Abstract
The notion of natural killer (NK)-cell education has recently emerged, and accumulating evidence indicates that the terminal differentiation of NK cells can be achieved in the periphery. This means that the proper function of these lymphocytes is dependent on their environment, opening a new door through which cancer cells can escape immunosurveillance.
Keywords: NK cells, TGFβ, anti-tumor immunity, breast cancer, education
The role of immune cells in suppressing the growth of malignant cells and shaping their immunogenicity has a direct impact on the prognosis of breast cancer patients.1 In particular, natural killer (NK) cells play a major part in breast cancer immunosurveillance. By analyzing invasive breast tumors in situ, we identified at least 3 different mechanisms whereby neoplastic cells can escape recognition (and hence lysis) by NK cells. These mechanisms are not mutually exclusive, but rather appear to be tightly interconnected.
First, NK cells from breast cancer patients exhibit decreased activity and altered expression of surface receptors, i.e., they express limited amount of activating receptors and increased levels of inhibitory receptors, exception made for killer inhibitory receptors (KIRs). These alterations are induced by the tumor itself, most likely upon the local overproduction of transforming growth factor β1 (TGFβ1), because they were reversed in cancer patients undergoing long-term remission.2
Second, breast cancer cells modulate their immunogenicity by several mechanisms, including the expression of ligands for NK cell-inhibitory receptors, the downregulation of ligands for NK cell-activating receptors, and the release of immunosuppressive or decoy molecules such as TGFβ1, soluble MHC class I polypeptide-related sequence A (MICAs) or soluble interleukin-1β receptor (IL1βR). Breast cancers can thus stimulate a state of immunological tolerance by rendering themselves invisible to NK cells and by synthesizing a dense magma of inhibitory molecules.3 These 2 types of alterations are frequently observed in solid malignancies.4
We recently reported a third process whereby malignant cells can escape immunosurveillance. In particular, we demonstrate that breast cancer cells can influence the terminal maturation of NK cells.2 While the absolute number of NK cells did not vary, we observed an increased proportion of poorly differentiated and non-cytotoxic NK-cell subsets in the peripheral blood, and even more so in the neoplastic lesions, of breast cancer patients. This might explain, at least in part, the poor cytotoxic functions observed in these patients. In addition, this observation highlights the heterogeneity and plasticity of the NK-cell compartment. The precise mechanisms involved in this phenomenon are still poorly defined.
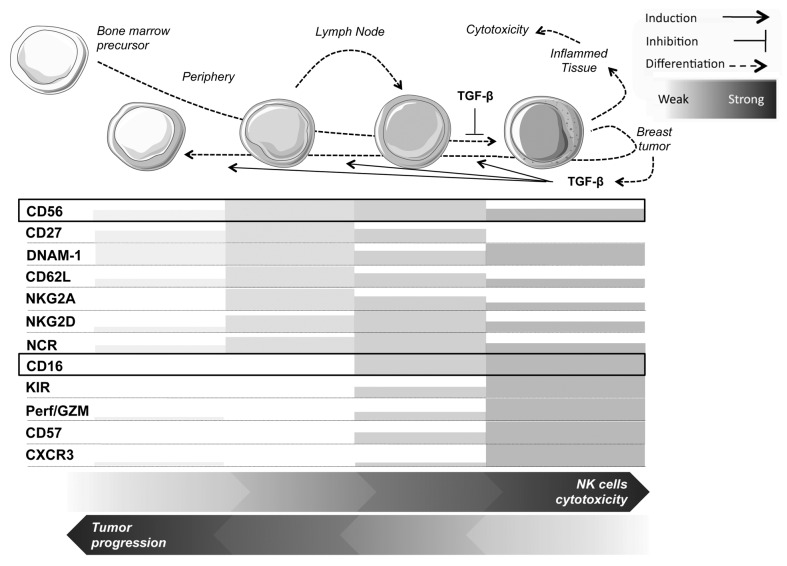
From what we know so far, NK cells usually show specific receptor expression patterns related to their maturation status (Fig. 1). These patterns were initially defined based on the expression of CD56 and CD16, and now also take into account the expression levels of KIRs and killer cell lectin-like receptor subfamily C, member 1 (KLRC1, best known as NKG2A). It is well known that non-terminally differentiated human NK cells collected from non-reactive normal lymph nodes (LNs) are mostly CD56brightCD16−KIR−NKG2A+ cells, whereas activated NK cells derived from normal but reactive/efferent LNs or the blood comprise a majority of CD56dimCD16+KIR+NKG2A+/− cells.5 The current belief is that immature CD56bright NK cells acquire the expression of CD16 and KIRs in the LNs during inflammation and then circulate as CD56dimCD16+KIR+NKG2A+/− effector cells. Depending on the anatomical location, the disturbance of the balance between effector CD56dimCD16+KIR+NKG2A+/− and precursor CD56brightCD16−KIR−NKG2A+ NK cells might thus constitute per se a marker of ongoing immune response. If so, inflamed tissues should be enriched in differentiated CD56dimCD16+KIR+NKG2A− NK cells. However, when comparing the NK-cell populations that infiltrate malignant and healthy breast tissues, things look more complicated than expected and a mosaic of different NK-cell subsets is found within breast carcinomas, with variable proportions of CD56brightCD16+/−KIR+/−NKG2A+/− and CD56dimCD16+/−KIR+/−NKG2A+/− NK cells. Notably, as compared with healthy mammary tissues, breast cancer lesions contain a decreased amount of mature CD56dimCD16+KIR+NKG2A− NK cells and an increased number of immature CD56brightCD16+ and CD56dimCD16− NK cells. The abundance of such immature NK-cell subset is also increased in the peripheral blood of patients with advanced breast cancer.
Figure 1. Expression pattern of NK cell-associated receptors and effectors according to maturation stage. Abbreviations: CXCR3, chemokine (C-X-C motif) receptor 3; GZM, granzyme; KIR, killer inhibitory receptor; NCR, natural cytotoxicity receptor; Perf, perforin.
Altogether, these data suggest that different NK-cell subsets correspond to sequential steps of NK-cell differentiation as induced by the tumor environment, which is most certainly able to reset/revert the terminal differentiation status of these lymphocytes.6 Although TGFβ1 is known to affect NK-cell maturation,7 precise mechanistic insights implying this cytokines in the regulation of critical transcription factors such as eomesodermin (EOMES), IKAROS family zinc finger 2 (IKZF2, best known as HELIOS) and T-box 21 (TBX21, best known as T-bet) are missing.
Our results are in line with recent findings showing that the developmental programming of NK cells is not completely irreversible and that mature NK cells can be re-educated by their microenvironment.8 Contrary to T lymphocytes, which are mostly primed within LNs, NK cells can directly be recruited to sites of inflammation, suggesting that their differentiation status can modified almost anywhere.9 We tested this hypothesis by looking at the chemokine receptors expressed on distinct NK-cell subsets in the peripheral blood of breast cancer patients. We found that, despite the preferential homing pattern to peripheral tissues for CD56dim NK cells and to LNs for CD56bright cells, all NK cells can migrate to virtually any tissues in response to an inflammatory gradient. The ample homing pattern of NK cells and their ability to undergo terminal differentiation in situ represent two major advantages with respect to their innate effector functions and their role in the initiation of immune responses. However, as mentioned above, this also means that the terminal differentiation of NK cells (and hence their effector functions) are dependent on an “appropriate” microenvironment. Of note, it has recently been shown that the binding of KIRs by MHC class I molecules (MHCI) plays a major role in controlling NK-cell responses and, paradoxically, in maintaining NK cells in a state of responsiveness to subsequent activation events, a process referred to as “education.”9 Interestingly, one of the most frequent alterations manifested by (breast) cancer cells is the downregulation of MHC class I molecules. Initially, this phenomenon was interpreted as a means to escape adaptive immune responses, but it may finally turn out to constitute a major mechanism whereby malignant cells escape NK cell-mediated antitumor immunity as well.
In conclusion, advanced breast cancers seem to harness a wide panel of mechanisms to escape antitumor immune responses. One well-known molecule is quasi systematically involved in almost all of them: TGFβ1.10 In light of our data, blocking TGFβ1 signaling in NK cells might constitute the bases for the development of novel immunotherapeutic strategies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Mamessier E, Bourgin C, Olive D. When breast cancer cells start to fend the educational process of NK cells off. OncoImmunology 2013; 2:e26688; 10.4161/onci.26688
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26688
References
- 1.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 2.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P, Romagné F, Thibault G, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–22. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mamessier E, Sylvain A, Bertucci F, Castellano R, Finetti P, Houvenaeghel G, Charaffe-Jaufret E, Birnbaum D, Moretta A, Olive D. Human breast tumor cells induce self-tolerance mechanisms to avoid NKG2D-mediated and DNAM-mediated NK cell recognition. Cancer Res. 2011;71:6621–32. doi: 10.1158/0008-5472.CAN-11-0792. [DOI] [PubMed] [Google Scholar]
- 4.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, André P, Dieu-Nosjean MC, Alifano M, Régnard JF, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–22. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 5.Romagnani C, Juelke K, Falco M, Morandi B, D’Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, et al. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–55. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 6.Mamessier E, Pradel LC, Thibult ML, Drevet C, Zouine A, Jacquemier J, Houvenaeghel G, Bertucci F, Birnbaum D, Olive D. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J Immunol. 2013;190:2424–36. doi: 10.4049/jimmunol.1200140. [DOI] [PubMed] [Google Scholar]
- 7.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–67. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207:2065–72. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas LM, Peterson ME, Long EO. Cutting Edge: NK Cell Licensing Modulates Adhesion To Target Cells. J Immunol. 2013 doi: 10.4049/jimmunol.1301159. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J Clin Invest. 1993;92:2569–76. doi: 10.1172/JCI116871. [DOI] [PMC free article] [PubMed] [Google Scholar]