The temperate grass, Brachypodium distachyon, is a useful model for studying gene networks controlling flowering.
Abstract
Timing of flowering is key to the reproductive success of many plants. In temperate climates, flowering is often coordinated with seasonal environmental cues such as temperature and photoperiod. Vernalization is an example of temperature influencing the timing of flowering and is defined as the process by which a prolonged exposure to the cold of winter results in competence to flower during the following spring. In cereals, three genes (VERNALIZATION1 [VRN1], VRN2, and FLOWERING LOCUS T [FT]) have been identified that influence the vernalization requirement and are thought to form a regulatory loop to control the timing of flowering. Here, we characterize natural variation in the vernalization and photoperiod responses in Brachypodium distachyon, a small temperate grass related to wheat (Triticum aestivum) and barley (Hordeum vulgare). Brachypodium spp. accessions display a wide range of flowering responses to different photoperiods and lengths of vernalization. In addition, we characterize the expression patterns of the closest homologs of VRN1, VRN2 (VRN2-like [BdVRN2L]), and FT before, during, and after cold exposure as well as in different photoperiods. FT messenger RNA levels generally correlate with flowering time among accessions grown in different photoperiods, and FT is more highly expressed in vernalized plants after cold. VRN1 is induced by cold in leaves and remains high following vernalization. Plants overexpressing VRN1 or FT flower rapidly in the absence of vernalization, and plants overexpressing VRN1 exhibit lower BdVRN2L levels. Interestingly, BdVRN2L is induced during cold, which is a difference in the behavior of BdVRN2L compared with wheat VRN2 during cold.
Flowering when conditions are most favorable is an important adaptive trait for reproductive success. Many plants adapted to temperate climates synchronize their flowering to coincide with seasons by monitoring cues such as temperature and photoperiod. One adaptation to a temperature cue is vernalization, the process by which plants become competent to flower after prolonged exposure to the cold of winter. This adaptation ensures that flowering occurs only under favorable conditions in spring (Amasino, 2004; Kim et al., 2009; Amasino and Michaels, 2010).
The genetic network underlying the vernalization response has been studied intensively in the crucifer Arabidopsis (Arabidopsis thaliana; Kim et al., 2009; Amasino and Michaels, 2010). In Arabidopsis, the MADS box transcription factor FLOWERING LOCUS C (FLC) is a major floral repressor that is active in the fall season prior to exposure of the cold of winter (Michaels and Amasino, 1999; Sheldon et al., 1999). The FRIGIDA (FRI) complex promotes high FLC expression in the fall (Michaels and Amasino, 1999; Johanson et al., 2000; Choi et al., 2011), resulting in repression of key floral promoting genes and thus preventing flowering (Helliwell et al., 2006; Searle et al., 2006). Allelic variation at FRI and FLC account for much of the natural variation in flowering time in natural accessions of Arabidopsis (Shindo et al., 2005). During winter, cold induces expression of VERNALIZATION INSENSITIVE3 (VIN3; Sung and Amasino, 2004) and noncoding RNAs, referred to as COLDAIR and COOLAIR, that initiate in the first intron and 3′ end of FLC, respectively (Swiezewski et al., 2009; Heo and Sung, 2011). VIN3 interacts with a Polycomb Repressive Complex 2 (PRC2) complex presumably to create a cold-specific PRC2 that is able to silence FLC (Wood et al., 2006; De Lucia et al., 2008). COLDAIR is thought to guide PRC2 to the FLC locus (Heo and Sung, 2011). PRC2 represses FLC by depositing H3K27 methylation at the FLC locus (De Lucia et al., 2008). Stable FLC repression after cold requires H3K9 methylation and the activities of VERNALIZATION1 (VRN1) and LIKE-HETEROCHROMATIN PROTEIN1 (Levy et al., 2002; Mylne et al., 2006; Sung et al., 2006). Stable repression of FLC permits induction of genes that promote flowering in the spring, such as FLOWERING LOCUS T (FT), which encodes the mobile florigen signal that moves from leaves to the meristem (Searle et al., 2006; Corbesier et al., 2007; Jaeger and Wigge, 2007; Mathieu et al., 2007; Tamaki et al., 2007). FT interacts with the basic leucine zipper domain (bZIP) transcription factor FD, and the resulting complex activates floral homeotic genes, such as APETALA1 (AP1), to promote floral meristem identity and flower development (Abe et al., 2005).
In addition to the Brassicaceae family, there are two other families of plants, Poaceae and Amaranthaceae, for which something is known about the vernalization system at the molecular level. Natural variation in the vernalization response in sugar beets (Beta vulgaris; Amaranthaceae) results from allelic variation at Beta vulgaris BOLTING TIME CONTROL1 (BvBTC1) (formerly the B locus). Dominant alleles of BvBTC1 result in an annual habit, whereas recessive alleles (Bvbtc1) confer a vernalization requirement and biennial growth habit. BvBTC1 encodes a pseudoresponse regulator with homology to the Arabidopsis PSEUDORESPONSE REGULATOR3 (PRR3) and PRR7 and barley (Hordeum vulgare) PHOTOPERIOD1-H1 genes (Pin et al., 2012). Dominant BvBTC1 alleles in annual sugar beets are associated with low mRNA levels of BvFT1, a repressor within the FT subfamily, and high mRNA levels of the Arabidopsis FT ortholog, BvFT2, and this is associated with rapid flowering without vernalization. In nonvernalized plants, which are delayed in flowering, recessive Bvbtc1 alleles are associated with high mRNA levels of BvFT1 and low mRNA levels of BvFT2 (Pin et al., 2012). BvFT1 is hypothesized to act upstream of BvFT2 because overexpression of BvFT1 results in lower level of BvFT2 mRNA (Pin et al., 2010). During cold in biennial beet, Bvbtc1 expression increases and BvFT1 expression decreases, permitting high BvFT2 expression in subsequent long days of spring to promote flowering (Pin et al., 2010, 2012).
In wheat (Triticum aestivum) and barley (Poaceae), three genes are thought to participate in a regulatory loop to control the timing of flowering, namely VRN1, VRN2, and FT (Dennis and Peacock, 2009; Distelfeld et al., 2009a; Greenup et al., 2009). Prior to cold exposure, high levels of VRN2 repress FT to prevent flowering (Yan et al., 2004b). VRN2 is in the CONSTANS-like gene family and contains a putative zinc finger and a CONSTANS, CONSTANS-LIKE, and TIMING OF CAB1 (CCT) domain (Yan et al., 2004b; Higgins et al., 2010). Although VRN2 acts similar to FLC and BvFT1 in that it is a floral repressor, it is unrelated to either gene, and thus far, no BvFT1 orthologs have been found in grasses, and it remains to be determined if recently discovered FLC-like genes play a functional role in the vernalization process in grasses (Greenup et al., 2010; Ruelens et al., 2013). That different genes are involved in establishing the vernalization requirement in Arabidopsis, beets, and cereals (as well as consideration of paleobotanic and paleoclimatic data) indicates that vernalization systems are likely to have evolved after these groups of plants diverged (Amasino, 2010).
During cold exposure in wheat and barley, VRN2 mRNA levels decrease and VRN1 mRNA levels increase; after cold exposure, VRN1 mRNA levels remain high and VRN2 mRNA levels remain low (Yan et al., 2003, 2004b; Dubcovsky et al., 2006; Trevaskis et al., 2006; Preston and Kellogg, 2008; Sasani et al., 2009). VRN1 is related to the AP1)/CAULIFLOWER (CAL)/FRUITFUL (FUL) family of MADS box transcription factors that control floral meristem identity in Arabidopsis (Yan et al., 2003; Preston and Kellogg, 2008). Whereas AP1/CAL/FUL are expressed primarily in meristems in Arabidopsis (Liljegren et al., 1999), in grasses, VRN1 is expressed in both meristems and leaves (Trevaskis et al., 2003; Yan et al., 2003; Preston and Kellogg, 2008; Sasani et al., 2009; Alonso-Peral et al., 2011). Cold activation of VRN1 is accompanied by changes in chromatin in a presumed regulatory region of its first intron (Oliver et al., 2009). Opposite to that of the cold-repressed FLC but expected for that of a cold-induced gene, VRN1 chromatin modifications shift from repressive (H3K27 methylation) to active (H3K4 methylation) during vernalization (Oliver et al., 2009).
In support of a model in which VRN1 is a repressor of VRN2, high levels of VRN1 early in development in lines harboring dominant VRN1 alleles are associated with low levels of VRN2 (Loukoianov et al., 2005; Hemming et al., 2008; Distelfeld et al., 2009a). Furthermore, VRN2 is not repressed after cold in vrn1 mutants, suggesting VRN1 plays a role in maintaining repression of VRN2 after vernalization (Chen and Dubcovsky, 2012). VRN2 levels do however decrease during cold exposure in vrn1 mutants, indicating there are additional factors that repress VRN2 during cold in wheat (Chen and Dubcovsky, 2012).
In spring after vernalization, low VRN2 levels allow for long-day photoperiod activation of FT in leaves and a switch from vegetative to floral meristem identity and flowering (Sasani et al., 2009). High levels of FT are associated with high levels of VRN1 expression in leaves, suggesting the possibility of a positive feedback loop between VRN1 and FT to “lock in” flowering (Yan et al., 2006; Shimada et al., 2009; Distelfeld and Dubcovsky, 2010). In vitro, FT physically interacts with the FD ortholog FD-like2, and this complex binds to the VRN1 promoter, lending support to this part of the feedback loop model (Li and Dubcovsky, 2008).
Epistatic relationships between VRN1, VRN2, and FT in leaves have been revealed through the study of existing allelic variants (Trevaskis et al., 2003; Yan et al., 2003, 2004b, 2006; Dubcovsky et al., 2005; Karsai et al., 2005; Hemming et al., 2008; Shimada et al., 2009). Particular varieties of spring barley and spring wheat, which do not require vernalization, carry either deletions of the VRN2 locus or point mutations in the CCT domain of VRN2 (Yan et al., 2004b; Dubcovsky et al., 2005; Karsai et al., 2005; von Zitzewitz et al., 2005; Distelfeld et al., 2009b). Therefore, an active VRN2 is necessary for a vernalization requirement. Other spring varieties have dominant alleles of VRN1 or FT that are constitutively activated (Yan et al., 2003, 2004a, 2006; Fu et al., 2005; Loukoianov et al., 2005; von Zitzewitz et al., 2005). Dominant VRN1 alleles bypass the requirement for vernalization, presumably by down-regulating VRN2 early in development (Loukoianov et al., 2005; Hemming et al., 2008). Plants overexpressing FT result in rapidly flowering plants with low levels of VRN2 and high levels of VRN1, indicating that FT can promote VRN1 expression in leaves (Shimada et al., 2009), whereas overexpression of VRN2 delays flowering (Hemming et al., 2008).
Although there has been great progress in understanding the vernalization pathway in wheat and barley, the extent to which vernalization pathways are conserved in grasses remains to be determined as well as identification of additional genes involved in flowering. Brachypodium distachyon is a small, wild relative of temperate grasses within the Pooideae, which includes important cereal crops such as wheat, barley, oats (Avena sativa), and rye (Secale cereale). Brachypodium spp. is a useful model grass because of its small, completely sequenced diploid genome (International Brachypodium Initiative, 2010), simple growth requirements, large collection of accessions, inbreeding nature, and high rate of recombination (Draper et al., 2001; Vogel et al., 2006, 2009; Opanowicz et al., 2008; Filiz et al., 2009; Brkljacic et al., 2011; Huo et al., 2011; Mur et al., 2011). All of these characteristics make Brachypodium spp. an attractive model for studying flowering, and the vernalization response in particular, in a temperate grass.
Several studies have characterized flowering behavior in a limited number of Brachypodium spp. accessions (Draper et al., 2001; Vogel et al., 2009; Schwartz et al., 2010). To advance Brachypodium spp. as a model for studying flowering, we have characterized flowering behavior in a range of accessions in response to different photoperiods and lengths of cold treatment. In addition, we have analyzed expression of VRN1, FT, and VRN2 genes in response to cold treatments and photoperiod to better understand what parts of the cereal regulatory loop might be conserved in Brachypodium spp.. Our results indicate there is rich natural diversity in photoperiod and vernalization requirements to exploit for understanding gene networks controlling flowering. In addition, we provide evidence for conserved roles of VRN1 and FT in promoting flowering, whereas the role of VRN2 is less clear.
RESULTS
Natural Variation of Vernalization and Photoperiod Requirement
Hundreds of diploid Brachypodium spp. accessions have been collected from its native range in Mediterranean Europe and the Middle East (Vogel et al., 2009; Mur et al., 2011). Preliminary studies identified a wide range of flowering behaviors in many accessions. Using this phenotypic diversity, we chose 13 accessions to evaluate in detail for flowering time under different photoperiods and the length of cold required to “saturate” the vernalization response. These 13 accessions were also chosen because they span the native range of Brachypodium spp. and represent the genotypic diversity within Brachypodium spp. based upon Simple Sequence Repeat (SSR) marker analysis (Supplemental Table S1; Vogel et al., 2009). Plants from each accession were subjected to cold treatment (5°C) in short days (8-h light, 16-h dark) as imbibed seeds in soil for varying lengths of time, followed by outgrowth in three different photoperiods: 20-h light/4-h dark, 16-h light/8-h dark, and 8-h light/16-h dark. Although a 20-h photoperiod is longer than the native photoperiod range experienced by Brachypodium spp. accessions, many Brachypodium spp. researchers use this photoperiod to accelerate growth and development, and thus including results from a 20-h photoperiod has practical value.
During cold treatments of 1 to 4 weeks, seedlings rarely emerged from the soil, but with longer cold treatments, the coleoptile typically emerged, as commonly observed in other cereals such as barley (Sasani et al., 2009; Greenup et al., 2010, 2011). During the longest cold treatments, seedlings typically reached the first leaf stage, with the second leaf visible but not fully expanded.
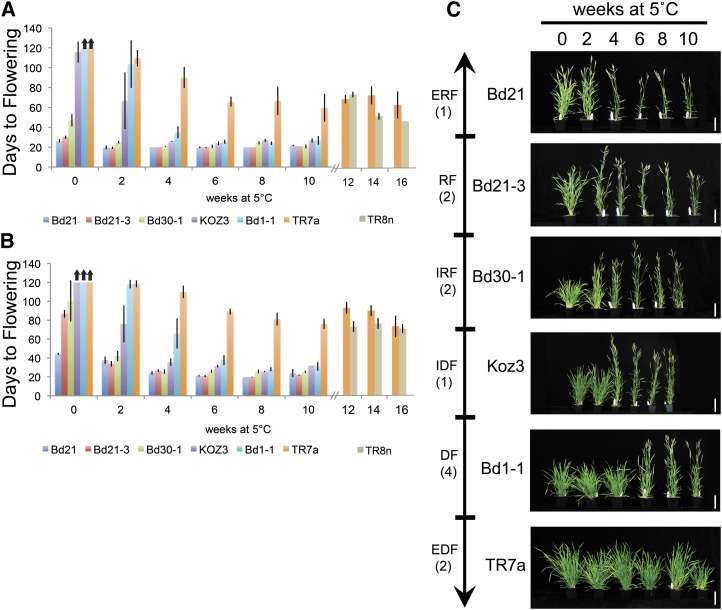
There is a great range of flowering behaviors among the 13 accessions. Although any categorization of this wide spectrum of flowering behavior is somewhat arbitrary, we find it useful to categorize the 13 accessions into six classes based upon the number of days it takes to flower without prior vernalization in both 16- and 20-h daylength and the length of cold exposure required to saturate the vernalization response. These classes are extremely rapid flowering, rapid flowering, intermediate rapid flowering, intermediate delayed flowering, delayed flowering and extremely delayed flowering. Flowering time was measured as days to flowering for all accessions (Fig. 1; Supplemental Fig. S1, A, B, E, and F) as well as primary leaf number on the main (or parent) shoot at flowering (Supplemental Fig. S1, C, D, G, and H). In all accessions tested, days to flowering was highly correlated with leaf number (r2 = 0.93; Supplemental Fig. S2).
Figure 1.
Vernalization and photoperiod interact to determine flowering time in a range of Brachypodium spp. accessions. A and B, Imbibed seeds of accessions were cold treated at 5°C in soil in an 8-h photoperiod for the indicated length of time (weeks), followed by outgrowth in a growth chamber set to 20-h light/4-h dark (A) or 16-h light/8-h dark (B). Flowering time is measured as the number of days to spike emergence (Zadoks scale = 50) from the end of cold treatment. Arrows indicate treatments where plants did not flower within the 120 d of the experiment. For TR8n in A and B, only flowering data for 12, 14, and 16 weeks of cold is presented (see Supplemental Fig. S1 for all flowering data for this accession.) C, Photographs of representative plants from each of the six flowering classes (described in the text) after 60 d of outgrowth in a 16-h photoperiod after vernalization. Number in parentheses below class name represents the number of accession(s) evaluated in this study that fall within that particular flowering class. ERF, Extremely rapid flowering; RF, rapid flowering; IRF, intermediate rapid flowering; IDF, intermediate delayed flowering; DF, delayed flowering; EDF, extremely delayed flowering. Bar = 5 cm.
Accession Bd21 is the sole member of the extremely rapid flowering class; it is the only accession tested that flowers rapidly (less than 45 d) in both 16-h and 20-h photoperiods without prior cold exposure, although there is a slight vernalization response when grown in a 16-h photoperiod (Fig. 1, A and B; Supplemental Fig. S1). Bd21-3 and Bd3-1 are in the rapid flowering class because they have a conditional vernalization requirement; these accessions do not require cold to flower rapidly in a 20-h photoperiod (20–30 d to flower [DTF]), but 2 to 3 weeks of cold significantly accelerates flowering in a 16-h photoperiod (Fig. 1, A and B). This is evident when considering both days to heading after vernalization and the total number of leaves produced by the main shoot at flowering (Supplemental Fig. S1, C and G). Bd21-3 and Bd3-1 therefore have a facultative vernalization requirement, dependent upon the photoperiod in which they are grown.
All of the other accessions tested have a strong vernalization response in a 20-h photoperiod as well as a 16-h photoperiod. We place accessions Bd30-1 and Bd2-3 in an intermediate rapid flowering class, as they are delayed in flowering compared with accessions in the rapid-flowering class, flowering within an average of 50 to 60 d with significant variability in flowering time without vernalization in a 20-h photoperiod (Fig. 1A). Bd30-1 and Bd2-3 exhibit delayed flowering when grown in 16 h of light (approximately 100 d), yet a short cold treatment of 2 to 3 weeks is sufficient to allow rapid flowering (Fig. 1B).
Without vernalization, none of the accessions within the three remaining delayed flowering classes flowered in a 20-h photoperiod upon termination of the experiment after 120 d of growth (Fig. 1A; Supplemental Fig. S1A). Plants that failed to flower continued to produce leaves and also produced a number of secondary tillers from the main shoot, resulting in a bushier plant (Fig. 1C; Supplemental Figs. S1C and S3). Given that none of these remaining classes flowered without vernalization, the length of cold required to saturate the vernalization response was the sole criterion used to categorize these accessions. Accessions BdTR12c and Koz-3 are placed in an intermediate delayed flowering class, given that as little as 2 to 4 weeks of cold exposure accelerated their flowering by over 50 d (Fig. 1, A and B; Supplemental Fig. S1). Accessions Bd1-1, RON-2, Tek-11, and Bd29-1 are placed in a delayed flowering class, as they require 6 to 8 weeks of cold to saturate their vernalization response (Fig. 1, A and B; Supplemental Fig. S1). In the case of BdTR8n and BdTR7a, flowering occurred after 60 d in a 20-h photoperiod even after 10 weeks of cold, in contrast to 20 to 30 d for the other accessions tested, and thus BdTR8n and BdTR7a are placed in an extremely delayed flowering class (Fig. 1A; Supplemental Fig. S1). To determine if longer cold treatments might further accelerate flowering of BdTR8n and BdTR7a, imbibed seeds were exposed to 12, 14, and 16 weeks of cold. Sixteen weeks of cold accelerated BdTR8n flowering by about 20 d in a 20-h photoperiod, whereas there was no acceleration of flowering of BdTR7a, which still flowered around 60 d after cold treatment (Fig. 1A). Photographs of representative plants from each of the six classes are presented in Figure 1C. Additional photographs of representative plants from all accessions × treatments at 60 d after cold treatment are shown in Supplemental Fig. S3.
None of the Brachypodium spp. accessions flowered in an 8-h photoperiod within 120 d even after 10 weeks of cold treatment (Supplemental Figs. S4 and S5). There are, however, varieties of wheat and barley in which inflorescence development is initiated in short days, but inflorescence emergence is severely delayed or does not occur in short days (Evans, 1987; Dubcovsky et al., 2006). Dissection of Brachypodium spp. meristems on the main shoot of at least three plants from each treatment after 120 d of outgrowth in short days revealed all of the meristems were vegetative with no morphological sign of floral transition (data not shown). However, spike emergence is observed in some Bd21 and Bd21-3 plants grown in short days after over 200 d of growth, and meristems show signs of morphological transition to flowering by 180 d of growth (data not shown). Thus, most of the accessions tested behave as obligate long-day plants, but the most rapid flowering accessions, Bd21 and Bd21-3, have a facultative long-day response flowering under short 8-h photoperiods when grown for long periods.
We also note that there is a range of heights among accessions (see also Draper et al., 2001; Filiz et al., 2009)), and cold treatment reduces the height of many accessions (Supplemental Fig. S6). Clearly, some of the variation in height is attributed to differences in flowering time. However, some accessions that received a saturating cold treatment and that flowered at the same time showed substantial variation in height. For example, Bd21-3 reached 23 cm and Bd29-1 reached 45 cm after saturating cold treatments, and both averaged six leaves at flowering (Supplemental Figs. S1C and S6C). To evaluate height differences without the complication of differences in flowering time, we measured height among accessions grown in a noninductive 8-h photoperiod, where all plants remained in a vegetative state. Again, there is a range of heights among accessions, and Bd3-1 and Bd2-3 are among the tallest accessions (Supplemental Figs. S5 and S6). However, cold treatment did not have a large effect on height within an accession in a noninductive 8-h photoperiod. Accounting for the minimum height that many of the accessions reach, even after long cold treatments, a minimum headspace of 70 cm is typically needed when growing many different accessions in a controlled environment chamber.
Interaction of Vernalization and Photoperiod
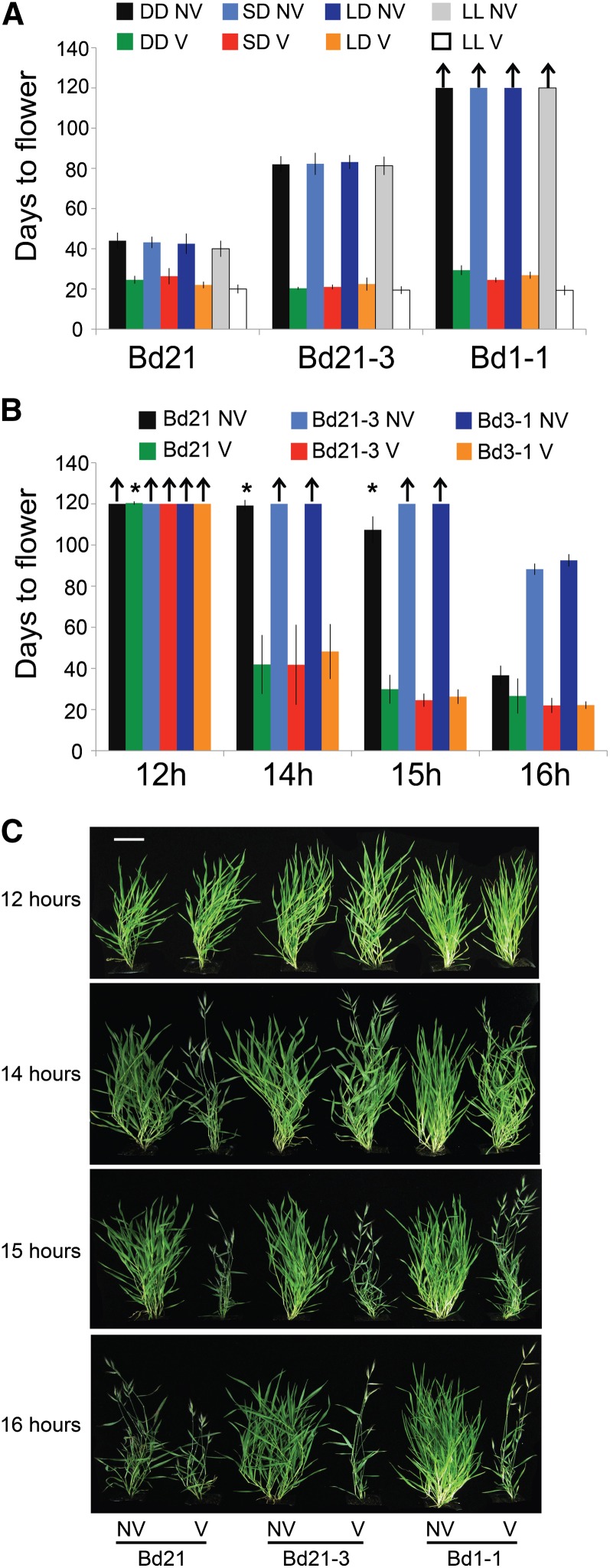
To test if there is any effect of photoperiod on the process of vernalization, imbibed seeds from Bd21, Bd21-3, and Bd1-1 were exposed to one of four light regimes during cold treatment: no light, short days (8-h light/16-h dark), long days (16-h light/8-h dark), and continuous light. All plants flowered rapidly after a saturating cold treatment ended, regardless of the photoperiod regime experienced during cold exposure, when compared with their respective nonvernalized control plants, which were exposed to the same photoperiod treatment for an equivalent developmental time (Fig. 2A; Supplemental Fig. S7). However, as discussed above, only a minimal amount of development occurs when vernalizing imbibed seeds; thus, although there is no effect of photoperiod on the effectiveness of vernalization on imbibed seeds, it is possible that the photoperiod during cold exposure of mature plants could affect flowering.
Figure 2.
Effect of different photoperiods during and after vernalization on flowering time. A, Imbibed seeds sown on plates were exposed to 5°C for 3 weeks (Bd21, Bd21-3) or 7 weeks (Bd1-1) in either no light (DD), 8-h light/16-h dark (SD), 16-h light/8-h dark (LD), or constant light (LL). Nonvernalized controls were exposed to a similar light regime to an equivalent stage of development that the vernalized seedlings reached by the end of the cold treatment. Plants were grown in a 16-h photoperiod following vernalization after transplanting to soil. B, Imbibed seeds sown in soil were exposed to 5°C for 3 weeks (under a 16-h photoperiod) before shifting to one of four photoperiods: 12-h light/12-h dark, 14-h light/10-h dark, 15-h light/9-h dark, or 16-h light/8-h dark. For A and B, flowering time reflects the number of days to spike emergence (Zadoks scale = 50) from the end of cold treatment. Arrows indicate treatments where plants did not flower during the 120 d of the experiment. Asterisk indicates some plants within a treatment did not flower after 120 d. C, Photos of 60-d-old plants from B. NV, Nonvernalized; V, vernalized. Bar = 5 cm.
Some Brachypodium spp. accessions flower more rapidly with increasing photoperiod, such as Bd21, Bd21-3, and Bd3-1 (Fig. 1, A and B). To explore the minimum photoperiod that promotes rapid flowering following vernalization, imbibed seeds from Bd21, Bd21-3, and Bd3-1 were sown in soil and exposed to 3 weeks of cold treatment (5°C, 16-h photoperiod) followed by growth in one of four photoperiods: 12-h light/12-h dark, 14-h light/10-h dark, 15-h light/9-h dark, or 16-h light/8-h dark. Fourteen hour or longer photoperiods significantly accelerate flowering following vernalization in Bd21, Bd21-3, and Bd3-1 (Fig. 2, B and C; Supplemental Fig. S8). By contrast, unvernalized plants and plants that experienced a 12-h photoperiod following vernalization exhibited delayed flowering (Fig. 2, B and C; Supplemental Fig. S8). Generally, longer photoperiods resulted in less variation and more rapid flowering among individuals after cold treatment.
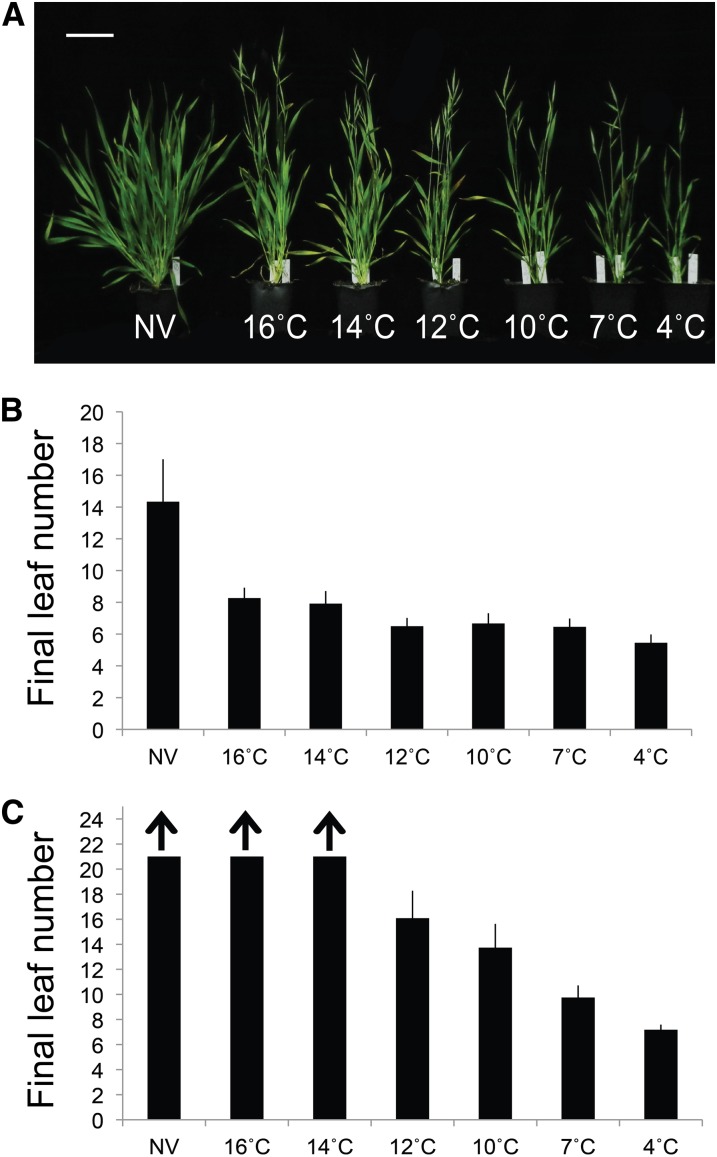
Temperature Range of the Vernalization Response
Several studies have defined a range of temperatures that satisfy the vernalization requirement in different plant species or accessions within a species (Ahrens and Loomis, 1963; Chujo, 1966; Trione and Metzger, 1970; Rawson et al., 1998; Wollenberg and Amasino, 2012). To define the temperature range effective for Brachypodium spp. vernalization, we exposed seedlings (Zadoks stage 10–11; Zadoks et al., 1974) from Bd21-3 and Bd1-1 to a range of temperatures (vernalization treatment under a long-day 16-h photoperiod) and assessed flowering compared with nonvernalized controls. Because time and temperature result in different growth rates among the different vernalization treatments, flowering time is presented as the number of primary leaves on the main shoot at flowering (see Supplemental Fig. S9 for flowering time presented as days to flowering).
Interestingly, temperatures averaging as high as 16°C had some accelerating effect on flowering in Bd21-3, but there was little, if any, effect in Bd1-1 (Fig. 3). In Bd1-1, acceleration of flowering was evident after a 12°C cold treatment (Fig. 3C). One caveat to this interpretation is that Bd21-3 and Bd1-1 vary in the time it takes to satisfy their vernalization requirement (3 versus 7 weeks, respectively), and hence additional growth that occurred during cold treatment of Bd1-1 plants may have influenced the ability to vernalize at higher temperatures. Regardless of this, it is clear that temperatures as high as 12°C accelerate flowering in Bd1-1.
Figure 3.
Temperature range that satisfies the vernalization requirement. Imbibed Bd21-3 or Bd1-1 seeds were sown in soil and allowed to germinate to Zadoks stages 07 to 10. Seedlings were then transferred to different vernalization temperatures under a 16-h photoperiod for 3 weeks (Bd21-3) or 7 weeks (Bd1-1) before shifting to outgrowth in a 16-h photoperiod. Outgrowth conditions averaged 22°C during the light period and 18°C during the dark period under a 16-h photoperiod. During vernalization, temperatures fluctuated diurnally in a 16-h photoperiod (data not shown). A, Representative images of Bd21-3 plants after different temperatures during cold treatment (all plants are the same age). Flowering time of Bd21-3 (B) or Bd1-1 (C) plants measured as the number of primary leaves produced by the main shoot at the time of flowering. Arrows indicate plants did not flower during the 100 d of the experiment. NV, Nonvernalized. [See online article for color version of this figure.]
VRN1 and FT Promote Flowering in Brachypodium spp.
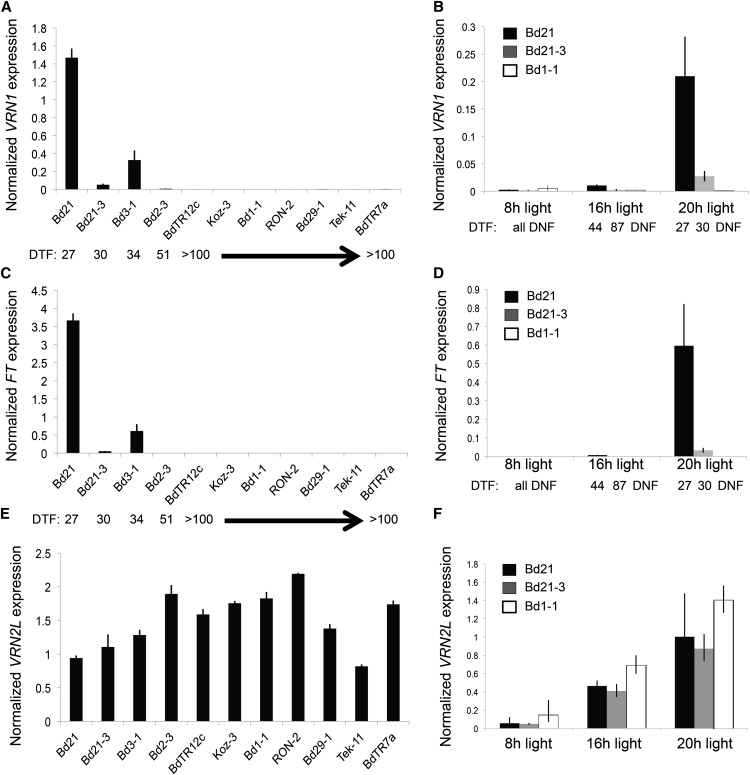
Brachypodium spp. has VRN1/AP1- and FT-containing gene families, in which clearly identifiable orthologs to wheat VRN1 and wheat FT are present (Higgins et al., 2010). To determine the relationship between flowering time and mRNA levels of VRN1 or FT in Brachypodium spp., we measured mRNA levels of these genes in various accessions grown without cold treatment in a 20-h photoperiod. These accessions were selected because they vary in flowering time and vernalization requirement (Fig. 1A; Supplemental Fig. S1). There was a clear correlation between elevated levels of VRN1 and FT and rapid flowering among the accessions. VRN1 and FT levels were highest in Bd21 (DTF = 27), followed by Bd3-1 (DTF = 34) and Bd21-3 (DTF = 30; Fig. 4, A and C). Accessions that showed delayed flowering (50 d or longer) had low levels of VRN1 and FT (Fig. 4, A and C). These data agree with the correlation between flowering time and VRN1 and FT levels previously published in a sample of Brachypodium spp. accessions (Schwartz et al., 2010).
Figure 4.
VRN1, VRN2L, and FT expression in different accessions and photoperiods without cold treatment. A,C, and E, RT-qPCR of VRN1 (A), FT (C), and VRN2L (E) expression in the second leaf of Brachypodium spp. accessions. Plants were grown nonvernalized in a 20-h photoperiod and harvested at leaf stage 2 (Zadoks stages = 12–13). B,D, and F, RT-qPCR of VRN1 (B), FT (D), and VRN2L (F) expression in the second leaf of Brachypodium spp. accessions (Bd21, Bd21-3, and Bd1-1) grown nonvernalized in 8-, 16-, or 20-h photoperiods and harvested at leaf stage 2 during the middle of the photoperiod. Bars represent the average of three biological replicates ± 1 sd. DNF, Did not flower during 120 d of growth.
As shown in Figures 1 and 2, accessions that are rapid flowering when grown in 20-h photoperiods exhibit delayed flowering under shorter photoperiods. For example, Bd21 and Bd21-3 flower later in a 16-h photoperiod (DTF = 44 and 87, respectively) compared with a 20-h photoperiod (DTF = 27 and 30, respectively) in the absence of cold treatment. Consistent with this, VRN1 and FT levels are significantly lower in Bd21 and Bd21-3 plants grown in a 16-h photoperiod compared with plants grown in a 20-h photoperiod (Fig. 4, B and D). FT and VRN1 are not detected in Bd21 or Bd21-3 plants grown in an 8-h photoperiod (Fig. 4, B and D), consistent with the observation that neither of these accessions flowered within 120 d when grown in this photoperiod (Supplemental Fig. S4). In addition, FT and VRN1 mRNA levels were undetectable in Bd1-1 in all photoperiods, correlating with the delayed flowering of this line in the absence of cold treatment (>120 d; Fig. 4, B and D).
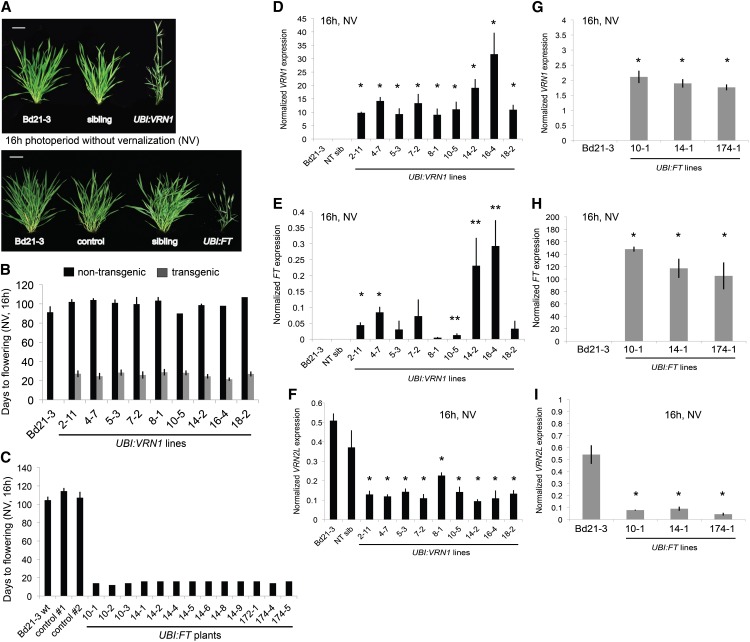
To evaluate the role of VRN1 and FT in Brachypodium spp. flowering, we generated transgenic Bd21-3 plants that express the VRN1 or FT complementary DNA (cDNA) under control of a Panicum virgatum ubiquitin promoter (Mann et al., 2012). We generated 10 independent transgenic lines that segregate for the VRN1 transgene UBI:VRN1 and three independent transgenic lines that segregate for the FT transgene UBI:FT. In a 16-h photoperiod, in which wild-type Bd21-3 is delayed in flowering without undergoing vernalization, transgenic plants harboring UBI:VRN1 flowered within an average of 21 to 29 d and produced an average of five to six leaves (Fig. 5, A and B; Supplemental Fig. S10A). Strikingly, UBI:FT transgenic plants flowered after about 12 d and produced four leaves (Fig. 5, A and C; Supplemental Fig. S10B).
Figure 5.
VRN1 and FT overexpression cause rapid flowering. A, Representative photos of Bd21-3 wild-type, nontransgenic sibling plants (sibling), empty pANIC10A vector transformed plants (control), and UBI:VRN1 and UBI:FT plants grown nonvernalized in a 16-h photoperiod. Bar = 4 cm. B, Flowering time of Bd21-3 wild-type compared with independent UBI:VRN1 transgenic lines. Dark gray indicates plants carrying UBI:VRN1, and light gray indicates sibling plants segregating from the same primary transformant mother plant that do not carry the transgene. C, Flowering time averages of Bd21-3 wild-type and empty vector control plants (control) compared with flowering time of individual UBI:FT transgenic plants from different transgenic lines. For B and C, plants were grown nonvernalized in a 16-h photoperiod, and bars represent the average ± 1 sd. D to I, RT-qPCR data of leaf 2 from two-leaf stage UBI-VRN1, UBI-FT, or Bd21-3 control plants grown nonvernalized in a 16-h photoperiod. VRN1 expression (D and G), FT expression (E and H), and VRN2L expression (F and I). For D to I, bars represent the average of three biological replicates ± 1 sd. Single asterisk indicates P value < 0.01, and two asterisks indicate P value < 0.05. NT sib, Nontransgenic sibling plant. [See online article for color version of this figure.]
VRN1 expression levels in the leaves of 10 independent UBI:VRN1 transgenic lines is significantly higher (P < 0.05) compared with wild-type Bd21-3 plants or sibling plants that did not harbor the transgene (Fig. 5D). FT expression is also significantly elevated in the leaves of the UBI:VRN1 transgenic plants compared with control plants (P < 0.05), consistent with the rapid flowering phenotype of these plants and indicating that VRN1 plays a role in promoting FT expression in leaves (Fig. 5E). FT expression levels in the leaves of three independent UBI:FT transgenic lines is significantly higher (P < 0.01) compared with wild-type Bd21-3 plants that did not harbor the transgene, correlating with the early flowering phenotype of these lines (Fig. 5H). Additionally, VRN1 expression is significantly up-regulated (P < 0.01) in the UBI:FT transgenic lines compared with wild-type Bd21-3 (Fig. 5G).
Effect of Vernalization (Cold) on VRN1 and FT in Brachypodium spp.
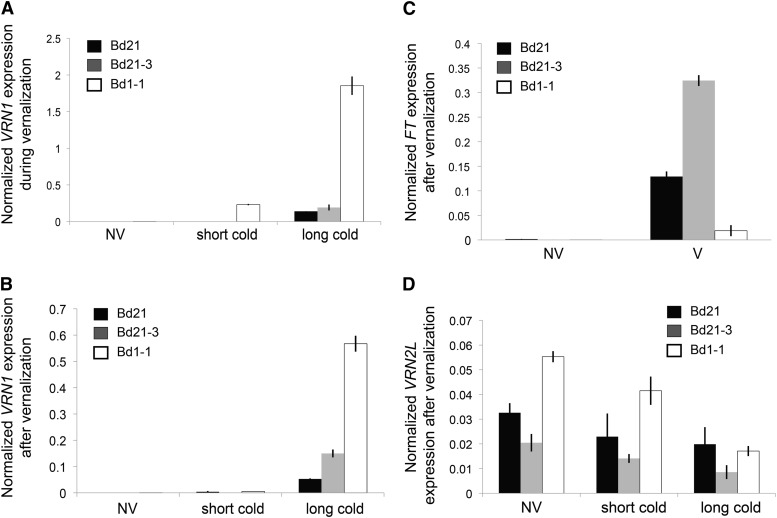
In wheat and barley, VRN1 levels increase during cold and remain elevated following cold (Trevaskis et al., 2003; Yan et al., 2003; Preston and Kellogg, 2008; Sasani et al., 2009), whereas FT levels are higher in vernalized plants following cold treatment compared with nonvernalized plants (Yan et al., 2006; Hemming et al., 2008; Sasani et al., 2009; Shimada et al., 2009). We tested the influence of cold treatment (performed under a 16-h long-day photoperiod) on expression of the VRN1 and FT orthologs in three Brachypodium spp. accessions.
VRN1 mRNA levels increase quantitatively during increasing length of cold treatment in Bd1-1 and were significantly higher in all accessions tested by the end of the saturating cold treatment (Fig. 6A). FT levels are undetectable in all samples at the end of the cold treatment (data not shown). After a saturating cold treatment of imbibed seeds, VRN1 and FT expression was significantly higher in the leaves of vernalized samples of all accessions compared with the respective nonvernalized samples (Fig. 6, B and C), correlating with rapid flowering. However, VRN1 levels were significantly lower in leaves of all accessions after a subsaturating cold treatment compared with saturating cold treatments (Fig. 6B). The lower VRN1 levels measured after a subsaturating cold treatment correlates with delayed flowering in these accessions after this treatment (Fig. 6B; Supplemental Fig. S11). VRN1 levels remained high in leaves of Bd21, Bd21-3, and Bd1-1 plants that were fully vernalized, and plants flowered rapidly after this cold treatment (Fig. 6B; Supplemental Fig. S11; data not shown).
Figure 6.
VRN1, FT, and VRN2L gene expression with and without cold treatment of imbibed seeds. Imbibed seeds were given a subsaturating (short cold) vernalization treatment (1 week at 5°C for Bd21 and Bd21-3; 3 weeks at 5°C for Bd1-1) or a saturating (long cold) vernalization treatment (3 weeks at 5°C for Bd21 and Bd21-3; 7 weeks at 5°C for Bd1-1). Leaf 2 was harvested 11 d after removing from cold (B–D) or from emerging shoot tissue at the end of the cold treatment (A). Tissue was harvested at a similar stage of development from nonvernalized controls. Plants were grown in a 16-h photoperiod during and after vernalization. For C, Bd1-1 was treated for 6 weeks of cold. A, RT-qPCR of VRN1 in shoot tissue of Bd21, Bd21-3, and Bd1-1 during cold treatment. B, RT-qPCR of VRN1 in leaf 2 of Bd21, Bd21-3, and Bd1-1 after cold treatment. C, RT-qPCR of FT in leaf 2 of Bd21, Bd21-3, and Bd1-1 after cold treatment. D, RT-qPCR of VRN2L in leaf 2 of Bd21, Bd21-3, and Bd1-1 after cold treatment. Bars represent mean of three biological replicates ± se. Black indicates Bd21, gray indicates Bd21-3, and white indicates Bd1-1. NV, Nonvernalized; V, vernalized.
A Gene with Homology to Wheat VRN2 Is Up-Regulated by Cold
The closest homolog of wheat VRN2 is Bradi3g10010 (Higgins et al., 2010; Ream et al., 2012). We refer to this gene as VRN2-like (VRN2L) because Bayesian phylogenetic studies using the CCT domain place Bradi3g10010 within the VRN2 clade; however, the VRN2 and Ghd7 (a photoperiod response regulator) clades are unresolved but may in fact be orthologous (for additional discussion, see Ream et al., 2012). Additionally, BLAST searches using VRN2L in the most recently released versions of the wheat (Brenchley et al., 2012) and barley genomes (Mayer et al., 2012) do not reveal a closer homolog to Bradi3g10010 than HvVRN2 and TmVRN2 (Zinc Finger-CCT1 [ZCCT1]/ZCCT2).
Given that there are no closer homologs to wheat VRN2 than BdVRN2L, we hypothesized that BdVRN2L might play a VRN2-like role in Brachypodium spp. As stated earlier, VRN1, VRN2, and FT are thought to form a regulatory loop in wheat and barley. If the same regulatory loop exists in Brachypodium spp., plants that overexpress VRN1 should have high levels of FT and low levels of VRN2L. VRN2L expression was significantly lower in all of the UBI:VRN1 lines (P < 0.05) and UBI:FT lines (P < 0.01) compared with wild-type Bd21-3 plants or nontransgenic sibling plants (Fig. 5, F and I). These data support the hypothesis that VRN2L plays a role analogous to wheat VRN2 in a feedback loop model with VRN1 and FT (Yan et al., 2006; Shimada et al., 2009; Distelfeld and Dubcovsky, 2010).
In wheat and barley, VRN2 expression is lower in short days versus long days (Dubcovsky et al., 2006; Trevaskis et al., 2006; Sasani et al., 2009). In the tropical cereals rice (Oryza sativa) and Zea mays, GHD7 represses flowering in long days and transcripts of GHD7 are correspondingly higher in long days versus short days (Xue et al., 2008; Hung et al., 2012). We measured VRN2L expression in Brachypodium spp. accessions Bd21, Bd21-3, and Bd1-1 under different photoperiods (Fig. 4F). VRN2L is more highly expressed with increasing photoperiod in all three accessions (Fig. 4F), consistent with behavior of wheat VRN2 and rice Ghd7.
We analyzed VRN2L levels in different accessions to determine if there was a correlation with flowering time in a 20-h photoperiod, as is the case with VRN1 and FT. VRN2L expression varied by less than 3-fold among the accessions tested (Fig. 4E). Furthermore, there was lack of a strong correlation between flowering time and VRN2L expression among different Brachypodium spp. accessions. For instance, Bd2-3 flowers at around 50 d without cold in a 20-h photoperiod but had higher levels of VRN2L than Tek-11 (Fig. 4E), a line that did not flower in a 20-h photoperiod unless exposed to at least 8 weeks of cold (Fig. 1). Thus, if VRN2L plays a repressive role in Brachypodium spp. flowering, VRN2L mRNA levels are not likely to be rate limiting.
In wheat and barley, VRN2 is down-regulated in response to cold and is stably repressed after cold (Yan et al., 2004b; Trevaskis et al., 2006). VRN2L expression was lower in leaves derived from vernalized imbibed seeds (measured after vernalization was completed) compared with nonvernalized plants (Fig. 6D). Furthermore, VRN2L mRNA levels measured in plants after the end of the cold treatment progressively decreased after exposure to increasing durations of cold in Bd1-1 and slightly decreased in Bd21 and Bd21-3 (Fig. 6D). VRN2L levels were very low or undetectable in shoot tissues emerging from a germinating seed during cold. Consistent with this, VRN2 levels in wheat and barley are very low or undetectable in similar tissues during cold (Sasani et al., 2009).
During cold, wheat and barley VRN2 levels decrease significantly in leaves (Yan et al., 2004b; Trevaskis et al., 2006), consistent with VRN2’s role as a floral repressor and similar to the decrease in FLC levels during vernalization in Arabidopsis (Michaels and Amasino, 1999; Sheldon et al., 1999). Surprisingly, VRN2L mRNA levels increase in leaves during cold exposure of Bd21-3 seedlings in both short and long days (Fig. 7A; Supplemental Fig. S12D). This pattern of expression is in stark contrast to that observed for VRN2 in wheat (cv G3116) or barley (cv Sonja), where VRN2 is repressed in leaves during cold exposure in long days (Supplemental Fig. S12, E and F; Yan et al., 2004b; Trevaskis et al., 2006; Sasani et al., 2009) and where VRN2 mRNA levels are very low or undetectable in short days, regardless of cold treatment (Dubcovsky et al., 2006; Trevaskis et al., 2006; Sasani et al., 2009). As a control for the cold treatment, we also measured VRN1 mRNA levels. Similar to wheat and barley VRN1, Brachypodium spp. VRN1 mRNA levels increased in the leaves by the end of cold (Supplemental Fig. S12, A–C).
Figure 7.
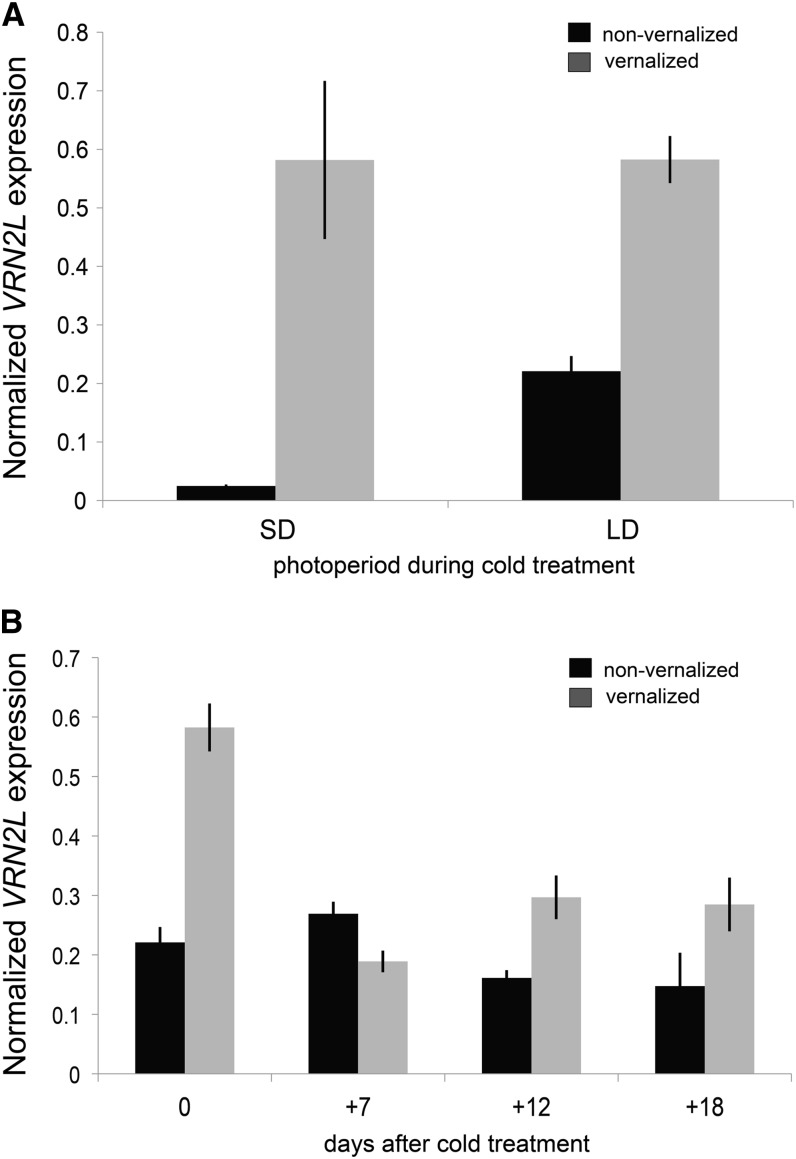
VRN2L gene expression in leaves of vernalized plants during and after cold treatment. Seedlings were grown to the two-leaf stage (Zadoks stage = 12) before transferring to 5°C for 3 weeks. After cold treatment, plants were grown in a 16-h photoperiod. A, RT-qPCR of VRN2L in leaf 2 of nonvernalized (black) versus vernalized (gray) Bd21-3 at the end of a saturating vernalization treatment conducted in short days (8-h photoperiod) or long days (16-h photoperiod). B, RT-qPCR of VRN2L in leaf 2 of nonvernalized (black) versus vernalized (gray) Bd21-3 after cold treatment performed in 16-h photoperiod. Bars represent the average of three biological replicates ± 1 sd.
Seven days after cold exposure of seedlings, VRN2L mRNA levels were slightly lower in vernalized versus nonvernalized plants (Fig. 7B), similar to the results presented in Figure 6 in leaves derived from vernalized imbibed seeds. However, this difference was abolished after 12 days following vernalization (Fig. 7B). Similar to observations with imbibed seed vernalization (Fig. 6, A and B), VRN1 mRNA levels were high in leaves of vernalized seedlings at the end of the cold treatment and remained high in vernalized plants following cold (Supplemental Fig. S13, A and B). FT mRNA levels were undetectable in leaves of seedlings at the end of cold treatment (data not shown) but were significantly higher after cold treatment in vernalized plants (Supplemental Fig. S13C).
To more precisely determine when VRN2L levels increase in leaves during cold, Bd21-3 seedlings were grown to the two-leaf stage and shifted to one of several lengths of cold in a 16-h photoperiod ranging from hours to weeks. VRN2L levels in leaf 2 peaked after 25 h of cold and were still higher in plants treated for 1 to 4 weeks of cold compared with nontreated plants (Fig. 8), similar to the patterns observed in Figure 7A and Supplemental Figure S12D. In addition, we analyzed VRN2L levels from a recently published microarray data set (Li et al., 2012), where Bd21 was treated with different periods of cold ranging from 0 to 24 hours. In this data set, VRN2L levels also increased during short cold, peaking between 10 and 24 h of cold treatment. In summary, VRN2L mRNA levels peak during short-term cold exposure, but levels remain elevated even after several weeks of cold compared with noncold-treated plants. We also note that the VRN2L cold induction pattern is different than the cold induction pattern of VRN1, where expression increases gradually over several weeks of cold (Supplemental Fig. S14).
Figure 8.
VRN2L expression in response to cold. A, RT-qPCR of VRN2L in leaf 2 in plants that received the indicated lengths of cold treatments (5°C) in a 16-h photoperiod. Expression values of each treatment are shown relative to noncold-treated plants harvested at the same time of day. Bars represent an average of three biological replicates ± 1 sd.
DISCUSSION
We have characterized flowering time variation in a variety of Brachypodium spp. accessions in relation to photoperiod and vernalization. There is a wide range of variation for flowering time behaviors, and flowering time can be manipulated quite readily by varying either cold exposure or photoperiod (Fig. 1). Our study provides a foundation for characterizing the genetic determinants of natural variation in flowering behavior at a molecular level.
Photoperiod and Temperature Influence on Flowering
For the majority of Brachypodium spp. accessions, 6 weeks of vernalization followed by a 20-h photoperiod will generally result in plants that flower at the same time and at a similar developmental stage (Fig. 1). Exceptions to this observation are accessions in the extremely delayed flowering class such as BdTR7a and BdTR8n, which require longer cold exposure to flower rapidly after a shift to warm conditions (Fig. 1, A and B; Supplemental Fig. S1). Although a 20-h photoperiod is not a native photoperiod where these accessions are found, we chose this photoperiod because it is a common daylength used by many laboratories to grow Brachypodium spp., and this photoperiod provides an additional parameter to classify flowering behavior among accessions. Conversely, all accessions tested will remain vegetative for at least 120 d if grown in an 8-h photoperiod (Supplemental Fig. S4). Vernalization is effective in imbibed seeds for all accessions as initially reported for Bd1-1 (Schwartz et al., 2010).
Brachypodium spp. accessions have previously been grouped into spring and winter classes based on flowering behavior in a 20-h photoperiod without vernalization (Schwartz et al., 2010). We have expanded this analysis and categorized Brachypodium spp. accessions into six different flowering time classes (Fig. 1C). Although the boundaries distinguishing these classes is arbitrary, this more discriminating classification system will inform genome-wide association studies and provide researchers with a framework for growing different accessions in controlled laboratory environments. Future studies using a larger set of accessions will explore in greater depth whether any associations exist between geography and flowering behavior. In our study, accessions from Iraq typically flowered most rapidly with little or no vernalization requirement. Turkish accessions showed a range of flowering behaviors (2 weeks of cold to greater than 10 weeks of cold to accelerate flowering), and two Spanish accessions also differed substantially in vernalization requirement.
Given the photoperiods within the native growth habitats of Brachypodium spp., most lines might actually behave as winter annuals. This is clearly supported by evidence of delayed flowering of Bd21, Bd21-3, and Bd3-1 in a 14-h photoperiod (compared with 16-h or 20-h photoperiods) and acceleration of flowering of these accessions in a 14-h photoperiod after vernalization (Fig. 2). In the absence of cold, some winter cereals transition to flowering more rapidly after a short-day treatment followed by growth in long days compared with growth in long days alone (Evans, 1987; Dubcovsky et al., 2006; Turner et al., 2013). Although a previous study did not provide evidence of this response when using long days of 20-h for accessions Bd21-3, Bd3-1, and Bd30-1 (formerly DNSCA-6; Schwartz et al., 2010), testing these accessions in shorter, native long-day photoperiods should be informative. We have observed natural variation in the ability of short days to replace cold in accelerating flowering in Brachypodium spp. under more native photoperiods (D.P. Woods, T.S. Ream, and R.M. Amasino, unpublished data).
None of the accessions tested flower under short 8-h daylength after 120 d of outgrowth, even after receiving long vernalization treatments. Additionally, meristems of all tested accessions remained vegetative throughout the 120-d growth period with no signs of floral transition. Together, this suggests that it is unlikely that the Brachypodium spp. accessions tested contain photoperiod-insensitive alleles. Photoperiod-insensitive alleles within specific genotypes of wheat and barley lead to rapid flowering in both long and short daylength and exhibit normal spike development (Dubcovsky et al., 2006; Beales et al., 2007; Turner et al., 2013). This contrasts with studies in wheat in which genotypes have been discovered that carry CArG-box mutations within the promoter region of VRN1, resulting in early initiation of spike development in short days but subsequent slower growth of the spikelet in short days compared with long days (Dubcovsky et al., 2006).
A range of temperatures will satisfy the vernalization requirement (Fig. 3), similar to that found in studies of wheat (Ahrens and Loomis, 1963; Chujo, 1966; Trione and Metzger, 1970; Rawson et al., 1998). Temperatures as high as 16°C have been reported to accelerate flowering via the vernalization pathway in Arabidopsis (Wollenberg and Amasino, 2012). In wheat, temperatures between 0°C and 12°C have been reported to be effective, although higher temperatures generally result in less effective vernalization (Chujo, 1966). This is not unexpected given that in nature, plants or seeds will experience a range of cold temperatures over the course of winter. Nonetheless, temperatures from 2°C to 6°C are optimal to ensure a complete vernalization response and thus more uniform rapid flowering in response to long days across a range of accessions.
VRN1 and FT Promote Flowering
The VRN1 ortholog in Brachypodium spp. has a conserved role similar to that in wheat and barley in promoting flowering. The Brachypodium spp. FT ortholog also has a floral promoting effect, as is the case across a range of flowering plant families. VRN1 and FT levels correlate with flowering behavior in nonvernalized and vernalized plants, and overexpression of these genes results in rapid-flowering plants (Figs. 4–6). Long-term cold induces VRN1 expression, and this correlates with higher FT expression after vernalization (Fig. 6; Supplemental Figs. S13 and S14). Overexpression of VRN1 and FT both lead to rapid flowering in a 16-h photoperiod. Consistent with studies from wheat and barley, which suggest a positive feedback loop exists between FT and VRN1, we observed high mRNA levels of FT in UBI:VRN1 lines and high mRNA levels of VRN1 in UBI:FT lines (Yan et al., 2006; Shimada et al., 2009; Distelfeld and Dubcovsky, 2010), suggesting this part of the model is conserved in Brachypodium spp. and throughout the Pooideae.
In wheat and barley, rapid-flowering lines often harbor natural allelic variants of VRN1 or FT (Yan et al., 2003, 2004a, 2006; Fu et al., 2005; Loukoianov et al., 2005; von Zitzewitz et al., 2005). Dominant alleles of VRN1 and FT in wheat and barley are able to bypass the vernalization requirement, even in the presence of functional VRN2 alleles. Future studies will examine whether there is association between certain alleles of VRN1, FT, and VRN2L with flowering. Sequences of many accessions are currently being generated and will enable such a study (http://genome.jgi.doe.gov/genome-projects/).
Brachypodium spp. VRN2L Is Distinct from Wheat/Barley VRN2
In contrast to VRN1 and FT, the role of VRN2L in Brachypodium spp. flowering is less clear. VRN2L, the closest homolog to wheat/barley VRN2 identified to date in Brachypodium spp., shares some features in common with wheat/barley VRN2 (Ream et al., 2012). Specifically, VRN2L is expressed more highly as days get longer (Fig. 4F; Ream et al., 2012), and after vernalization of imbibed seeds, VRN2L mRNA levels are lower in leaves (Fig. 6). Searches of assemblies of other Brachypodium spp. accessions with a range of flowering behaviors has so far not revealed a gene with closer homology to wheat VRN2, nor have these searches revealed a VRN2 homolog in the region of synteny that is deleted in Bd21 (for discussion, see Ream et al., 2012).
On the other hand, several features of Brachypodium spp. VRN2L are distinct from VRN2 in wheat and barley. First, VRN2 mRNA levels decrease during cold in wheat and barley and remain low at least 14 days after vernalization in wheat (Yan et al., 2004b); by contrast, Brachypodium spp. VRN2L mRNA levels increase during cold exposure (Fig. 7; Supplemental Fig. S12D). The cold-mediated increase occurs quite rapidly (within a day) relative to the time it takes to satisfy the vernalization requirement (several weeks; Fig. 8). The cold-induced expression of VRN2L in leaves is reminiscent, at least to some degree, to that of the C-REPEAT BINDING FACTOR genes that have roles in cold acclimation (Thomashow, 2010). Further tests are necessary to determine whether VRN2L in Brachypodium spp. has any role in cold acclimation.
Second, Brachypodium spp. VRN2L levels are only slightly lower after seedling vernalization, and this repression is not observed past 12 days following the end of cold exposure (Fig. 7). A confounding variable regarding seedlings that have undergone vernalization (but not in the case of imbibed seeds where VRN2L mRNA is essentially not detected at this developmental stage) is the several-fold higher mRNA levels of VRN2L following cold treatment relative to nonvernalized plants. After moving to warm temperatures, VRN2L levels do drop relative to the higher levels present immediately at the end of cold treatment in vernalized plants, but 7 d after cold exposure, they are only marginally lower than the mRNA levels in nonvernalized plants. By 12 d following cold exposure, as discussed above, VRN2L levels are not lower than the nonvernalized controls. This pattern of expression is not indicative of a gene acting as a floral repressor that is targeted by a vernalization response pathway.
Brachypodium spp. VRN2L mRNA levels in leaves do not always correlate with VRN1 or FT expression in the absence of cold, highlighting another feature distinguishing Brachypodium spp. VRN2L from wheat or barley VRN2, where high mRNA levels of FT and VRN1 consistently correlate with low mRNA levels of VRN2 (Trevaskis et al., 2006; Yan et al., 2006; Hemming et al., 2008; Shimada et al., 2009). The inconsistent correlation of VRN2L with VRN1 and FT is highlighted by two examples. In the first case, rapid-flowering accessions Bd21, Bd21-3, and Bd3-1 have high mRNA levels of VRN1 and FT but not correspondingly lower levels of VRN2 when compared with other accessions (Fig. 4E). However, in the second case, high mRNA levels of VRN1 and FT (i.e. generated via a UBI:VRN1 or UBI:FT transgene) do correlate with lower VRN2L mRNA levels (Fig. 5F).
In summary, Brachypodium spp. is a useful model for understanding the genetic networks conferring a vernalization response in a temperate grass. It is likely that the roles of VRN1 and FT are conserved in vernalization in Brachypodium spp. compared with cereals. Future studies will reveal the extent of conservation in the vernalization network between Brachypodium spp. and economically important cereals such as wheat, oats, and barley.
MATERIALS AND METHODS
Plant Growth
Accession information is located in Supplemental Table S1. Seeds were imbibed overnight in distilled water at 5°C prior to sowing. Individual plants were grown in MetroMix 360 (Sungrow) in plastic pots and fertilized weekly with Peters Excel 15-5-15 Cal-Mag and Peters 10-30-20 Blossom Booster (RJ Peters). Growth chamber temperatures averaged 22°C during the light period and 18°C during the dark period. Plants were grown under T5 fluorescent bulbs (5,000 K, Lithonia), and light intensities averaged around approximately 150 μmol m–2 s–1 at plant level. To minimize light intensity differences within each chamber, plants were rotated several times per week. For experiments in Figures 2 and 3, plants were grown under T8 fluorescent bulbs, and light intensities averaged around 150 μmol s–2 at plant level. Developmental stage was recorded using the Zadoks scale (Zadoks et al., 1974). Nonvernalized control plants were synchronized to the developmental stage plants had reached when they were removed from vernalization. In Figure 3, plants were at the same developmental stage when entering the vernalization treatment. Additionally, for the rest of the experiments all plants entered the cold at the same developmental stage.
Plant Measurements
Flowering time of vernalized plants was measured as the number of days from the end of vernalization (or emergence of the coleoptile in nonvernalized plants) to the first day upon which emergence of the spike was detected (Zadoks scale = 50; Zadoks et al., 1974). The number of primary leaves derived from the main shoot was recorded at the time of heading to control for development. For plants that flowered, height was measured from the base of the plant to the tallest point of the plant. For nonflowering plants, height was measured from the soil level to the highest point of the tallest leaf.
RT-qPCR assays
RNA was isolated from leaf tissue or whole shoot tissue using Plant Concert RNA Reagent (Life Technologies) following the manufacturer’s recommendations. RNA samples were treated with Turbo DNase (Life Technologies) according to the manufacturer’s protocol prior to first-strand cDNA synthesis. RNA integrity was assessed by a combination of A260/A280 and A260/A230 measurements and running samples on a denaturing formaldehyde-agarose gel as recommended in the Qiagen RNAeasy Mini Handbook. Only those samples with 260/280 and 260/230 ratios greater than 1.7 and showing no degradation by gel electrophoresis were used. For each sample, no real-time (RT) controls were performed. cDNA was made using 1 μg of total RNA per sample and M-MLV Reverse Transcriptase (Promega) and poly-T primers (IDT). For quantitative PCR (qPCR), 3 μL cDNA (diluted 5-fold following first-strand synthesis) was amplified using Takara ExTaq II in a 20-μL reaction containing 6 μL water, 10 μL SybrGreen Takara ExTaqII Master Mix, and 1 μL 10 μm forward and reverse primers. qPCR reactions were amplified on an ABI 7500 Fast system with the following parameters: 95°C for 30 s and 40 cycles of 95°C for 5 s, 60°C for 5 s, and 72°C for 34 s, followed by a melting curve program. Fifty-five degrees Celsius was used for annealing in all samples for Supplemental Figure S12. Fluorescence data were collected during the 72°C extension steps and during the melting curve program. Gene expression was quantified using the ΔΔCt method (Livak and Schmittgen, 2001), and all primer efficiencies ranged from 95% to 110%. Expression data for each gene is shown normalized to UBIQUITIN-CONJUGATING ENZYME18 (Brachypodium distachyon; Hong et al., 2008; Schwartz et al., 2010), Actin (barley [Hordeum vulgare]; Trevaskis et al., 2006), or TRANSLATION ELONGATION FACTOR1 (wheat [Triticum aestivum]; Distelfeld and Dubcovsky, 2010). Primer pairs used to amplify each gene are listed in Supplemental Table S2.
Generation of UBI:VRN1 and UBI:FT Transgenics
VRN1 and FT cDNAs were amplified from Bd21-3 cDNA pooled from vernalized and nonvernalized leaf tissue. cDNAs were gel extracted (Qiagen) and were cloned into pENTR-D-TOPO (Life Technologies) using the manufacturer’s protocol. Clones were verified by sequencing. pENTR-cDNAs were recombined into pANIC10A (Mann et al., 2012) using Life Technologies LR Clonase II following the manufacturer’s protocol. Clones were verified by sequencing in pANIC10A and then transformed into chemically competent Agrobacterium tumefaciens strain Agl-1. Plant callus transformation was performed as described (Vogel and Hill, 2008). Independent transgenic lines were genotyped for the transgene using a cDNA-specific forward and pANIC vector AcV5 tag reverse primer (Supplemental Table S1). Primer pairs used to clone each cDNA are listed in Supplemental Table S1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Vernalization timecourses of Brachypodium spp. accessions recorded as days to flowering and leaf count on the main shoot grown in 20-h and 16-h photoperiods.
Supplemental Figure S2. Correlation of days to flowering and final leaf number.
Supplemental Figure S3. Photographs of Brachypodium spp. accessions after 60 d of outgrowth post-vernalization.
Supplemental Figure S4. Vernalization timecourse of Brachypodium spp. accessions grown in an 8-h photoperiod.
Supplemental Figure S5. Photographs of Brachypodium spp. accessions grown in an 8-h photoperiod after 120 d of outgrowth.
Supplemental Figure S6. Height of Brachypodium spp. accessions in different photoperiods following cold treatment.
Supplemental Figure S7. Effect of different photoperiods during vernalization on flowering time.
Supplemental Figure S8. Effect of different photoperiods after vernalization on flowering time.
Supplemental Figure S9. Effect of different temperatures during vernalization on flowering time.
Supplemental Figure S10. Primary leaf number on the main shoot at flowering of UBI:VRN1 and UBI:FT.
Supplemental Figure S11. Vernalization timecourse for Bd21-3 and Bd1-1.
Supplemental Figure S12. VRN1 and VRN2L gene expression in leaves of wheat, barley, and Brachypodium spp. during cold.
Supplemental Figure S13. VRN1 and FT gene expression in leaves of vernalized plants during and after cold treatment.
Supplemental Figure S14. VRN1 expression in response to varying lengths of cold.
Supplemental Table S1. Origin of Brachypodium spp. accessions used in this study and assignment to flowering class.
Supplemental Table S2. Primer Sequences.
Acknowledgments
We thank members of the Amasino and Donna Fernandez labs for useful discussions and the Gordon and Betty Moore Foundation and the Life Sciences Research Foundation for their postdoctoral fellowship support.
Glossary
- DTF
d to flower
- cDNA
complementary DNA
- RT
real-time
- qPCR
quantitative PCR
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 [DOI] [PubMed] [Google Scholar]
- Ahrens JL, Loomis WE. (1963) Floral induction and development in winter wheat. Crop Sci 3: 463–466 [Google Scholar]
- Alonso-Peral MM, Oliver SN, Casao MC, Greenup AA, Trevaskis B. (2011) The promoter of the cereal VERNALIZATION1 gene is sufficient for transcriptional induction by prolonged cold. PLoS ONE 6: e29456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasino R. (2004) Vernalization, competence, and the epigenetic memory of winter. Plant Cell 16: 2553–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasino R. (2010) Seasonal and developmental timing of flowering. Plant J 61: 1001–1013 [DOI] [PubMed] [Google Scholar]
- Amasino RM, Michaels SD. (2010) The timing of flowering. Plant Physiol 154: 516–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales J, Turner A, Griffiths S, Snape JW, Laurie DA. (2007) A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor Appl Genet 115: 721–733 [DOI] [PubMed] [Google Scholar]
- Brenchley R, Spannagl M, Pfeifer M, Barker GL, D’Amore R, Allen AM, McKenzie N, Kramer M, Kerhornou A, Bolser D, et al. (2012) Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 491: 705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brkljacic J, Grotewold E, Scholl R, Mockler T, Garvin DF, Vain P, Brutnell T, Sibout R, Bevan M, Budak H, et al. (2011) Brachypodium as a model for the grasses: today and the future. Plant Physiol 157: 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Dubcovsky J. (2012) Wheat TILLING mutants show that the vernalization gene VRN1 down-regulates the flowering repressor VRN2 in leaves but is not essential for flowering. PLoS Genet 8: e1003134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kim J, Hwang HJ, Kim S, Park C, Kim SY, Lee I. (2011) The FRIGIDA complex activates transcription of FLC, a strong flowering repressor in Arabidopsis, by recruiting chromatin modification factors. Plant Cell 23: 289–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chujo H. (1966) Difference in vernalization effect in wheat under various temperatures. Journal of the Crop Science Society of Japan 35: 177–186 [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al. (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- De Lucia F, Crevillen P, Jones AM, Greb T, Dean C. (2008) A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci USA 105: 16831–16836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ES, Peacock WJ. (2009) Vernalization in cereals. J Biol 8: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelfeld A, Dubcovsky J. (2010) Characterization of the maintained vegetative phase deletions from diploid wheat and their effect on VRN2 and FT transcript levels. Mol Genet Genomics 283: 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelfeld A, Li C, Dubcovsky J. (2009a) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12: 178–184 [DOI] [PubMed] [Google Scholar]
- Distelfeld A, Tranquilli G, Li C, Yan L, Dubcovsky J. (2009b) Genetic and molecular characterization of the VRN2 loci in tetraploid wheat. Plant Physiol 149: 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper J, Mur LA, Jenkins G, Ghosh-Biswas GC, Bablak P, Hasterok R, Routledge AP. (2001) Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiol 127: 1539–1555 [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky J, Chen C, Yan L. (2005) Molecular characterization of the allelic variation at the VRN-H2 vernalization locus in barley. Mol Breed 15: 395–407 [Google Scholar]
- Dubcovsky J, Loukoianov A, Fu D, Valarik M, Sanchez A, Yan L. (2006) Effect of photoperiod on the regulation of wheat vernalization genes VRN1 and VRN2. Plant Mol Biol 60: 469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LT. (1987) Short day induction of inflorescence initiation in some winter-wheat varieties. Aust J Plant Physiol 14: 277–286 [Google Scholar]
- Filiz E, Ozdemir BS, Budak F, Vogel JP, Tuna M, Budak H. (2009) Molecular, morphological, and cytological analysis of diverse Brachypodium distachyon inbred lines. Genome 52: 876–890 [DOI] [PubMed] [Google Scholar]
- Fu D, Szucs P, Yan L, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM, Dubcovsky J. (2005) Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol Genet Genomics 273: 54–65 [DOI] [PubMed] [Google Scholar]
- Greenup A, Peacock WJ, Dennis ES, Trevaskis B. (2009) The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Ann Bot (Lond) 103: 1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenup AG, Sasani S, Oliver SN, Talbot MJ, Dennis ES, Hemming MN, Trevaskis B. (2010) ODDSOC2 is a MADS box floral repressor that is down-regulated by vernalization in temperate cereals. Plant Physiol 153: 1062–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenup AG, Sasani S, Oliver SN, Walford SA, Millar AA, Trevaskis B. (2011) Transcriptome analysis of the vernalization response in barley (Hordeum vulgare) seedlings. PLoS ONE 6: e17900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell CA, Wood CC, Robertson M, James Peacock W, Dennis ES. (2006) The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J 46: 183–192 [DOI] [PubMed] [Google Scholar]
- Hemming MN, Peacock WJ, Dennis ES, Trevaskis B. (2008) Low-temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiol 147: 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JB, Sung S. (2011) Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 331: 76–79 [DOI] [PubMed] [Google Scholar]
- Higgins JA, Bailey PC, Laurie DA. (2010) Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PLoS ONE 5: e10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SY, Seo PJ, Yang MS, Xiang F, Park CM. (2008) Exploring valid reference genes for gene expression studies in Brachypodium distachyon by real-time PCR. BMC Plant Biol 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung HY, Shannon LM, Tian F, Bradbury PJ, Chen C, Flint-Garcia SA, McMullen MD, Ware D, Buckler ES, Doebley JF, et al. (2012) ZmCCT and the genetic basis of day-length adaptation underlying the postdomestication spread of maize. Proc Natl Acad Sci USA 109: E1913–E1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo N, Garvin DF, You FM, McMahon S, Luo MC, Gu YQ, Lazo GR, Vogel JP. (2011) Comparison of a high-density genetic linkage map to genome features in the model grass Brachypodium distachyon. Theor Appl Genet 123: 455–464 [DOI] [PubMed] [Google Scholar]
- International Brachypodium Initiative (2010) Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 463: 763–768 [DOI] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA. (2007) FT protein acts as a long-range signal in Arabidopsis. Curr Biol 17: 1050–1054 [DOI] [PubMed] [Google Scholar]
- Johanson U, West J, Lister C, Michaels S, Amasino R, Dean C. (2000) Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290: 344–347 [DOI] [PubMed] [Google Scholar]
- Karsai I, Szucs P, Mészáros K, Filichkina T, Hayes PM, Skinner JS, Láng L, Bedo Z. (2005) The Vrn-H2 locus is a major determinant of flowering time in a facultative × winter growth habit barley (Hordeum vulgare L.) mapping population. Theor Appl Genet 110: 1458–1466 [DOI] [PubMed] [Google Scholar]
- Kim DH, Doyle MR, Sung S, Amasino RM. (2009) Vernalization: winter and the timing of flowering in plants. Annu Rev Cell Dev Biol 25: 277–299 [DOI] [PubMed] [Google Scholar]
- Levy YY, Mesnage S, Mylne JS, Gendall AR, Dean C. (2002) Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science 297: 243–246 [DOI] [PubMed] [Google Scholar]
- Li C, Dubcovsky J. (2008) Wheat FT protein regulates VRN1 transcription through interactions with FDL2. Plant J 55: 543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Rudi H, Stockinger EJ, Cheng H, Cao M, Fox SE, Mockler TC, Westereng B, Fjellheim S, Rognli OA, et al. (2012) Comparative analyses reveal potential uses of Brachypodium distachyon as a model for cold stress responses in temperate grasses. BMC Plant Biol 12: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljegren SJ, Gustafson-Brown C, Pinyopich A, Ditta GS, Yanofsky MF. (1999) Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 11: 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Loukoianov A, Yan L, Blechl A, Sanchez A, Dubcovsky J. (2005) Regulation of VRN-1 vernalization genes in normal and transgenic polyploid wheat. Plant Physiol 138: 2364–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DG, Lafayette PR, Abercrombie LL, King ZR, Mazarei M, Halter MC, Poovaiah CR, Baxter H, Shen H, Dixon RA, et al. (2012) Gateway-compatible vectors for high-throughput gene functional analysis in switchgrass (Panicum virgatum L.) and other monocot species. Plant Biotechnol J 10: 226–236 [DOI] [PubMed] [Google Scholar]
- Mathieu J, Warthmann N, Küttner F, Schmid M. (2007) Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol 17: 1055–1060 [DOI] [PubMed] [Google Scholar]
- Mayer KF, Waugh R, Brown JW, Schulman A, Langridge P, Platzer M, Fincher GB, Muehlbauer GJ, Sato K, Close TJ, et al. (2012) A physical, genetic and functional sequence assembly of the barley genome. Nature 491: 711–716 [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11: 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LA, Allainguillaume J, Catalán P, Hasterok R, Jenkins G, Lesniewska K, Thomas I, Vogel J. (2011) Exploiting the Brachypodium tool box in cereal and grass research. New Phytol 191: 334–347 [DOI] [PubMed] [Google Scholar]
- Mylne JS, Barrett L, Tessadori F, Mesnage S, Johnson L, Bernatavichute YV, Jacobsen SE, Fransz P, Dean C. (2006) LHP1, the Arabidopsis homologue of HETEROCHROMATIN PROTEIN1, is required for epigenetic silencing of FLC. Proc Natl Acad Sci USA 103: 5012–5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver SN, Finnegan EJ, Dennis ES, Peacock WJ, Trevaskis B. (2009) Vernalization-induced flowering in cereals is associated with changes in histone methylation at the VERNALIZATION1 gene. Proc Natl Acad Sci USA 106: 8386–8391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opanowicz M, Vain P, Draper J, Parker D, Doonan JH. (2008) Brachypodium distachyon: making hay with a wild grass. Trends Plant Sci 13: 172–177 [DOI] [PubMed] [Google Scholar]
- Pin PA, Benlloch R, Bonnet D, Wremerth-Weich E, Kraft T, Gielen JJ, Nilsson O. (2010) An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science 330: 1397–1400 [DOI] [PubMed] [Google Scholar]
- Pin PA, Zhang W, Vogt SH, Dally N, Büttner B, Schulze-Buxloh G, Jelly NS, Chia TY, Mutasa-Göttgens ES, Dohm JC, et al. (2012) The role of a pseudo-response regulator gene in life cycle adaptation and domestication of beet. Curr Biol 22: 1095–1101 [DOI] [PubMed] [Google Scholar]
- Preston JC, Kellogg EA. (2008) Discrete developmental roles for temperate cereal grass VERNALIZATION1/FRUITFULL-like genes in flowering competency and the transition to flowering. Plant Physiol 146: 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson HM, Zajac M, Penrose LDJ. (1998) Effect of seedling temperature and its duration on development of wheat cultivars differing in vernalization response. Field Crops Res 57: 289–300 [Google Scholar]
- Ream TS, Woods DP, Amasino RM. (2012) The molecular basis of vernalization in different plant groups. Cold Spring Harb Symp Quant Biol 77: 105–115 [DOI] [PubMed] [Google Scholar]
- Ruelens P, de Maagd RA, Proost S, Theißen G, Geuten K, Kaufmann K. (2013) FLOWERING LOCUS C in monocots and the tandem origin of angiosperm-specific MADS-box genes. Nat Commun 4: 2280. [DOI] [PubMed] [Google Scholar]
- Sasani S, Hemming MN, Oliver SN, Greenup A, Tavakkol-Afshari R, Mahfoozi S, Poustini K, Sharifi HR, Dennis ES, Peacock WJ, et al. (2009) The influence of vernalization and daylength on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare). J Exp Bot 60: 2169–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CJ, Doyle MR, Manzaneda AJ, Rey PJ, Mitchell-Olds T, Amasino RM. (2010) Natural variation of flowering time and vernalization responsiveness in Brachypodium distachyon. Bioenergy Research 3: 38–46 [Google Scholar]
- Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, Amasino RA, Coupland G. (2006) The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev 20: 898–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES. (1999) The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11: 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Ogawa T, Kitagawa S, Suzuki T, Ikari C, Shitsukawa N, Abe T, Kawahigashi H, Kikuchi R, Handa H, et al. (2009) A genetic network of flowering-time genes in wheat leaves, in which an APETALA1/FRUITFULL-like gene, VRN1, is upstream of FLOWERING LOCUS T. Plant J 58: 668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo C, Aranzana MJ, Lister C, Baxter C, Nicholls C, Nordborg M, Dean C. (2005) Role of FRIGIDA and FLOWERING LOCUS C in determining variation in flowering time of Arabidopsis. Plant Physiol 138: 1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Amasino RM. (2004) Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427: 159–164 [DOI] [PubMed] [Google Scholar]
- Sung S, He Y, Eshoo TW, Tamada Y, Johnson L, Nakahigashi K, Goto K, Jacobsen SE, Amasino RM. (2006) Epigenetic maintenance of the vernalized state in Arabidopsis thaliana requires LIKE HETEROCHROMATIN PROTEIN 1. Nat Genet 38: 706–710 [DOI] [PubMed] [Google Scholar]
- Swiezewski S, Liu F, Magusin A, Dean C. (2009) Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature 462: 799–802 [DOI] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. (2007) Hd3a protein is a mobile flowering signal in rice. Science 316: 1033–1036 [DOI] [PubMed] [Google Scholar]
- Thomashow MF. (2010) Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol 154: 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES. (2003) MADS box genes control vernalization-induced flowering in cereals. Proc Natl Acad Sci USA 100: 13099–13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Hemming MN, Peacock WJ, Dennis ES. (2006) HvVRN2 responds to daylength, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiol 140: 1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trione EJ, Metzger RJ. (1970) Wheat and barley vernalization in a precise temperature gradient. Crop Sci 10: 390–392 [Google Scholar]
- Turner AS, Faure S, Zhang Y, Laurie DA. (2013) The effect of day-neutral mutations in barley and wheat on the interaction between photoperiod and vernalization. Theor Appl Genet 126: 2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J, Hill T. (2008) High-efficiency Agrobacterium-mediated transformation of Brachypodium distachyon inbred line Bd21-3. Plant Cell Rep 27: 471–478 [DOI] [PubMed] [Google Scholar]
- Vogel JP, Garvin DF, Leong OM, Hayden DM. (2006) Agrobacterium-mediated transformation and inbred line development in the model grass Brachypodium distachyon. Plant Cell Tissue Organ Cult 84: 199–211 [Google Scholar]
- Vogel JP, Tuna M, Budak H, Huo N, Gu YQ, Steinwand MA. (2009) Development of SSR markers and analysis of diversity in Turkish populations of Brachypodium distachyon. BMC Plant Biol 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zitzewitz J, Szucs P, Dubcovsky J, Yan L, Francia E, Pecchioni N, Casas A, Chen TH, Hayes PM, Skinner JS. (2005) Molecular and structural characterization of barley vernalization genes. Plant Mol Biol 59: 449–467 [DOI] [PubMed] [Google Scholar]
- Wollenberg AC, Amasino RM. (2012) Natural variation in the temperature range permissive for vernalization in accessions of Arabidopsis thaliana. Plant Cell Environ 35: 2181–2191 [DOI] [PubMed] [Google Scholar]
- Wood CC, Robertson M, Tanner G, Peacock WJ, Dennis ES, Helliwell CA. (2006) The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc Natl Acad Sci USA 103: 14631–14636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, et al. (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40: 761–767 [DOI] [PubMed] [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. (2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci USA 103: 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Helguera M, Kato K, Fukuyama S, Sherman J, Dubcovsky J. (2004a) Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor Appl Genet 109: 1677–1686 [DOI] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J. (2004b) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303: 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100: 6263–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. (1974) Decimal code for growth stages of cereals. Weed Res 14: 415–421 [Google Scholar]