteric/supraclavicular lymph nodes, local recurrences, abdominal wall metastases, or other organ sites (adrenals, bone, brain, pleural/peritoneal/omental).
TNM data was available in 72/90 (80%) patients, as many patients had treatments for the primary CRC in outside institutions, and data was not retrievable in all patients. Most CRC patients had a pT3 (43 [60%]) or pT4 (20 [28%]) depth of invasion of the primary tumor, whereas the minority had a pT1 (5 [7%]) or pT2 (4 [5%]) invasion depth. The nodal status of the primary tumor was negative in 25 (34%), and 49 (66%) primary CRC had metastatic lymph spread to begin with.
CEA serum levels at the time of CTC detection were available in 87/90 (97%) patients. The mean CEA serum level at the time of CTC detection was 147.8 ng/ml (SEM ± 65.6) (range 1–4967.7 ng/ml). CA19.9 serum levels at the time of CTC detection were available in 25/90 (28%). The mean CA19.9 serum level was 1150.8 U/ml (± 635) (range 1–15 022 U/ml).
Mutational status (KRAS, BRAF, PI3K, and NRAS), microsatellite instability (MSI), and EGFR, ERCC1, and TS (thymidylate synthetase) expression was reviewed. MSI (MSI-H and MSI-L) was determined in 6/30 (20%). Mutational analysis showed that 16/63 (25%) tumors had a KRAS mutation (codon 12 or 13), 6/46 (13%) had a BRAF mutation (codon 600), 1/19 (5%) carried an NRAS mutation (codon 12, 13, and 61), and 1/21 (5%) had a PI3K mutation (codon 1047). Protein expression analysis data was available in 33/90 (37%) patients. EGFR expression was high in 24 (73%) and low in 9 (27%), ERCC1 high in 5 (15%) and low in 28 (85%), and TS was high in 11 (33%) and low in 22 (67%) patients.
Imaging (CT and PET/CT scans) and biopsy results were reviewed in multidisciplinary conferences staffed by several specialties (surgical oncologists, medical oncologists, radiation oncologists, radiologists, interventional radiologists, gastroenterologists, and pulmonologists), to determine specific metastatic organ involvement. Nineteen (21%) patients had lung metastases only, 31 (34%) had liver metastases, and 40 (45%) patients had combined or different sites involved. Sites of extrapulmonary and extrahepatic metastases were mediastinal/retroperitoneal/pelvic/mesenteric/supraclavicular lymph nodes, abdominal wall metastases, or other organ sites (adrenals, bone, brain, or pleural/peritoneal/omental) and local recurrences.
Correlation of CTCs with different metastatic spread patterns
CTCs were determined in 7.5 ml of blood with the CellSearch® system, and CTCs were detectable in 36/90 (40%) patients (Table 2). With regard to presence vs. absence of CTCs, patients with limited lung metastases had the lowest rate of presence of CTCs in the blood (2/19 [11%]), followed by liver metastases (10/31 [32%]). Patients with diffuse metastases had the significantly highest prevalence of CTC presence (24/40 [60%]; P < 0.001 [Fisher exact test]). In stage IV CRC patients, ≥3 CTCs/7.5 ml blood has been shown to be of negative prognostic significance.6 Overall, 13/90 (14%) of all patients had ≥3 CTCs detectable. None of the patients with lung metastases had ≥3 CTCs (0/19), 4/31 (13%) with isolated liver metastases, and 9/40 (23%) of all others, not reaching a level of statistical significance (P = 0.07 [Fisher exact test]).
Table 2. CTC prevalence and numbers are lowest in stage IV CRC patients with lung and liver metastases.
| Total | Lung metastases | Liver metastases | Diffuse metastases | P value | ||
|---|---|---|---|---|---|---|
| n | 90 | 19 (21%) | 31 (34%) | 40 (45%) | ||
| CTCs detectable | 36/90 (40%) | 2/19 (11%) | 10/31 (32%) | 24/40 (60%) | <0.001a | |
| CTCs ≥ 3 | 13/90 (14%) | 0/19 (0%) | 4/31 (13%) | 9/40 (23%) | 0.07a | |
| CTC number: | ||||||
| Mean (± SEM) | 2.0 (± 0.8) | 0.1 (± 0.1) | 0.9 (± 0.3) | 3.7 (± 1.7) | ||
| Median (range) | 0 (0–56.3) | 0 (0–1.0) | 0 (0–7.0) | 0.6 (0–56.3) | 0.001b |
SEM, standard error of the mean. aFisher exact test; bKruskal–Wallis test.
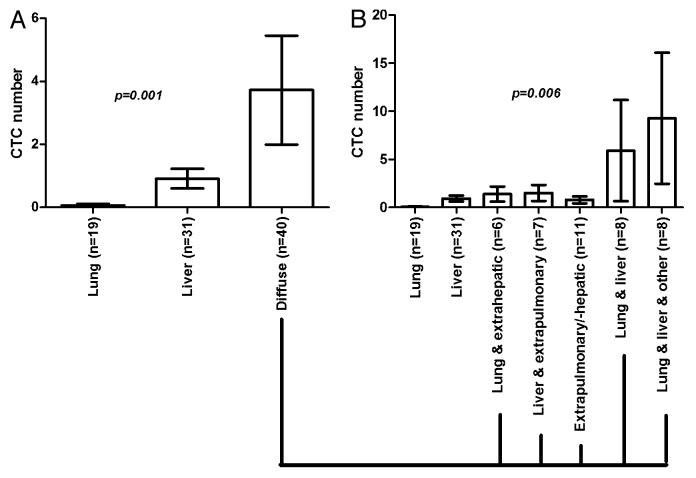
The mean CTC number detected in all 90 patients was 2.0 (range 0–56.3). Nineteen patients had isolated metastases to the lung with a mean CTC number of 0.1 (SEM ± 0.1; range 0–1.0). 31 patients with liver metastases had a mean CTC number of 0.9 (± 0.3; range 0–7.0). 40 patients with diffuse metastases had a mean CTC number of 3.7 (± 1.7; range 0–56.3).
Statistical analysis showed a significantly higher CTC number for patients with diffuse metastases in contrast to CRC patients with limited lung or liver metastases (P = 0.001; Kruskal–Wallis test) (Fig. 1).
Figure 1. Bar graphs showing different metastatic spread patterns in 90 stage IV CRC patients, as determined by biopsy results and imaging. CTCs were determined in 7.5 ml of blood by EpCAM-based and FDA-approved CellSearch® analysis. (A) Patients with diffuse metastases had significantly higher CTC numbers than patients with metastases limited to the lung or liver. (B) Represents subclassification of the metastatic patterns in patients with diffuse metastases. Analysis revealed that patients with lung and liver metastases, and patients with lung and liver and additional extrapulmonary/-hepatic disease had the highest CTC numbers, in contrast to patients with isolated lung or liver, or absence of lung or liver metastases. Patient numbers are provided in brackets. Shown are mean values with standard error of the mean (SEM). P values were calculated by nonparametric Kruskal–Wallis test.
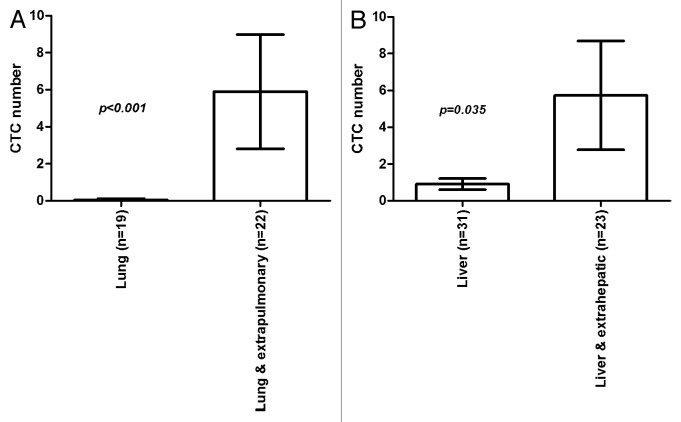
As demonstrated in Figure 2 and outlined in Table 3, we also compared CTC numbers in patients with metastases limited to the lungs (n = 19; CTCs detectable in 2 [11%] patients; mean CTC number: 0.1 [± 0.1]; range 0–1.0) with the group with lung and extrapulmonary (all sites) metastases (n = 22; CTCs detectable in 15 [68%] patients; mean CTC number: 5.9 [± 3.1]; range 0–56.3), and patients with metastases limited to the liver (n = 31; CTCs detectable in 10 [32%] patients; mean CTC number: 0.9 [± 0.3]; range 0–7.0) with the cohort having liver and extrahepatic (all sites) metastases (n = 23; CTCs detectable in 15 [65%] patients; mean CTC number 5.7 [± 3.0]; range 0–56.3). In both statistical analyses with the Fisher exact test for presence of CTCs (lung vs. lung and extrapulmonary: P < 0.001; liver vs. liver and extrahepatic: P < 0.001), and Kruskal–Wallis test for overall CTC numbers (lung vs. lung and extrapulmonary: P = 0.027; liver vs. liver and extrahepatic: P = 0.035), a significant higher rate of presence and number of CTCs was determined for patients with additional spread to other sites. Patients with metastases limited to the lungs or liver revealed to have significantly less CTCs in the peripheral blood, also indicating that CTCs are a marker for diffuse spread.
Figure 2. Bar graphs demonstrating comparison of CTC numbers determined in 7.5 ml of blood by FDA-approved CellSearch® analysis in patients with (A) isolated lung metastases vs. lung and extrapulmonary spread, and (B) isolated liver metastases vs. liver and extrahepatic spread. Stage IV CRC patients with isolated lung or liver in comparison to extrapulmonary or -hepatic metastases had significantly higher CTC numbers than patients with isolated lung or purely extrapulmonary metastases. Patient numbers are provided in brackets. Shown are mean values with standard error of the mean (SEM). P values were calculated by nonparametric Kruskal–Wallis test.
Table 3. CTC prevalence and numbers are higher in stage IV CRC patients with lung and extrapulmonary vs. lung only, and liver and extrahepatic vs. liver metastases.
| Total | Lung metastases | Lung and extrapulmonary | P value | Liver metastases | Liver and extrahepatic | P value | |
|---|---|---|---|---|---|---|---|
| n | 90 | 19 (21%) | 22 (24%) | 31 (34%) | 23 (26%) | ||
| CTCs detectable (n) | 36/90 (40%) | 2/19 (11%) | 15/22 (68%) | <0.001a | 10/31 (32%) | 15/23 (65%) | 0.027a |
| CTC number: | |||||||
| Mean (± SEM) | 2.0 (± 0.8) | 0.1 (± 0.1) | 5.9 (± 3.1) | 0.9 (± 0.3) | 5.7 (± 3.0) | ||
| Median (range) | 0 (0–56.3) | 0 (0–1) | 1 (0–56.3) | <0.001b | 0 (0–7) | 1 (0–56.3) | 0.035b |
SEM, standard error of the mean. aFisher exact test; bKruskal–Wallis test.
CTCs and other factors
CTC numbers did not correlate with serum CEA levels (nonparametric Spearmen correlation = 0.15, P = N.S.). No association between CTC presence and age, gender, primary tumor location, nodal metastases, mutational status (KRAS, BRAF, NRAS, and PI3K), or expression of response predictors (EGFR, ERCC1, and TS) was noted (P = N.S.; Fisher exact test).
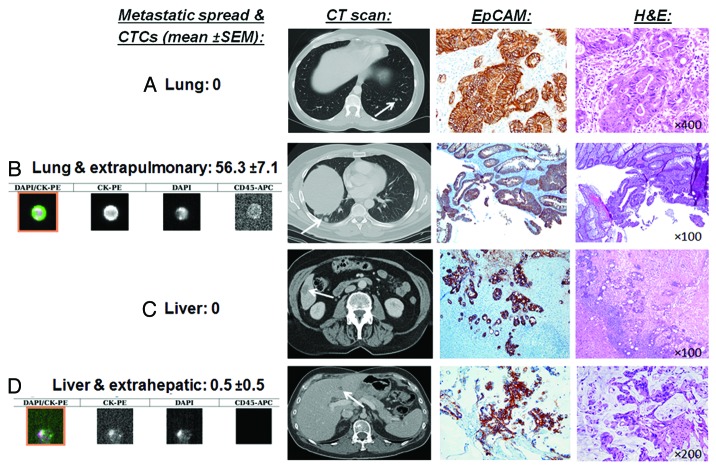
As the CellSearch® detection system is based on immunomagnetic bead selection of exclusively EpCAM+ CTCs, EpCAM immunostaining of the primary CRC tumors with a monoclonal antibody (BerEP4) was performed to confirm positivity. The primary tumors were chosen for immunostaining because actual metastases tissue was not available in most patients. CRC primary tumors of the stage IV patients analyzed for CTC by CellSearch® expressed EpCAM, demonstrating that it was not downregulated. Figure 3 illustrates different examples of stage IV CRC patients with lung or liver metastases with high or zero CTCs that all had consistently EpCAM expression.
Figure 3. EpCAM is consistently expressed in CRC primary tumors, also in patients that have no CTCs detectable by EpCAM-based CellSearch® system. Illustrated are CTC images and mean CTC numbers (± SEM), CT scans of liver and lung metastases, EpCAM, and hematoxylin and eosin (H&E) stains of CRC tumors. Shown are patients with (A) resectable (oligometastatic) lung metastases with no additional spread and with zero CTCs (good surgical candidate for lung resection), (B) lung metastases and extrapulmonary spread with mean CTCs of 56.3 (poor surgical candidate for lung resection), (C) resectable (oligometastatic) liver metastases with no additional spread with zero CTCs (good surgical candidate for liver resection), and (D) liver metastases and extrahepatic spread with mean CTCs of 0.5 (poor surgical candidate for liver resection).
Survival analysis was also performed. All patients were followed by the Survivorship Program at our institution. Median follow-up time was 11.3 mo. Patient with diffuse metastases had a significantly worse overall survival than patients with metastases limited to the lung or liver (P = 0.0042; log-rank test) (data not shown). Comparing survival of CRC patients with lung and extrapulmonary metastases with patients with isolated lung metastases, no statistically significant correlation was found (P = 0.0901). A statistically significant poorer survival was noted for patients with liver and extrahepatic metastases, in contrast to patients with isolated liver metastases (P = 0.0006). Subanalysis of CTC presence and survival was not found to have enough power for analysis, due to short periods of follow-up intervals and low number of mortality events.
Discussion
The study demonstrates that stage IV CRC patients with diffuse metastases harbor the highest CTC quantity, in contrast to patients with metastases limited to the lungs or liver that consistently have a low CTC number. At the same time, patients with diffuse metastases were the ones with the poorest survival. The association of diffuse spread with high CTC numbers could be of clinical relevance, as patients with potentially resectable CRC metastases limited to the lung and liver with presence of CTCs, but no detectable cancer at other sites by current staging modalities, might be at high risk for early recurrence after metastasectomy. In these patients, CTCs could be an indicator of micrometastatic disease that is undetectable with current routine staging techniques. In support of this hypothesis, a recent meta-analysis demonstrated that the detection of CTCs in patients with resectable CRC liver metastases is associated with disease progression and poor survival.9 In addition, a recent case report described a patient that underwent CRC lung metastasectomy with adequate preoperative staging and a normal CEA serum level, and the only marker that predicted a recurrence within 6 mo after surgery were elevated preoperative CTC numbers.10 Interestingly, in our cohort patients with the highest CTC number were those with simultaneous liver and lung metastases, irrespective of presence of additional extrapulmonary and extrahepatic disease. A past study demonstrated that even patients with simultaneous liver and lung metastases may well benefit from metastasectomy in both organs.11 The exact role of CTCs in patients with simultaneous CRC lung and liver metastases needs to be further clarified, and survival data and recurrence patterns should be correlated with CTC data. From a clinician’s standpoint, the authors believe that patients with simultaneous lung and liver metastases should be looked at cautiously, and the indication for metastasectomy should be well established.
In our patient group, CEA serum level did not correlate with CTC numbers, although investigators have published both similar and differing results.12,13 One reason could be the application of different statistical tests by investigators, and we found a nonparametric correlation analysis to be most adequate.
One limitation with CTC detection is that CTC numbers are low in the peripheral blood.7,8 The FDA-approved CellSearch® technology does not detect CTCs in many patients with widely metastatic disease, while few have exceedingly high CTC numbers in the peripheral blood.14 Intraoperative CTC isolation could be an exceptional opportunity to isolate higher numbers of CTCs due to surgical manipulation and access to blood in proximity to the tumor outflow. Other studies on few patients demonstrated that CTCs can be isolated more frequently in tumor outflow than in the peripheral blood during surgery of primary CRC.15,16 To further address this we initiated an ongoing clinical trial on intraoperative CTC isolation during CRC lung and liver metastasectomy, comparing the CellSearch® system with a size-based CTC isolation technique. (ClinicalTrials.gov identifier: NCT01722903).
CTC isolation has significance for future CRC staging and chemosensitivity testing. Correlation of single CTCs to biomarker expression profiles in cancer tissue can explain what kind of metastases cause CTC dissemination and lead to the detection of novel markers for prognostication and therapy monitoring. Findings on CTCs can also lead to clinical trials on CRC patients receiving adjuvant chemotherapy after resection, as well as studies on local treatments, such as transarterial drug application to the liver based on CTC numbers.
Due to the CellSearch® system’s limited capture efficiency to detect rare CTCs, future studies will need to evaluate innovative technologies, such as novel filtration-based CTC isolation techniques that isolate CTCs by size criteria.17 These may have a higher CTC capture efficiency and allow isolation of viable cells for further chemosensitivity and response testing. By analyzing biomarker expression in CTCs and metastases, molecular changes required for re-forming metastases will be elucidated, and there is potential for identification of therapeutic molecular targets. A number of techniques have been developed for the isolation of CTCs in peripheral blood since the first attempts in the late 1800s.18 These include reverse transcriptase polymerase chain reaction (rt-PCR), immunocytochemistry, flow cytometry, microchips, and size-based filtration methods.17,19-21
Several studies have demonstrated the clinical significance of CTC numbers as a prognostic marker when enumerated by the CellSearch® system in metastatic tumors of breast, colon and prostate.6,22,23 However, CellSearch® is limited by the selection of EpCAM+ CTCs only. A recent study has shown that EpCAM–CTCs may have a crucial role in metastasis, and that these CTCs have an important function for development of breast cancer brain and lung metastases.24,25 In our study, CRC patients with metastases limited to the lungs had the lowest number of CTCs detected by CellSearch®, but EpCAM-independent CTC isolation techniques might detect, isolate and characterize EpCAM–CTCs in patients with CRC lung metastasis in future. However, our study showed that the primary CRC of the patients tested with CellSearch®—including the ones that did not have CTCs in the peripheral blood and had diffuse spread—expressed EpCAM by immunohistochemistry, suggesting—but not proving—that EpCAM downregulation might not have contributed to absence of CTCs in our patients. In the present study, the actual metastasis tissue was not available, and future studies should analyze EpCAM expression in metastases specimens for definitive clarification. Finally, a subset of CTCs may still downregulate EpCAM and escape the detection system, even if the primary and metastatic tumors express EpCAM.26
The survival follow-up time was short due to recent initiation of the study. Nevertheless, our outcome data already revealed that patients with diffuse metastases had significantly worse overall survival than patients with metastases limited to the lungs or liver. This indicates that our patient cohort is representative for stage IV CRC, and that patients with high CTCs are the ones that have the poorest outcome. However, the observation period was not sufficient for a statistically meaningful survival analysis with regard to presence or quantity of CTCs and metastatic spread patterns. We hypothesize that longitudinal follow-up will further clarify the impact of CTCs on survival, as has been shown by other investigators, and what spread patterns are associated with presence of CTCs in patients with metastases limited to the lung or liver.6
The present analysis reveals that stage IV CRC patients with metastases limited to the liver and lungs have a very low CTC quantity, in contrast to patients with diffuse metastases. In general, the study allows the conclusion that patients with diffuse spread have more CTCs than patients with metastases limited to the liver or lungs. At the same time these results also direct the next step. In particular patients with limited and potentially resectable CRC lung and liver metastases with presence of CTCs, but no detectable cancer at other sites by current staging modalities, might be at high risk for early recurrence after metastasectomy. This will need to be investigated in a future analysis that will need to correlate CTC numbers with recurrence data specifically in patients that undergo CRC liver or lung metastasectomy. One of the major criteria for lung or liver metastasectomy is the absence of diffuse disease leading to early relapses, as these patients should not undergo high-risk thoracic or abdominal surgery. Correlation of CTC numbers with post-surgery recurrence data in a cohort of patients undergoing CRC liver or lung metastasectomy that actually carry CTCs will be able to assess whether CTCs are predictive of recurrence in patients with limited and resectable cancer spread. In general, future studies will need to characterize individual CTCs to identify useful biomarkers for therapeutic targets.
Methods
Patient selection
Institutional Review Board (IRB) approval was obtained at Penn State Hershey Medical Center. Between January 2011 and March 2013, venous blood (7.5 ml) was prospectively drawn from stage IV CRC patients that were evaluated for metastasectomy at the multidisciplinary CRC clinics of the Program for Liver, Pancreas and Foregut (Lung and Esophageal) Tumors at the Penn State Hershey Cancer Institute. Inclusion criteria were stage IV CRC with active/persistent disease proven by biopsy or imaging criteria. Patients who had chemotherapy or other cancer-directed treatments within 4 weeks before CTC detection were excluded.
CTC detection
CTC detection was performed with FDA-approved CellSearch® (Veridex) system. Seven and a half milliliters of peripheral vein or Mediport blood were obtained after the first 5 ml of blood was discarded to avoid contamination with normal epithelial cells. Samples were collected in CellSave® tubes (Veridex), and all samples were analyzed within 3 d using the standard CellSearch® protocol and the CTC Epithelial Cell Kit (Veridex). The CellSearch® system qualifies a cell as a CTC if it has an evident nucleus by 4’,6-diamidino-2-phenylindole (DAPI) and is EpCAM+, cytokeratin 8,18/19+, and CD45−. Analysis and enumeration of CTCs was conducted by a certified assay operator (DT Dicker).
Clinicopathological data
Clinical and pathological data were collected by reviewing electronic records, including primary tumor characteristics (TNM) and CEA serum levels. Imaging (CT, MRI, and PET/CT scans) was reviewed in a multidisciplinary conference (including a radiologist) to determine metastatic organ involvement. Mutational status (KRAS, BRAF, PI3K, and NRAS), microsatellite instability (MSI) and EGFR, ERCC1, and TS (thymidylate synthetase) expression were determined within the Department of Pathology, or by sending out specimens to Response DX Colon®, or Quest Diagnostics®. All patients are being followed by our Survivorship Clinic at the Cancer Institute, which consists of 3–6 mo regular clinical visits. Survival data was generated.
EpCAM immunostaining
The respective CRC primary tumors were chosen as the metastases tissue was not available in most stage IV cases, assuming that the primary tumor represents EpCAM expression of the metastases. All tissue specimens were fixed in buffered formalin, routinely processed and embedded in paraffin. Sections from representative tumor blocks of all cases were cut at 4-μm thickness, and hematoxylin and eosin stain was performed per routine histology protocol. Antigen retrieval was done with EDTA (pH 8.0), and immunohistochemical staining for EpCAM with a monoclonal mouse antihuman antibody (clone BerEP4) (Dako) diluted 1:100 was performed on Dako Autostainer Plus® using the streptavidin-biotin-peroxidase system, and the signal was visualized with 3,3’-Diaminobenzidine (DAB) detection kit, applied according to the manufacturer’s manual. The staining was visualized with Olympus microscope and the images were captured with Olympus DP26 digital camera. All slides were examined by a board-certified pathologist (Z Yang).
Statistical analysis
Statistical analysis was performed using statistical software SAS version 9.3 (SAS Institute). Significance statements refer to a P value of <0.05. Statistical tests applied were nonparametric Kruskal–Wallis test, Fisher exact test, log-rank test, and nonparametric linear correlation (Spearman Correlation) analysis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Financial Support
This study was supported by the Commonwealth Universal Research Enhancement Program (CURE/Tobacco Funds of the State of Pennsylvania) (JT Kaifi). WS El-Deiry is an American Cancer Society Research Professor.
Acknowledgments
We thank all participating patients for their involvement in this study, and our Clinical Program Coordinator Teresa Smink, BSN, OCN, for assisting in identifying the patients.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/26884
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Chun YS, Vauthey JN. Extending the frontiers of resectability in advanced colorectal cancer. Eur J Surg Oncol. 2007;33(Suppl 2):S52–8. doi: 10.1016/j.ejso.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–40. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 4.Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, Rao SB, Eng-Wong J, Seillier-Moiseiwitsch F, Noone AM, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27:5153–9. doi: 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes DF, Smerage JB. Circulating tumor cells. Prog Mol Biol Transl Sci. 2010;95:95–112. doi: 10.1016/B978-0-12-385071-3.00005-8. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–21. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 7.Lianidou ES, Mavroudis D, Sotiropoulou G, Agelaki S, Pantel K. What’s new on circulating tumor cells? A meeting report. Breast Cancer Res. 2010;12:307. doi: 10.1186/bcr2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192:373–82. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groot Koerkamp B, Rahbari NN, Büchler MW, Koch M, Weitz J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol. 2013;20:2156–65. doi: 10.1245/s10434-013-2907-8. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto M, Tanaka F, Yoneda K, Kondo N, Takuwa T, Matsumoto S, Kuroda A, Noda M, Tomita N, Hasegawa S. Circulating tumor cells as a potential biomarker in selecting patients for pulmonary metastasectomy from colorectal cancer: report of a case. Case Rep Oncol. 2012;5:542–5. doi: 10.1159/000343677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Headrick JR, Miller DL, Nagorney DM, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg. 2001;71:975–9, discussion 979-80. doi: 10.1016/S0003-4975(00)02522-4. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal C, Meropol NJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann Oncol. 2013;24:420–8. doi: 10.1093/annonc/mds336. [DOI] [PubMed] [Google Scholar]
- 13.Lu CY, Uen YH, Tsai HL, Chuang SC, Hou MF, Wu DC, Juo SH, Lin SR, Wang JY. Molecular detection of persistent postoperative circulating tumour cells in stages II and III colon cancer patients via multiple blood sampling: prognostic significance of detection for early relapse. Br J Cancer. 2011;104:1178–84. doi: 10.1038/bjc.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joosse SA, Pantel K. Biologic challenges in the detection of circulating tumor cells. Cancer Res. 2013;73:8–11. doi: 10.1158/0008-5472.CAN-12-3422. [DOI] [PubMed] [Google Scholar]
- 15.Rahbari NN, Bork U, Kircher A, Nimitz T, Schölch S, Kahlert C, Schmidt T, Steinert G, Ulrich AB, Reissfelder C, et al. Compartmental differences of circulating tumor cells in colorectal cancer. Ann Surg Oncol. 2012;19:2195–202. doi: 10.1245/s10434-011-2178-1. [DOI] [PubMed] [Google Scholar]
- 16.Jiao LR, Apostolopoulos C, Jacob J, Szydlo R, Johnson N, Tsim N, Habib NA, Coombes RC, Stebbing J. Unique localization of circulating tumor cells in patients with hepatic metastases. J Clin Oncol. 2009;27:6160–5. doi: 10.1200/JCO.2009.24.5837. [DOI] [PubMed] [Google Scholar]
- 17.Williams A, Balic M, Datar R, Cote R. Size-based enrichment technologies for CTC detection and characterization. Recent Results Cancer Res. 2012;195:87–95. doi: 10.1007/978-3-642-28160-0_8. [DOI] [PubMed] [Google Scholar]
- 18.Wicha MS, Hayes DF. Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J Clin Oncol. 2011;29:1508–11. doi: 10.1200/JCO.2010.34.0026. [DOI] [PubMed] [Google Scholar]
- 19.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–9. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin HK, Zheng S, Williams AJ, Balic M, Groshen S, Scher HI, Fleisher M, Stadler W, Datar RH, Tai YC, et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res. 2010;16:5011–8. doi: 10.1158/1078-0432.CCR-10-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 23.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Ridgway LD, Wetzel MD, Ngo J, Yin W, Kumar D, Goodman JC, Groves MD, Marchetti D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med. 2013;5:80ra48. doi: 10.1126/scitranslmed.3005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mego M, De Giorgi U, Dawood S, Wang X, Valero V, Andreopoulou E, Handy B, Ueno NT, Reuben JM, Cristofanilli M. Characterization of metastatic breast cancer patients with nondetectable circulating tumor cells. Int J Cancer. 2011;129:417–23. doi: 10.1002/ijc.25690. [DOI] [PubMed] [Google Scholar]
- 26.Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, Krahn T, von Ahsen O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. doi: 10.1186/1471-2407-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]