Abstract
It has been reported that schistosomiasis-associated PAH (Sch-PAH) has a more benign clinical course compared with idiopathic PAH (IPAH). We therefore hypothesized that Sch-PAH subjects would present with less impaired cardiopulmonary and metabolic responses to exercise than IPAH patients, even with similar resting pulmonary hemodynamic abnormalities. The aim of this study was to contrast physiologic responses to incremental exercise on cycle ergometer between subjects with Sch-PAH and IPAH. We performed incremental cardiopulmonary exercise tests (CPET) in subjects newly diagnosed with IPAH (n = 9) and Sch-PAH (n = 8), within 1 month of the hemodynamic study and before the initiation of specific therapy for PAH. There were no significant between-group differences in cardiac index, pulmonary vascular resistance or mean pulmonary artery pressure. However, mean peak oxygen uptake (VO2) was greater in Sch-PAH than IPAH patients (75.5±21.4 vs 54.1±16.1% predicted, p = 0.016), as well as the ratio of increase in VO2 to work rate (8.2±1.0 vs 6.8±1.8 mL/min/W, p = 0.03). Additionally, the slope of the ventilatory response as a function of CO2 output was lower in Sch-PAH (40.3±3.9 vs 55.6±19.8; p = 0.04), and the heart rate response for a given change in VO2 was also diminished in Sch-PAH compared to IPAH (80.1±20.6 vs 123.0±39.2 beats/L/min; p = 0.02). In conclusion, Sch-PAH patients had less impaired physiological responses to exercise than IPAH subjects with similar resting hemodynamic dysfunction. Our data suggest a more preserved cardiopulmonary response to exercise in Sch-PAH which might be related to its better clinical course compared to IPAH.
Introduction
Schistosomiasis is potentially the most common cause of pulmonary arterial hypertension (PAH) worldwide. [1], [2] Although 80 years have passed since the first description of cardiopulmonary involvement caused by schistosoma species, this association has not been well studied. [3] Thus, little is known about the pathophysiology, natural history and therapeutic response of schistosomiasis-associated PAH (Sch-PAH).
A recent study demonstrated that naïve Sch-PAH patients had similar survival rates compared to idiopathic PAH (IPAH) subjects treated with vasodilators. In an additional analysis, the authors suggested that Sch-PAH patients have a more benign clinical course than those with IPAH. [4] The mechanisms related to this finding were not described, but it might be related to a slower progression of arteriopathy in Sch-PAH.
Clinicians evaluate functional impairment in PAH patients using the New York Heart Association functional class assessment (NYHA-FC), six-minute walking distance (6MWD) and/or cardiopulmonary exercise testing (CPET). [5], [6] However, only CPET is able to properly identify the physiological mechanisms linked to functional impairment, including ventilatory limitation, circulatory dysfunction or impaired oxygen utilization in peripheral muscles. [7], [8] In fact, CPET has emerged as a surrogate method to evaluate functional impairment in many diseases, and there is a robust body of evidence addressing the diagnostic and prognostic usefulness of CPET in PAH patients.[7]–[13] Moreover, several abnormal CPET findings have been linked to PAH pathophysiology and disease severity. Compared to healthy subjects, patients with PAH show a reduced aerobic capacity, a high slope of minute-ventilation change as a function of CO2 production [(ΔVE/ΔVCO2), a marker of ventilatory inefficiency], an abnormally diminished end-tidal partial pressure of CO2 (PETCO2) at the anaerobic threshold and a steeper increase in heart rate as a function of the metabolic demand (ΔHR/ΔVO2). These abnormalities have been ascribed to the inability of the right ventricle to increase cardiac output through the pulmonary vascular bed, inefficient gas exchange due to ventilation-perfusion mismatching, and/or abnormalities in the control of ventilation. [7], [8] In this context, it is conceivable that patients with Sch-PAH would present with less severe hemodynamic abnormalities during incremental exercise compared with those with IPAH. To the best of the authors’ knowledge, this assertion has not been previously investigated. So, the aim of this study was to contrast the physiological responses during CPET of Sch-PAH and IPAH patients. We hypothesized that Sch-PAH subjects would have better functional performance compared to IPAH patients as indicated by higher maximal aerobic capacity and less impaired ventilatory, cardiovascular and gas exchange responses to exercise.
Materials and Methods
Subjects
This was a cross-sectional study in which incremental CPET was performed in subjects diagnosed with IPAH and Sch-PAH between 2007 and 2010. The test was performed within 1 month of the hemodynamic study and before the initiation of specific therapy for PAH.
PAH was defined as a mean pulmonary artery pressure (mPAP) ≥25 mmHg with a pulmonary capillary wedge pressure (PCWP) ≤15 mmHg. [5], [6] Patients were classified as having IPAH when a routine investigation did not find any cause for PAH. Sch-PAH was characterized by the presence of PAH associated with liver ultrasonographic findings suggestive of hepatoesplenic schistosomiasis (periportal fibrosis and/or left lobe enlargement) and at least one of the following: exposure to a region endemic for schistosomiasis, previous treatment for schistosomiasis or the presence of Schistosoma mansoni eggs in a stool examination or rectal biopsy. [1], [4].
Subjects were excluded from study if they had evidence of any of the following: right-to-left intracardiac shunt, resting hypoxemia, hospitalization in the last month, or an orthopedic limitation to cycling. None of the patients had ever been admitted to a physical rehabilitation program.
Measurements
At the baseline evaluation, we obtained a medical history that included an NYHA-FC assessment, transthoracic echocardiogram, spirometry, single breath carbon monoxide diffusing capacity (DLCO), resting arterial blood gases and 6MWD from all patients. [14].
Hemodynamic Evaluation
A right-sided heart catheterization was performed using standard techniques. Cardiac output (CO) was determined by the Fick method, with arterial and venous line, and estimated VO2 (125x body surface area). [15]–[17] Acute vasodilator responsiveness was evaluated using inhaled nitric oxide for 10 minutes, with a positive response defined as a fall in the mPAP of 10 mmHg or more (to a value ≤40 mmHg) without a decrease in CO. [5], [6].
Cardiopulmonary Exercise Testing (CPET)
Patients performed a symptom-limited ramp-incremental CPET on an electronically braked cycle ergometer (Corival 400, Lode, The Netherlands) at 60 rpm. O2 uptake (VO2, L/min), CO2 output (VCO2, L/min), minute ventilation (VE, L/min), the respiratory exchange ratio (RER, VCO2/VO2) and the end-tidal partial pressures for CO2 (PETCO2, mmHg) and O2 (PETO2, mmHg) were measured using a computer-based system, with breath-by-breath analysis (CardiO2 System™, Medical Graphics, St. Paul, MN) and were recorded as mean of 15 s. Heart rate (HR, bpm) was recorded from the R-R distance in 12-lead ECG tracing with on-line calculation of O2 pulse (VO2/HR, ml/beat) and also sampled as mean of 15 s. The peak VO2 was compared to Brazilian standards. [18].
The anaerobic threshold (AT) was identified using the modified V-slope and the ventilatory method as previously described. The ΔHR/ΔVO2, ΔVE/ΔVCO2 and the slope of VO2 as a function of work rate (ΔVO2/ΔWR) during the incremental phase were calculated from the start of increases in VO2 to the respiratory compensation point, according to standard procedures. [7],[19].
Cuff blood pressure and pulse oximetry were monitored and recorded. At the end of the test, subjects were asked to rate their “dyspnea” and “leg fatigue” using the 0–10 Borg’s category-ratio scale. [20].
Ethics Statement
The study protocol was approved by the Institutional Medical Ethics Committee: Hospital São Paulo – UNIFESP (n° 04878612.4.0000.5505) and written informed consent was obtained from all subjects.
Statistical Analysis
Statistical analysis was performed using the SPSS 19 statistical package (SPSS, Inc., Chicago, IL). All continuous variables had normal distribution as stated by the Kolmogorov-Smirnov test. Therefore, an unpaired t-test was used for the comparison of resting hemodynamics and exercise responses. We used ANOVA with Bonferroni correction for multiple comparisons. Pearson’s product moment correlation or Spearman’s rank correlation was used according to assess the level of correlation between variables. A p value of less than 0.05 was considered statistically significant.
Results
Patient Population
In our cohort, there were 9 subjects with IPAH and 8 subjects with Sch-PAH who met the study criteria and were able to enter the study. At the baseline evaluation, the demographic characteristics of the IPAH and Sch-PAH groups were similar (Table 1). Most patients were in functional class II or III, and there were no differences in 6MWD between the 2 groups. None of the patients with Sch-PAH had anemia or abnormal liver function tests.
Table 1. Baseline patient characteristics.
| Sch-PAH | IPAH | p value | |
| Age, years | 51±8 | 44±19 | 0.36 |
| Gender, n | 0.07 | ||
| Male | 4 | 2 | |
| Female | 4 | 7 | |
| BMI, kg/m2 | 24.6±4 | 23.7±2 | 0.62 |
| NYHA functional class | 0.95 | ||
| II | 3 | 3 | |
| III | 3 | 4 | |
| IV | 2 | 2 | |
| FVC, % | 81.5±13 | 91.8±25 | 0.32 |
| FEV1, % | 78.4±13 | 88.8±22 | 0.29 |
| FEV1/FVC | 0.79±0.1 | 0.83±0.1 | 0.23 |
| DLCO, % | 63±11 | 61±10 | 0.72 |
| PaCO2, mmHg | 33±3 | 33±6 | 0.93 |
| PaO2, mmHg | 75±10 | 78±10 | 0.44 |
| Six-minute-walk distance, m | 474±62 | 462±78 | 0.40 |
Definition of Abbreviations: BMI, body mass index; NYHA, New York Heart Association; FVC, forced vital capacity; FEV, forced expiratory volume; DLCO, carbon monoxide diffusing capacity; PaCO2, arterial carbon dioxide partial pressure; PaO2, arterial oxygen partial pressure.
Resting Pulmonary Hemodynamics
Hemodynamic parameters are summarized in Table 2. There were no significant differences in mean pulmonary pressure (mPAP), pulmonary vascular resistance (PVR), or cardiac index (CI) between groups. No patient displayed positive acute vasodilator response to inhaled nitric oxide.
Table 2. Hemodynamic characteristics.
| Parameter | Sch-PAH | IPAH | p value |
| Mean pulmonary artery pressure, mmHg | 57.5±18.5 | 63.8±17.1 | 0.47 |
| Pulmonary capillary wedge pressure, mmHg | 12.6±2.8 | 9.9±3.3 | 0.10 |
| Right atrial pressure, mmHg | 11.9±5 | 9.7±2 | 0.26 |
| Pulmonary vascular resistance, IU | 12.3±5.6 | 14.8±8.1 | 0.46 |
| Cardiac output, L/min | 4.00±0.6 | 4.09±1.2 | 0.84 |
| Cardiac index, L/min/m2 | 2.37±0.5 | 2.49±0.7 | 0.69 |
| Acute vasodilator response, n | 0 | 0 |
Physiological Responses to Exercise
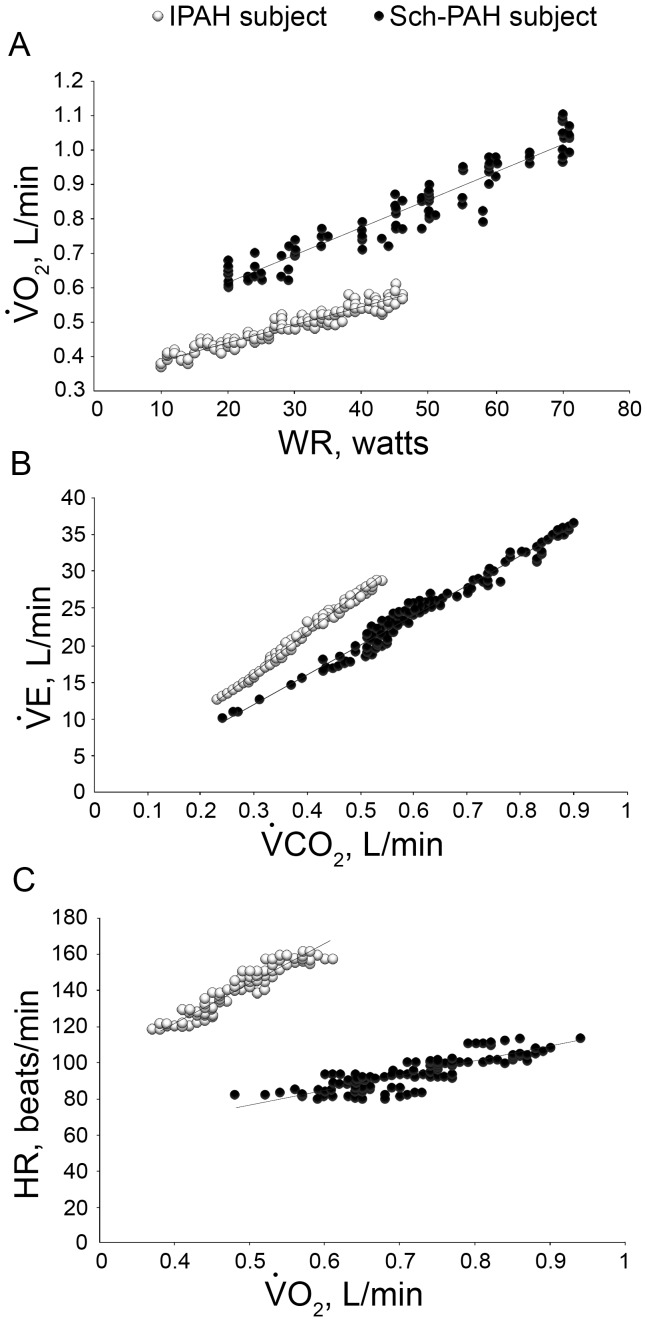
All subjects exhibited diminished aerobic capacity, with the test being interrupted by symptoms of breathlessness in 66% of IPAH subjects and leg effort in 75% of Sch-PAH subjects. The measurements of CPET variables in each group are presented in Table 3 and the typical physiological responses of Sch-PAH and IPAH patients to selected variables obtained during the CPET are presented in Figure 1.
Table 3. Cardiopulmonary exercise testing parameters.
| Sch-PAH | IPAH | p value | |
| WR, watts | 84±29 | 52±17 | 0.006 |
| Metabolic parameters | |||
| VO2 peak, % predicted | 75.5±21.4 | 54.0±16.1 | 0.016 |
| VO2 peak, mL/kg//min | 17.5±5.3 | 12.6±3.2 | 0.016 |
| VO2 at AT, % predicted | 51.3±9.3 | 40.3±13.0 | 0.040 |
| ΔVO2/ΔWR, mL/min/W | 8.2±1.0 | 6.8±1.8 | 0.030 |
| Ventilatory parameters | |||
| VT at AT, L | 1.1±0.3 | 0.9±0.2 | 0.090 |
| VE at AT, L/min | 25.6±6.2 | 22.6±5.2 | 0.300 |
| VE/MMV peak | 0.64±0.17 | 0.53±0.17 | 0.220 |
| VE/VO2 at AT | 33.6±6.0 | 42.3±9.4 | 0.056 |
| VE/VCO2 at AT | 38.8±5.5 | 46.6±10.8 | 0.055 |
| ΔVE/ΔVCO2 | 40.3±3.9 | 55.6±19.8 | 0.020 |
| Cardiovascular parameters | |||
| HR at peak, % predicted | 85.6±9.7 | 79.0±10.0 | 0.200 |
| O2 Pulse, % predicted | 87.5±20.9 | 65.5±16.4 | 0.015 |
| ΔHR/ΔVO2, beat/L/min | 80.1±20.6 | 123.0±39.2 | 0.020 |
| Gas exchange parameters | |||
| PETCO2 at AT, mmHg | 31.0±3.8 | 28.5±6.1 | 0.18 |
| PETO2 at AT, mmHg | 102.1±4.2 | 104.1±6.8 | 0.50 |
| O2 sat at rest, % | 96±2 | 96±1 | 0.64 |
| O2 sat at peak exercise, % | 93±4 | 94±4 | 0.58 |
| Subjective parameters | |||
| Borg - Dyspnea at peak | 7±2 | 6±2 | 0.52 |
| Borg - Leg effort at peak | 7±2 | 5±2 | 0.09 |
Definition of Abbreviations: WR, work rate; VO2, oxygen uptake; VE, minute ventilation; AT, anaerobic threshold; VT, tidal volume; MMV, maximal minute ventilation; VCO2, carbon dioxide uptake; HR, heart rate; PETCO2, end-expiratory pressure for carbon dioxide; PETO2, end-expiratory pressure for oxygen; O2 sat, oxyhemoglobin saturation by pulse oximetry.
Figure 1. Contrasting exercise responses in two subjects with similar resting hemodynamic impairment (CI 2.24 L/min/m2 for Sch-PAH subject, and CI 2.21 L/min/m2 for IPAH patient).
Upper panel: the ratio of increase in VO2 to work rate (ΔVO2/ΔWR); middle panel: slope of VE-VCO2; lower panel: increases in heart rate as a function of VO2.
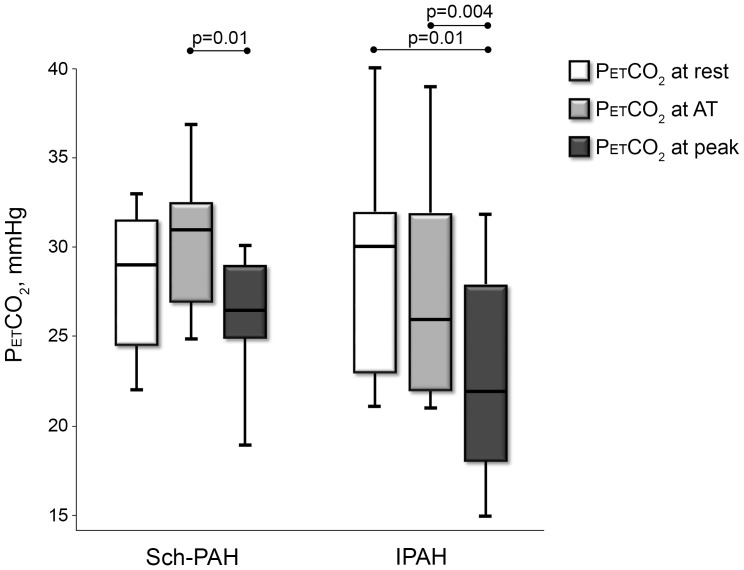
Compared with IPAH patients, Sch-PAH subjects had greater peak VO2 and higher ΔVO2/ΔWR. Also, VO2 was higher in the Sch-PAH group at the AT. The ΔVE/ΔVCO2 was lower in Sch-PAH patients than IPAH patients. Although VE/VCO2 and PETCO2 at AT were smaller in Sch-PAH patients, these variables did not reach statistical significance. There was a trend for different PETCO2 behavior between the groups during exercise (Figure 2).
Figure 2. The behavior of PETCO2 between rest and maximal exercise in Sch-PAH and IPAH subjects.
Sch-PAH patients had a shallower heart rate response for a given change in VO2 and, accordingly, a greater peak O2 pulse compared to IPAH subjects (p<0.05). No difference in SpO2 at rest and peak exercise was found between the groups. Mild hypoxemia during the test was only noted in 3 patients, 2 of which were from the Sch-PAH group.
Resting cardiac index was significantly correlated to peak VO2 only in IPAH subjects. Likewise, there was a correlation between peak VO2 and resting CI with VO2-WR and VE-VCO2 slopes in patients with IPAH, but not in subjects with Sch-PAH. In both groups, there were correlations between resting CI and peak VO2, with NYHA-FC, but not with 6MWT (Table 4; Table 5).
Table 4. Correlation coefficients between resting CI and key exercise variables obtained during CPET, NYHA-FC and 6MWT in patients with Sch-PAH and IPAH.
| Sch-PAH | IPAH | |
| NYHA-FC | −0.85* | −0.74* |
| 6MWD, m | −0.03 | 0.25 |
| VO2 peak, % predicted | 0.51 | 0.72* |
| ΔVO2/ΔWR | 0.61 | 0.58* |
| ΔVE/ΔVCO2 | 0.08 | −0.60* |
p<0.05;
Definition of Abbreviations: NYHA-FC, New York Heart Association functional class; VO2, oxygen uptake; WR, work rate; VE, minute ventilation; VCO2, carbon dioxide uptake.
Table 5. Correlation coefficients between VO2 peak (% predicted) with: NYHA-FC, 6MWT and selected CPET variables in patients with Sch-PAH and IPAH.
| Sch-PAH | IPAH | |
| NYHA-FC | −0.82** | −0.83** |
| 6MWT, m | −0.12 | −0.05 |
| ΔVO2/ΔWR | 0.36 | 0.87** |
| ΔVE/ΔVCO2 | −0.28 | −0.95** |
p<0.05;
p<0.01;
Definition of Abbreviations: NYHA-FC, New York Heart Association functional class; VO2, oxygen uptake; WR, work rate; VE, minute ventilation; VCO2, carbon dioxide uptake.
Discussion
To the best of our knowledge, this is the first study to comparatively assess the physiological adjustments to progressive exercise in subjects with Sch-PAH and IPAH. Although it is an initial study with a small sample, our results indicated that Sch-PAH patients exhibit less impaired exercise responses than IPAH patients, even if they had similar resting pulmonary hemodynamic abnormalities. These data might provide a mechanistic explanation for the reported differences in disease progression.
There are potentially more than twice the number of people with Sch-PAH than IPAH worldwide. [2], [3] Even in non-endemic regions, the prevalence of schistosomiasis is relevant, as demonstrated in a recent report from the French National Center for PAH that showed that schistosomiasis was present in 11% of patients with PAH attributed to non-cirrhotic portal hypertension. [21] Little is known about the pathophysiology of Sch-PAH, and it was previously classified as PAH attributed to embolic phenomena – schistosoma eggs from the portal-hepatic system. [22], [23] However, later studies showed that the histopathological features of Sch-PAH were similar to IPAH, even with the absence of eggs in the pulmonary arteries. It is possible that the presence of schistosoma eggs in the portal veins could trigger an inflammatory response or an imbalance in the vasoactive mediators in the pulmonary arteries. [23].
Fernandes et al. [4] showed that Sch-PAH patients present with less severe disease, exhibiting more preserved resting pulmonary hemodynamics and a trend toward better functional status than IPAH patients. They reported similar survival rates between naïve Sch-PAH subjects and IPAH subjects treated with vasodilators, suggesting a more benign clinical course for Sch-PAH. The baseline characteristics of our patients in both groups were similar to those described by Fernandes et al. [4], and we did not find a trend toward better NYHA-FC, 6MWD and resting hemodynamics in Sch-PAH patients. We did, however, find striking differences between groups during the evaluation of some of the exercise responses obtained in the CPET, as described below.
Differences in Aerobic Capacity and Work Efficiency
In healthy subjects, the VO2 increases as a function of cardiac output and O2 extraction, responding to muscle demand during exercise. In PAH, this response could mainly be impaired due to the failure of the right heart to increase cardiac output during exercise. Sun et al. [8] analyzed CPET findings in PAH patients with a mean cardiac index of 2.2 L/min/m2 and found a peak VO2 of about 44%, slightly lower than the values we found in our IPAH subjects. In our study, the peak VO2 was significantly higher in Sch-PAH patients compared to IPAH patients, even when expressed as mL/kg/min or a percent-predict value, which minimizes any heterogeneity between the groups. Furthermore, our subjects with IPAH had lower VO2 values at AT than our Sch-PAH patients, indicating the earlier development of lactic acidosis during exercise.
Moreover, the slope of VO2-WR, an index known to reflect circulatory or peripheral dysfunction, has been used to evaluate aerobic efficiency. This slope in IPAH patients was shallower than in Sch-PAH patients, reflecting a higher dependence on anaerobic metabolism during exercise in IPAH. [8], [19].
We did not find differences in 6MWD between the groups. Moreover, there were no correlations between 6MWD and parameters of aerobic capacity assessed by CPET, even with NYHA-FC. Although its importance in day practice, it is possible that the submaximal nature of the test, with lesser cardiovascular and ventilatory stress, had limited its capacity to detect disparities between the groups when compared to CPET. [24], [25].
Differences in Ventilatory Efficiency and Gas Exchange
PAH patients often have a ventilatory inefficiency, which is defined as an abnormally high ventilatory response relative to CO2 output and expressed during CPET as either a ratio at the AT or a slope obtained below the AT. This response has been related to areas of high V/Q mismatching or increased alveolar ventilation associated with several mechanisms, and is proportional to PAH severity. [7], [8], [26] A VE-VCO2 slope greater than 48 is associated with limited survival in PAH [13]. In our study, this slope was above 48 in 55% of IPAH patients, however all Sch-PAH subjects had this slope bellow this level. Moreover, this slope was significantly steeper in IPAH subjects than in Sch-PAH subjects (Table 3; Figure 1). Severe arterial hypoxemia and the development of right-to-left shunting during exercise could be additional explanations for the increases in ΔVE/ΔVCO2 [27] but they were not present in our patients.
Excessive ventilation in PAH patients is often accompanied by a reduction in PETCO2. In healthy subjects, a rise in PETCO2 is expected between rest and the AT, with a subsequent reduction in PETCO2 at maximal exercise. [8], [28] In our study, there was a trend toward lower PETCO2 values at AT in IPAH patients. Moreover, there was a tendency for PETCO2 to rise between rest and AT in Sch-PAH patients, contrasting with a progressive fall in PETCO2 in IPAH subjects (Figure 2), suggesting more preserved gas exchange function in Sch-PAH. Hansen et al. [29] emphasized the exercise-induced decline in PETCO2 as a marker for PAH as opposed to COPD and left ventricular failure. Furthermore, Yasunobu et al. [28] showed that the exercise-induced fall in PETCO2 that is observed in PAH is proportional to the severity of the disease.
Differences in Cardiovascular Responses to Exercise
O2 pulse (VO2/HR) is determined by stroke volume and O2 extraction. In our study, as there was an absence of severe oxyhemoglobin desaturation, the higher peak O2 pulse seen in Sch-PAH subjects probably reflects greater increases in stroke volume during exercise compared to IPAH subjects. Likewise, the inability to augment stroke volume during exercise leads to a disproportional increase in heart rate as a function of the metabolic demand (ΔHR/ΔVO2). This response was predominantly observed in our patients with IPAH, as opposed to Sch-PAH (Table 3; Figure 2).
Correlations between Resting Hemodynamics and Exercise Measurements
The resting cardiac index is known to correlate well with functional limitation in IPAH. [10]. Accordingly, in our patients, a correlation of resting CI to peak VO2 was observed in IPAH subjects, but not in Sch-PAH. Also, we found a significant correlation between peak VO2 and resting CI with ventilatory (ΔVE/ΔVCO2) and aerobic efficiency (ΔVO2/ΔWR) only in IPAH patients (Tables 4 and 5). Although no definite conclusions can be made on the correlation values, this is additional evidence of a more pronounced cardiovascular limitation in IPAH, indicating that patients with Sch-PAH have a greater ability to increase pulmonary blood flow during exercise, even with similar resting pulmonary hemodynamics (Table 2). Moreover, these finding supports the contention that selected CPET variables should be used with caution when assessing the prognosis or severity of patients with non-idiopathic PAH, as stated in a recent study involving various etiologies of PAH. [30].
Study Limitations
There are some limitations in our study that need to be acknowledged. The single-center nature of the study and our location in an area of Brazil that is not endemic for schistosomiasis may account for some selection bias; however, this bias is minimized by intern migratory practices to a cosmopolitan city like São Paulo. Also, as a result of restricted selection criteria, this study included a small number of patients, which limit the extrapolation of the results for different phenotypes of Sch-PAH subjects; moreover, the probability of a type II error must be considered in some comparisons between the groups. The female preponderance in IPAH group might explain some differences observed during CPET; however, the responses in percent-predict (gender-adjusted) were consistent with those found in absolute values, which minimizes this heterogeneity. Also, we were unable to evaluate arterial blood gases in all subjects during exercise, making it impossible to accurately assess the behavior of VD/VT.
In conclusion, patients with Sch-PAH had higher aerobic capacity, lower submaximal ventilatory and cardiovascular stresses, and less impaired pulmonary gas exchange abnormalities than IPAH subjects with similar resting hemodynamic dysfunction. These findings suggest that the more benign clinical course of Sch-PAH could be due to the less impaired ability of the right ventricle to increase pulmonary blood flow during exercise, as compared to IPAH subjects. This hypothesis deserves further investigation in larger epidemiological studies.
Funding Statement
The authors have no support or funding to report.
References
- 1. Lapa M, Dias B, Jardim C, Fernandes CJC, Dourado PMM, et al. (2009) Cardiopulmonary manifestations of hepatosplenic schistosomiasis. Circulation 119: 1518–1523. [DOI] [PubMed] [Google Scholar]
- 2. Lopes AA, Bandeira AP, Flores PC, Santana MVT (2010) Pulmonary hypertension in Latin America: pulmonary vascular disease: the global perspective. Chest 137: 78S–84S. [DOI] [PubMed] [Google Scholar]
- 3. Shaw AP, Ghareeb A (1938) The pathogenesis of pulmonary schistosomiasis in Egypt with special reference to Ayerza’s disease. J Pathol Bacteriol 46: 401–424. [Google Scholar]
- 4. Fernandes CJS, Jardim CV, Hovnanian A, Hoette S, Dias BA, et al. (2010) Survival in schistosomiais-associated pulmonary arterial hypertension. J Am Coll Cardiol 56: 715–720. [DOI] [PubMed] [Google Scholar]
- 5. Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, et al. (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 34: 1219–1263. [DOI] [PubMed] [Google Scholar]
- 6. Simmoneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, et al. (2009) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54: 43–54. [DOI] [PubMed] [Google Scholar]
- 7.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ (1999) Principles of the exercise testing and interpretation. Baltimore: Lippincott Williams &Wilkins. 585p.
- 8. Sun XG, Hansen JE, Oudiz RJ, Wasserman K (2001) Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation 104: 429–435. [DOI] [PubMed] [Google Scholar]
- 9. D’Alonzo GE, Gianotti LA, Pohil RL, Reagle RR, DuRee SL, et al. (1987) Comparison of progressive exercise performance of normal subjects and patients with primary pulmonary hypertension. Chest 92: 57–62. [DOI] [PubMed] [Google Scholar]
- 10. Arena R, Lavie CJ, Milani RV, Myers J, Guazzi M (2010) Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: an evidence-based review. J Heart Lung Transpl 29: 159–173. [DOI] [PubMed] [Google Scholar]
- 11. Dantzker DR, D’Alonzo GE (1985) Pulmonary gas exchange and exercise performance in pulmonary hypertension. Chest 88: 255S–257S. [DOI] [PubMed] [Google Scholar]
- 12. Wensel R, Optiz CF, Anker SD, Winkler J, Höeffken G, et al. (2002) Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation. 106: 319–324. [DOI] [PubMed] [Google Scholar]
- 13. Groepenhoff H, Vonk-Noordegraaf A, Boonstra A, Spreeuwenberg MD, Postmus PE, et al. (2008) Exercise testing to estimate survival in pulmonary hypertension. Med Sci Sports Exerc 40: 1725–1732. [DOI] [PubMed] [Google Scholar]
- 14. ATS statement: guidelines for the six-minute walk test (2002) Am J Respir Crit Care Med. 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 15. Chemla D, Castelain V, Herve P, Lecarpentier Y, Brimioulle S (2002) Haemodynamic evaluation of pulmonary hypertension. Eur Respir J 20: 1314–1331. [DOI] [PubMed] [Google Scholar]
- 16. Wilkinson J (2001) Haemodynamic calculations in the catheter laboratory. Heart 85: 113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira RK, Ferreira EP, Ramos RP, Messina CM, Kapins CE, et al.. (2013) Usefulness of pulmonary capillary wedge pressure as a correlate of left ventricular filling pressures in pulmonary arterial hypertension. J Heart Lung Transplant: In press. doi:10.1016/j.healun.2013.10.008. [DOI] [PubMed]
- 18. Neder JA, Nery LE, Silva AC, Andreoni S, Whipp BJ (1999) Maximal aerobic power and leg muscle mass and strength related to age in non-athlethic males and females. Eur J Appl Physiol Occup Physiol 79: 522–530. [DOI] [PubMed] [Google Scholar]
- 19. Neder JA, Nery LE, Peres C, Whipp BJ (2001) Reference values for dynamic responses to incremental cycle ergometry in males and females aged 20 to 80. Am J Respir Crit Care Med. 164: 1481–1486. [DOI] [PubMed] [Google Scholar]
- 20. Borg G (1990) Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health 16: 55–58. [DOI] [PubMed] [Google Scholar]
- 21. Le Pavec J, Souza R, Herve P, Lebrec D, Savale L, et al. (2008) Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med 178: 637–643. [DOI] [PubMed] [Google Scholar]
- 22. Simmoneau G, Galiè N, Rubin L, Langleben D, Seeger W, et al. (2004) Clinical classification of pulmonary hypertension. J Am Coll Cardiol 43: 5S–12S. [DOI] [PubMed] [Google Scholar]
- 23. Graham BB, Bandeira AP, Morrel NW, Butrous G, Tuder RM (2010) Schistosomiasis-associated pulmonary hypertension: pulmonary vascular disease: the global perspective. Chest 137: 20S–29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oudiz RJ (2005) The role of exercise testing in the management of pulmonary arterial hypertension. Semin Respir Crit Care Med 26: 379–84. [DOI] [PubMed] [Google Scholar]
- 25. Deboeck G, Niset G, Vachiery JL, Moraine JJ, Naeije R (2005) Physiological response to the six-minute walk test in pulmonary arterial hypertension. Eur Respir J 26: 667–72. [DOI] [PubMed] [Google Scholar]
- 26. Theodore J, Robin ED, Morris AJ, Burke CM, Jamielson SW, et al. (1986) Augmented ventilatory response to exercise in pulmonary hypertension. Chest 89: 39–44. [DOI] [PubMed] [Google Scholar]
- 27. Sun XG, Hansen JE, Oudiz RJ, Wasserman K (2002) Gas exchange detection of exercise-induced right-to-left shunt in patients with primary pulmonary hypertension. Circulation 105: 54–60. [DOI] [PubMed] [Google Scholar]
- 28. Yasunobu Y, Oudiz RJ, Sun XG, Hansen JE, Wasserman K (2005) End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest 127: 1637–1646. [DOI] [PubMed] [Google Scholar]
- 29. Hansen JE, Ulubay G, Chow BF, Sun XG, Wasserman K (2007) Mixed-expired and end-tidal CO2 distinguish between ventilation and perfusion defects during exercise testing in patients with lung and heart diseases. Chest 132: 977–983. [DOI] [PubMed] [Google Scholar]
- 30. Deboeck G, Scoditti C, Huez S, Vachiéry JL, Lamotte M, et al. (2012) Exercise testing to predict outcome in idiopathic versus associated pulmonary arterial hypertension. Eur Respir J 40: 1410–9. [DOI] [PubMed] [Google Scholar]