Modulating the synthesis of sterols affects the ability of a fungal protein to induce plant innate immunity.
Abstract
Plant-microbe interactions involve numerous regulatory systems essential for plant defense against pathogens. An ethylene-inducing xylanase (Eix) of Trichoderma viride is a potent elicitor of plant defense responses in specific cultivars of tobacco (Nicotiana tabacum) and tomato (Solanum lycopersicum). We demonstrate that tomato cyclopropyl isomerase (SlCPI), an enzyme involved in sterol biosynthesis, interacts with the LeEix2 receptor. Moreover, we examined the role of SlCPI in signaling during the LeEix/Eix defense response. We found that SlCPI is an important factor in the regulation of the induction of defense responses such as the hypersensitive response, ethylene biosynthesis, and the induction of pathogenesis-related protein expression in the case of LeEix/Eix. Our results also suggest that changes in the sterol composition reduce LeEix internalization, thereby attenuating the induction of plant defense responses.
Plant innate immunity is activated upon the recognition of pathogen- and microbe-associated molecular patterns by surface-localized immune receptors or the stimulation of cytoplasmic immune receptors by pathogen effector proteins (Jones and Dangl, 2006; Thomma et al., 2011). Leucine-rich repeat (LRR) receptor kinases and leucine-rich repeat receptor proteins (LRR-RLPs) respond to conserved microbe-associated molecular patterns by producing a defense response upon detection (Altenbach and Robatzek, 2007; Bittel and Robatzek, 2007; Robatzek et al., 2007; Geldner and Robatzek, 2008). One such LRR-RLP is the ethylene-inducing xylanase (Eix) receptor LeEix2. The fungal protein Eix (Dean et al., 1989) is a well-known protein elicitor of defense response reactions in tobacco (Nicotiana tabacum) and tomato (Solanum lycopersicum; Bailey et al., 1990; Avni et al., 1994). Eix induces ethylene biosynthesis, extensive electrolyte leakage, pathogenesis-related (PR) gene expression, reactive oxygen species (ROS), and the hypersensitive response (HR; Bailey et al., 1990; Ron et al., 2000). Eix was shown to specifically bind to the plasma membrane of responsive cultivars of both tomato and tobacco (Hanania and Avni, 1997). The response to Eix in tobacco and tomato cultivars is controlled by an LRR-RLP encoded by a single locus, termed LeEix (Ron and Avni, 2004). Previously, we showed that Eix triggers internalization of the LeEix2 receptor and its localization to endosomes (Bar and Avni, 2009).
Endocytic processes and vesicular transport in general require the participation of membrane components that form transport vesicles with a capability to store and process a number of molecules known to participate in cell signaling (Anderson, 1993; Patel et al., 2008; Hansen and Nichols, 2010). Sterols are lipophilic membrane components that have many important functions in an array of eukaryotes. Changes in membrane-bound sterol levels and composition can have effects on the activity of membrane proteins and on signal transduction processes. The interaction between sterols and phospholipids forms microdomains termed lipid rafts (Simons and Ikonen, 1997). In response to cellular stimuli, lipid rafts can change the protein microenvironment, leading to the initiation of signaling cascades (Simons and Toomre, 2000; Mongrand et al., 2010; Simon-Plas et al., 2011). Sterols also provide precursors for the biosynthesis of steroid hormones such as mammalian estrogens and glucocorticoids and plant brassinosteroids (Bishop and Yokota, 2001; Benveniste, 2004; Suzuki and Muranaka, 2007). One of the enzymes involved in sterol biosynthesis is cyclopropyl isomerase (CPI; Lovato et al., 2000; Benveniste, 2004).
A variety of endocytic pathways have been described in mammalian and fungal cells that differ mainly in the size, shape, and composition of endocytic vesicles and in the participation of different proteins (Conner and Schmid, 2003; Soldati and Schliwa, 2006). Cholesterol, the main mammalian sterol, has an important role in most internalization steps through both caveolae and clathrin-coated pits (Murata et al., 1995; Subtil et al., 1999). Cholesterol depletion alters endocytic structures and reduces the polar delivery of target proteins (Keller and Simons, 1998; Pichler and Riezman, 2004). Plant sterols are reported to be internalized into endosomes and distributed throughout the endocytic pathway in an actin-dependent manner (Grebe et al., 2003). The sterol endocytic pathway has been shown to interrupt the internalization, trafficking, and polar recycling of PIN2, an auxin efflux facilitator and polarity marker, in developing root epidermal cells of Arabidopsis (Arabidopsis thaliana; Grebe et al., 2003; Men et al., 2008). Sterols were shown to function in the trafficking of an ATP-binding cassette (ABCB19) from the trans-Golgi to the plasma membrane (Yang et al., 2013).
Here, we report the isolation of SlCPI, which interacts with the LeEix2 receptor. Modulating the expression or function of SlCPI affects the induction of plant defense responses mediated by Eix.
RESULTS
Two-Hybrid Screen for LeEix-Interacting Proteins
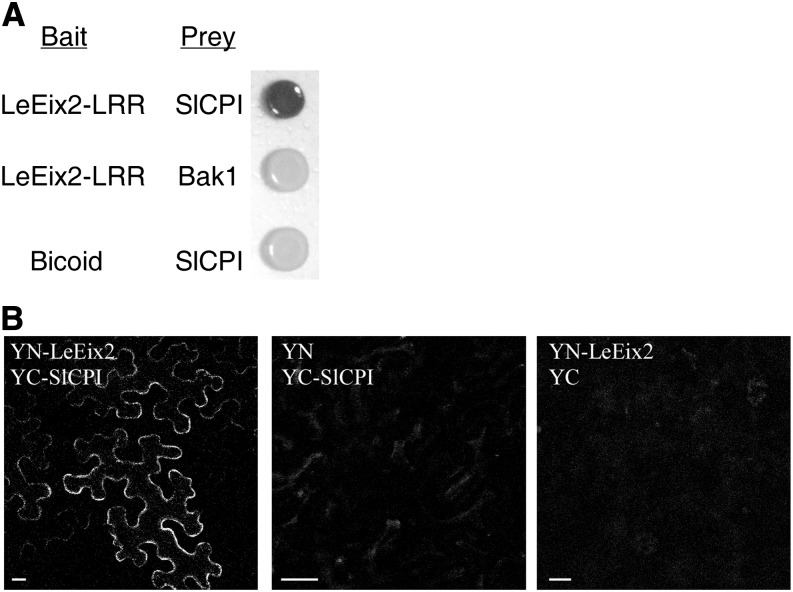
To study the signal transduction pathway leading to the induction of innate immunity mediated by Eix, we searched for proteins interacting with the LeEix2 receptor (Ron and Avni, 2004). The complementary DNA (cDNA) encoding the LRR part of the LeEix2 receptor (amino acids 110–913) was fused in frame to the C terminus of the DNA-binding domain of LexA in the “bait” plasmid pEG202 and transformed into the yeast (Saccharomyces cerevisiae) strain EGY48 (Gyuris et al., 1993). We screened approximately 1 × 106 independent clones of the library. Five positive clones (which appeared blue on Gal medium containing 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) and grew on selective medium) were isolated and sequenced. One cDNA that was identified as SlCPI showed 72% sequence identity and 89% similarity to AtCPI1 of Arabidopsis, which is involved in sterol biosynthesis.
The tomato CPI gene was identified by BLAST analysis, using the tomato genome sequence and the tomato EST database. We cloned the full-length SlCPI cDNA to examine direct interaction with the LRR domain of the LeEix2 receptor. Yeast strains carrying the LeEix2 bait and the full-length SlCPI prey grew in the absence of Leu, indicating LEU2 reporter gene activation. When grown on X-gal plates, these yeast cells were blue as a result of LacZ reporter gene activation (Fig. 1A). In contrast, a control yeast strain expressing the arbitrary bait, Bicoid (LexA fused to a transcriptionally inert fragment of the Drosophila melanogaster Bicoid product), and the SlCPI prey did not activate the LEU2 or LacZ reporter gene (Fig. 1A). Expression was dependent upon growth on Gal medium, indicating that the expression of SlCPI was required for expression of the reporter genes. Thus, SlCPI physically interacts with LeEix2 protein in the yeast two-hybrid system.
Figure 1.
Identification of LeEix-interacting proteins. A, EGY48 yeast cells containing LeEix2-LRR (in pEG202; bait) and CPI (in pJG4-5; prey), or LeEix2-LRR (in pEG202) and Bak1 (Bar et al., 2010; in pJG4-5), or Bicoid (in pJG4-5) and CPI (in pJG4-5) were grown on Gal medium lacking the relevant amino acids and supplemented with X-gal. B, N. benthamiana leaves transiently expressing YN-LeEix2 and YC-YC-SlCPI as indicated. Leaf sections were visualized 48 h after transformation using a laser scanning confocal microscope (Zeiss). Bars = 20 μm.
The bimolecular fluorescence complementation (BiFC) system (Bracha-Drori et al., 2004) was used to verify the interaction between LeEix2 and SlCPI in planta. We tested the interaction of SlCPI with LeEix2 (Ron and Avni, 2004) in the presence of Eix. SlCPI interacted with LeEix2 in the cell membrane (Fig. 1B). Four different experiments were analyzed, and at least 50 positive cells were visualized in each experiment. LeEix2 and SlCPI were examined for fluorescence with the complementary half of the yellow fluorescent protein, and the results were negative (Fig. 1B). Thus, BiFC assay results confirmed the yeast two-hybrid data demonstrating that LeEix2 interacts with SlCPI.
SlCPI Stimulates LeEix2 Signaling and Endocytosis
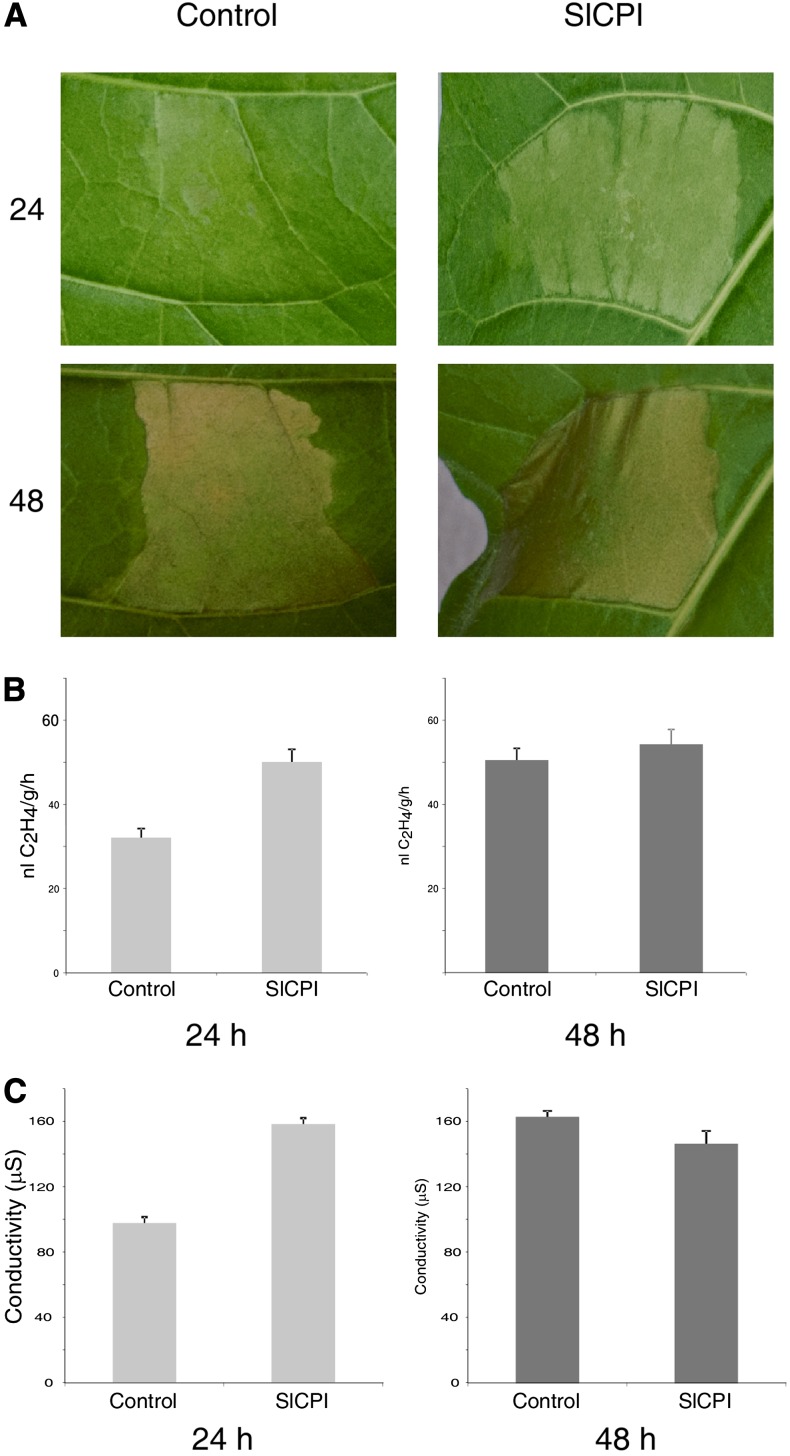
To gain insight into the role of SlCPI in the regulation of Eix-induced defense responses, we overexpressed SlCPI in tobacco plants. Leaves transiently expressing a mixture of Pro35S:tvEix and Pro35S:SlCPI or Pro35S:GFP (control) were tested for their effect on the induction of the HR, ethylene biosynthesis, electrolyte leakage, and the expression of the PR1-b gene by Eix. Induction of these responses by Eix was monitored 24 to 48 h after injection (Fig. 2). Leaves transiently expressing a mixture of Pro35S:SlCPI and Pro35S:tvEix developed the HR 24 h after injection, while leaves transiently expressing a mixture of Pro-35S:GFP and Pro-35S:tvEix developed the HR 48 h after injection (Fig. 2A). Tobacco leaves transiently transformed with Pro35S:GFP (control) or Pro35S:SlCPI were sampled 24 and 48 h after transformation. Leaf discs were floated on a 250 mm sorbitol solution supplemented with 2.5 μg mL−1 Eix. Ethylene biosynthesis was measured after 4 h (Fig. 2B). Additional leaf discs were floated on water, and electrolyte leakage was measured after 24 and 48 h (Fig. 2C) or cDNA was prepared 4 h after Eix application followed by semiquantitative reverse transcription-PCR using specific primers to the PR1-b gene (Supplemental Fig. S1). Overexpression of SlCPI for 24 h enhances HR development, ethylene synthesis, ion leakage, and PR1-b expression (Fig. 2; Supplemental Fig. S1). Interestingly, this enhancement diminished after 48 h (Fig. 2).
Figure 2.
SICPI overexpression stimulates LeEix2-induced defense responses. A, Tobacco transiently transformed with Pro35S:tvEix and either Pro35S:SlCPI or Pro35S:GFP. Induction of HR was monitored 24 and 48 h after transformation. B, Leaf discs of transiently transformed tobacco leaves with either Pro35S:GFP (control) or Pro35S:SlCPI were floated 24 or 48 h after transformation on a 250 mm sorbitol solution supplemented with 2.5 μg mL−1 Eix. Ethylene biosynthesis was measured after 4 h. Values represent averages ± se of four different experiments. C, Electrolyte leakage was measured 24 and 48 h after transformation. Values represent average conductivity ± se of three different experiments.
We next wished to examine whether CPI has a similar effect on the induction of defense responses in additional pattern recognition receptors (Jones et al., 1994). The LRR-RLPs LeEix2 and Cf9 are involved in the recognition of fungal effectors and possess structural similarities, such as extracellular LRRs and a short cytoplasmic domain (Ron and Avni, 2004; van der Hoorn et al., 2005). Pto was chosen as a control, since it is a cytoplasmic protein kinase R gene (Martin et al., 1993) and its signaling is probably not affected by endocytosis. Leaves transiently expressing a mixture of Pro35S:SlCPI, Pro35S:Avr9, and Pro35S:Cf9 developed HR 24 h after injection (Supplemental Fig. S2A), while control leaves developed HR after 48 h. HR development was not influenced by the expression of CPI in leaves transiently expressing a mixture of Pro35S:AvrPto and Pro35S:AvrPto (Supplemental Fig. S2B).
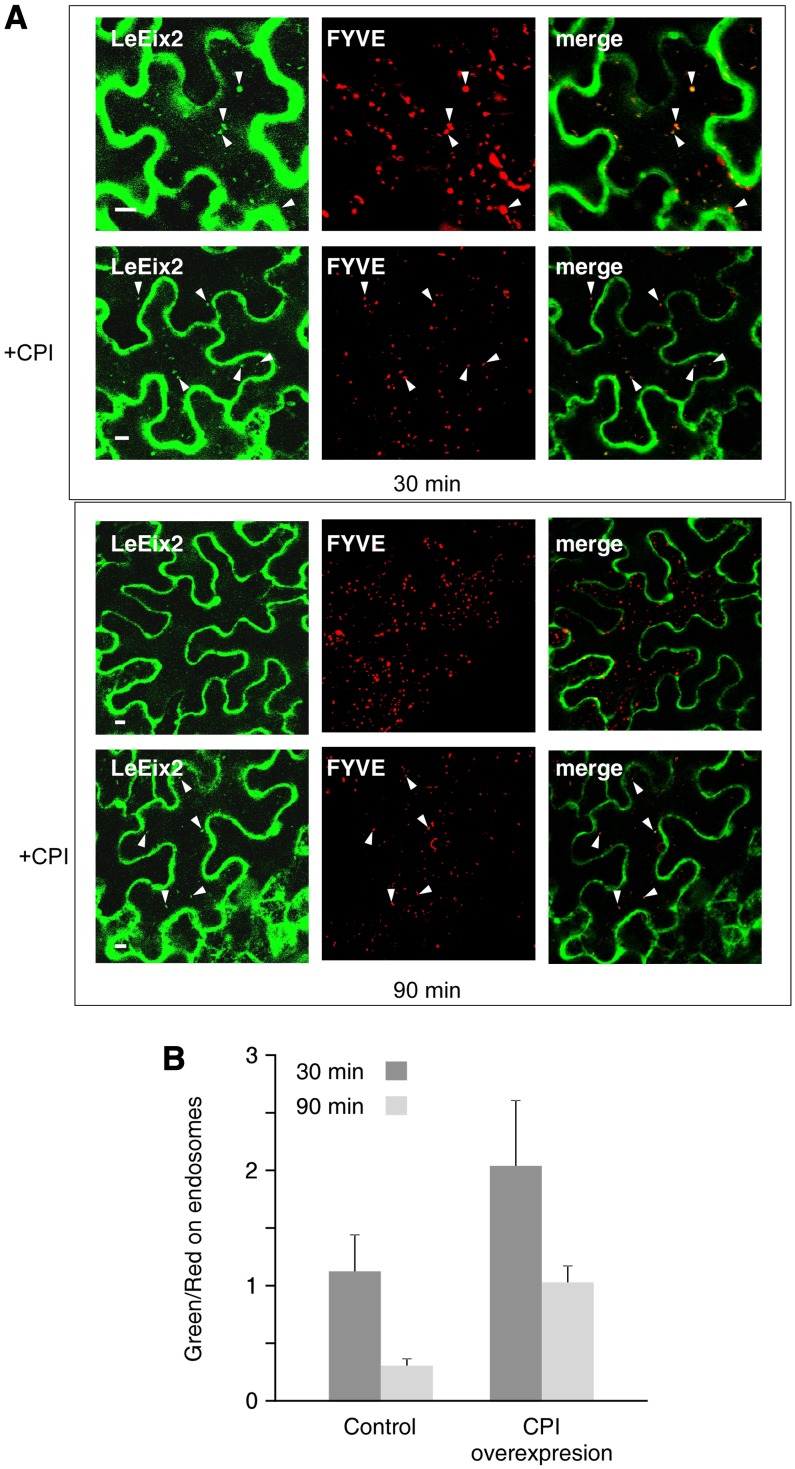
Previously, we showed that application of Eix triggers the endocytosis of the LeEix2 receptor within 30 min. GFP-tagged LeEix2 was found to colocalize with the FYVE-DsRed marker (Voigt et al., 2005) on endosomes (Bar and Avni, 2009). The peak of LeEix2 endocytosis occurs 15 to 30 min after Eix application, and about 60 min later the amount of GFP-tagged LeEix2 on endosomes declines (Bar and Avni, 2009). To determine whether SlCPI has an effect on the endocytosis of LeEix2, the colocalization of GFP-LeEix2 with FYVE-DsRed-tagged endosomes in leaves overexpressing SlCPI after the application of Eix was monitored (Fig. 3). Overexpression of SlCPI leads to a greater presence of GFP-LeEix2 on the endosomes at a later time point (90 min). In order to verify this, the green-to-red ratio (i.e. the ratio of LeEix2-GFP endosomal labeling to FYVE endosomal labeling) was quantified with and without CPI overexpression. Quantification parameters are described in “Materials and Methods.” As can be seen in Figure 3B, overexpression of CPI leads to a greater presence of GFP-LeEix2 on endosomes for greater periods. We previously demonstrated that preventing the endocytosis of the receptor inhibits Eix-induced signaling, while interfering with vesicle trafficking enhanced Eix-induced signaling (Sharfman et al., 2011). The presence of the receptor for a longer period on endosome correlates with the enhanced induction of defense responses in leaves overexpressing SlCPI (Fig. 2).
Figure 3.
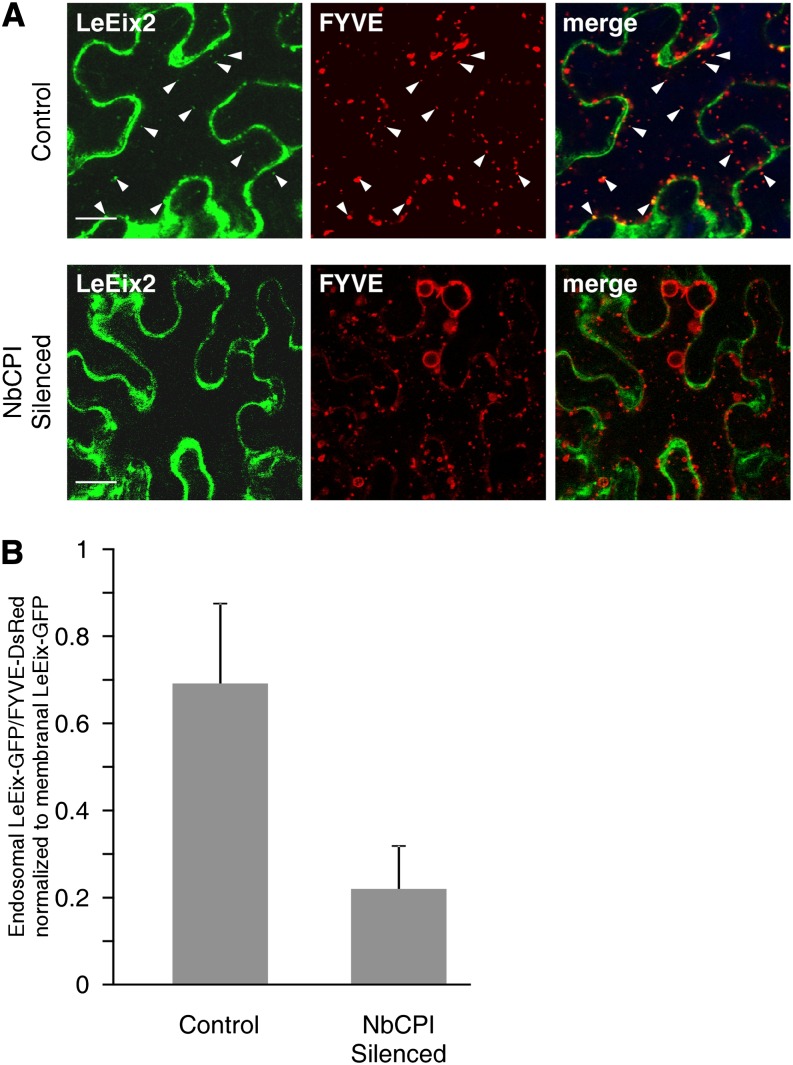
SICPI overexpression affects LeEix2 endocytosis. N. benthamiana was transiently transformed with a combination of Pro35S:GFP-LeEix2, Pro35S:SlCPI, and Pro35S:FYVE-DsRed (as indicated). A, Internalization of LeEix2-GFP on FYVE-DsRed-tagged endosomes was examined 30 min (top) or 90 min (bottom) after Eix application. Arrowheads indicate the colocalization of LeEix2- and FYVE-DsRed-tagged endosomes Bars = 20 μm. B, LeEix2-GFP and FYVE-DsRed fluorescence from at least five to 10 cells of each sample was quantified using ImageJ software. All images were captured using identical conditions.
Silencing NbCPI Expression Attenuates Eix Signaling
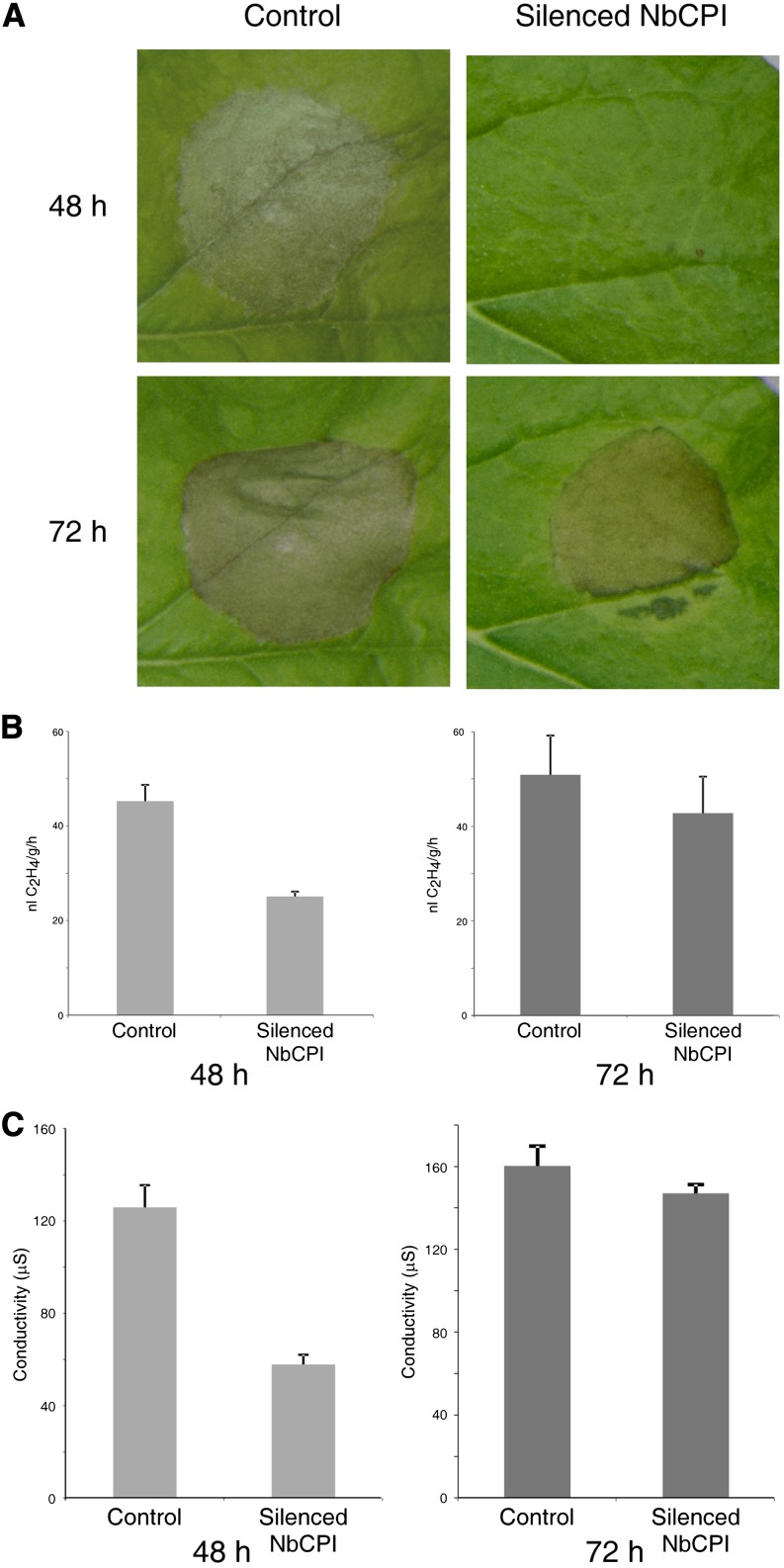
Virus-induced gene silencing (VIGS) was used in Nicotiana benthamiana plants (Liu et al., 2002a) as a complementary approach to show the role of CPI in the LeEix2/Eix system. An N. benthamiana fragment of 187 bp from NbCPI that showed 88% homology to SlCPI was used to silence NbCPI. The silenced plants exhibited a growth inhibition phenotype with small leaves, as observed in Arabidopsis cpi1-1 (Men et al., 2008), and a reduced level of NbCPI mRNA (Supplemental Fig. S3). Overexpression of LeEix2 and tvEix induce HR development 48 h after transformation, while NbCPI-silenced plants expressing LeEix2 and tvEix developed HR 72 h after transformation (Fig. 4A). Ethylene biosynthesis was measured 4 h after Eix application (Fig. 4B). Additional leaf discs were floated on water, and electrolyte leakage was measured after 48 and 72 h (Fig. 4C). Ethylene biosynthesis and ion leakage in NbCPI-silenced plants were reduced to about 44% and 54%, respectively, as compared with control plants. Overexpression of SlCPI enhanced the induction of defense responses (Fig. 2), while silencing CPI delayed the induction of defense responses (Fig. 4).
Figure 4.
HR monitoring, ethylene production, and ion leakage in NbCPI-silenced plants. A, NbCPI-silenced and control N. benthamiana plants were transformed with a mixture of Pro35S:LeEix2 and Pro-35S:tvEix. Induction of the HR was monitored 48 and 72 h after injection. B, Leaf discs from NbCPI-silenced and control plants, transiently transformed with either Pro35S:GFP or Pro35S:LeEix2 and Pro-35S:tvEix, were floated on a 250 mm sorbitol solution supplemented with 2.5 μg mL−1 Eix at 48 and 72 h after transformation. Ethylene biosynthesis was measured after 4 h. Values represent averages ± se of five different experiments. C, Electrolyte leakage was measured after 24 and 48 h. Values represent averages ± se of three different experiments.
We examined the effect of silencing the expression of NbCPI on the endocytosis of LeEix2 (Fig. 5). In N. benthamiana NbCPI-silenced plants, the colocalization of LeEix2-GFP with FYVE-DsRed-tagged endosomes after Eix application was inhibited (Fig. 5), suggesting that sterols are needed for the endocytosis of LeEix2. In order to verify this, the green-to-red ratio (i.e. the ratio of LeEix2-GFP endosomal labeling to FYVE-DsRed-tagged endosomal labeling) was quantified in N. benthamiana NbCPI-silenced plants (Fig. 5B). Quantification parameters are described in “Materials and Methods.” As can be seen in Figure 5B, silencing the expression of CPI leads to a decrease in the presence of GFP-LeEix2 on the endosomes.
Figure 5.
Silencing NbCPI attenuates LeEix2 internalization in response to Eix. N. benthamiana NbCPI-silenced and control plants were transiently transformed with a combination of Pro35S:LeEix2-GFP and Pro35S:FYVE-DsRed (as indicated). Eix (2.5 μg mL−1) was applied 24 after transformation. A, Internalization of LeEix2-GFP on FYVE-DsRed-tagged endosomes was examined 30 min after Eix application. Arrowheads indicate the colocalization of LeEix2-GFP and FYVE-DsRed on endosomes. Bars = 20 µm. B, The ratio of GFP-LeEix2 fluorescence to FYVE-DsRed fluorescence from at least five to 10 cells of each sample was quantified using ImageJ software. All images were captured using identical conditions.
Effect of Filipin on Eix Signaling
Previously, we showed that various inhibitors prevent the internalization of the LeEix2 receptor (Bar and Avni, 2009); this inhibition also prevents endosomal signaling in Eix-triggered defense responses (Sharfman et al., 2011). Here, we use filipin, a polyene antibiotic, known as a sterol-dependent endocytosis inhibitor. While filipin is a broad drug that can also cause membranal deformities, it is used for fluorescent and ultrastructural detection of sterols in animal cells (Nichols et al., 2001), yeast cells (Wachtler et al., 2003), and plant cells (Grebe et al., 2003; Liu et al., 2009; Boutté et al., 2011). In animals, filipin is a sterol-binding agent (Miller, 1984), and high filipin concentrations are used to deplete, redistribute, or remove plasma membrane cholesterol, resulting in endocytosis inhibition (Keller and Simons, 1998; Orlandi and Fishman, 1998; Nichols et al., 2001; Marella et al., 2002). In plants, filipin binds free sterols (Grebe et al., 2003) and is used as a sterol-dependent endocytosis inhibitor (Boutté and Grebe, 2009; Ovecka et al., 2010).
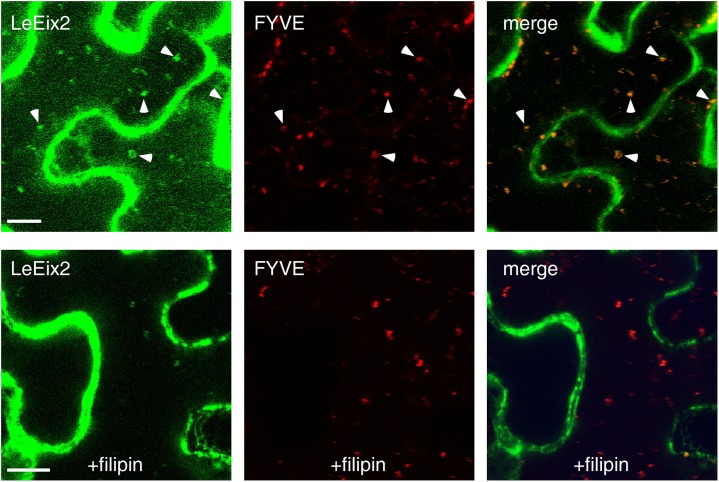
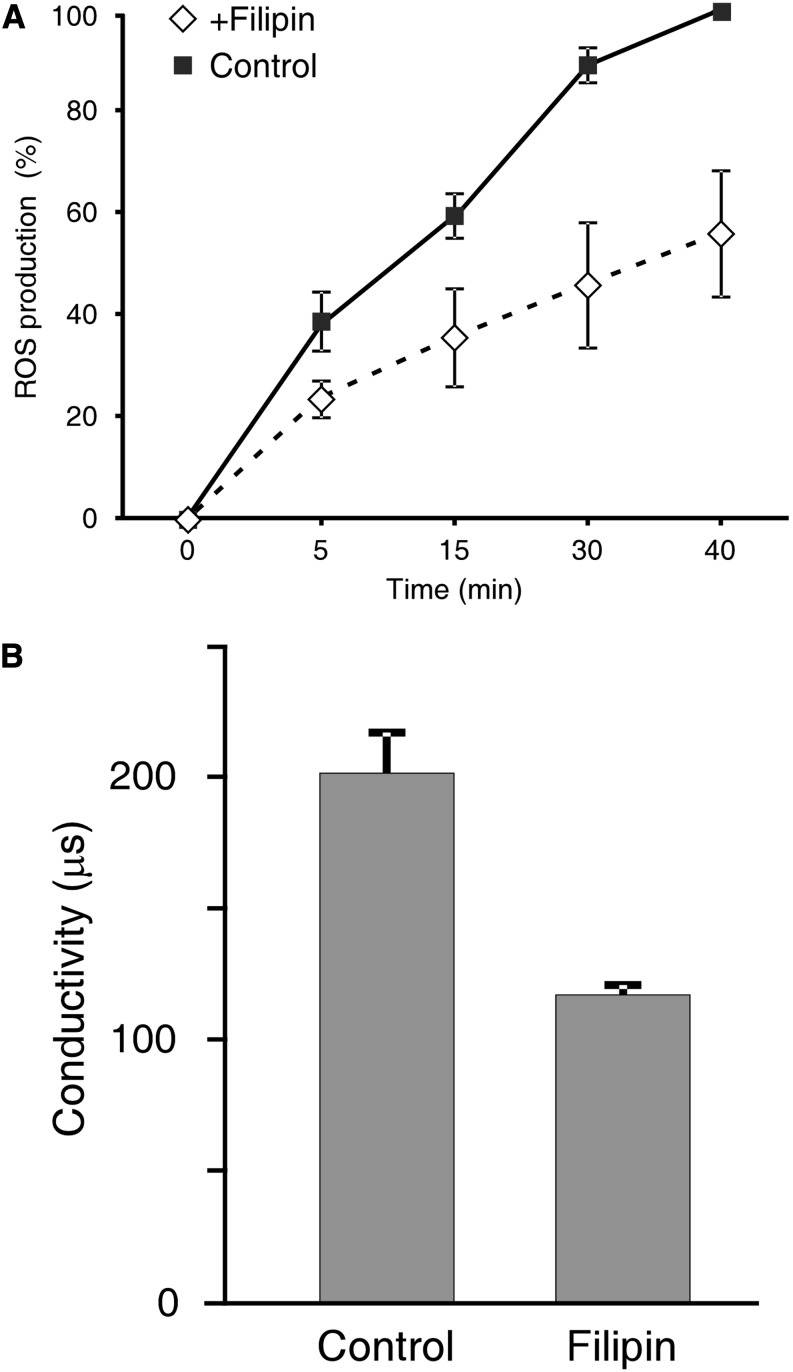
We compared the localization of LeEix2-GFP- and FYVE-DsRed-tagged endosomes after treatment with Eix alone or treatment with filipin and Eix (Fig. 6). Application of filipin (20 μg mL−1) to detached leaves 10 min prior to Eix treatment reduced LeEix2-GFP endocytosis in response to Eix treatment, while in the absence of filipin, LeEix2-GFP vesicles colocalized with the FYVE-DsRed-tagged endosomes after the application of Eix. Although filipin can have a broader effect on membranes, the cells observed remained normal in appearance within the time frame of the experiment. This result suggests that LeEix2 endocytosis is sterol dependent. The involvement of sterols in the Eix/LeEix system was also examined by ROS production and ion leakage. Eix was shown to induce ROS production (Laxalt et al., 2007; Bar and Avni, 2009; Yordanova et al., 2009; Sharfman et al., 2011) and ion leakage (Bar et al., 2010). ROS production was monitored between 10 and 40 min after the application of Eix in the presence of filipin in tobacco cells. Electrolyte leakage was measured 24 h after Eix application. The addition of filipin inhibited ROS production by 50% and electrolyte leakage by 40% in response to Eix as compared with control plants (Fig. 7). The effect of filipin in this system suggests that LeEix2 signaling is sterol dependent.
Figure 6.
Localization of LeEix2 with the endosomal marker FYVE-DsRed in leaf tissue treated with filipin. N. benthamiana leaves transiently expressing LeEix2-GFP and FYVE-DsRed were treated with Eix (2.5 μg g−1) or filipin (20 μg mL−1) and Eix (2.5 μg g−1). Leaf sections were visualized using a laser scanning confocal microscope 30 min after treatment. Arrowheads indicate the colocalization of LeEix2-GFP and FYVE-DsRed on endosomes. Bars = 10 µm.
Figure 7.
The effect of filipin treatment on the induction of defense responses mediated by Eix. A, Tobacco cells were incubated for 30 min with filipin (20 μg mL−1) followed by the addition of 2.5 μg mL−1 Eix. Cells were incubated in the presence of 5-amino salicylic acid (100 µm) for different time points as indicated. Changes in the color of the culture result from a chemical reaction indicating the presence of ROS. Images were quantified using ImageJ software. Each point represents the average ± se of three different experiments. B, Tobacco leaf discs were treated with filipin (20 μg mL−1) prior to the addition of Eix (2.5 μg mL−1). Electrolyte leakage was measured 24 h post Eix application. Values represent average conductivity ± se of three different experiments.
DISCUSSION
We have previously demonstrated that the internalization of endocytic vesicles is an obligatory requirement for Eix/LeEix2 signaling and defense responses (Bar and Avni, 2009; Sharfman et al., 2011). This work describes the identification of the LeEix2 interactor tomato CPI and its role in LeEix/Eix signaling and endocytosis. Arabidopsis CPI1, a membrane protein (Cattel et al., 1976), displays cycloeucalenol-obtusifoliol isomerase activity in vitro (Lovato et al., 2000). CPI is required for proper polar PIN2 localization and endocytosis (Men et al., 2008). An Arabidopsis cpi1-1 mutant showed altered sterol composition, which affects the postcytokinetic acquisition of PIN2 polarity by endocytosis (Men et al., 2008).
We have found that SlCPI interacts with LeEix2 and that the activity of SlCPI modulates Eix signaling by affecting its endocytosis. Overexpression of SlCPI stimulates the signaling of LeEix and Cf9, LRR-RLP receptors. However, it does not affect the signaling of the cytoplasmic receptor Pto. In the absence of SlCPI, LeEix signaling is attenuated.
The research into endocytosis and plant trafficking focuses on clathrin-dependent endocytic pathways and clathrin-independent mechanisms that may occur via lipid rafts (Grebe et al., 2003; Geldner, 2004; Aniento and Robinson, 2005; Ovecka et al., 2010). Multiple functions of sterols in mammalian and yeast endocytosis have been suggested; sterol has a role in forming membrane domains and in the internalization of these membrane structures. Upon cholesterol depletion, caveolae structures disappear and clathrin-coated endocytic structures are flattened (Rodal et al., 1999). Additionally, cholesterol depletion alters the efficiency of membrane transport steps. Sterols together with sphingolipids are responsible for protein recruitment to specialized membrane domains and their functionality in the endocytic pathway (Pichler and Riezman, 2004).
Filipin has an inhibitory role in the internalization step of endocytosis in plants. Application of filipin reduces the internalization of FM4-64, PIN1, and PIN2 (Kleine-Vehn et al., 2006; Men et al., 2008). Changes in the cellular sterol composition by filipin reduce LeEix2 internalization. Filipin was also found to be involved in ROS production in both mammalian and plant cells (Milovanova et al., 2008; Liu et al., 2009). Sterol sequestration with filipin disrupted membrane microdomain polarization, depressed tip-based ROS formation, dissipated the tip-focused cytosolic calcium gradient, and thereby arrested tip growth (Liu et al., 2009). Here, we show that ROS production induced by Eix was attenuated after treatment with filipin.
In plants, both clathrin-coated pits and ordered domains that are enriched with structural sterols on the plasma membrane serve as internalization platforms for plasma membrane-associated molecules and cargo. Plant sterols are reported to be internalized into endosomes and distributed within the endocytic pathway in an actin-dependent manner (Grebe et al., 2003)
SlCPI attenuates LeEix/Eix signaling and endocytosis, which was demonstrated to be sterol dependent and plays an important role in the development of plant defense responses. Previously, we showed that inhibiting clathrin-mediated endocytosis inhibits LeEix signaling (Sharfman et al., 2011). Here, we suggest that sterols are involved in the endocytosis of the LeEix2 receptor. Sterols might be involved in clathrin-mediated endocytosis or nonclathrin endocytosis, which may suggest that not all of the LeEix2 receptors undergo endocytosis via the clathrin pathway. Further experimentation is needed in order to pinpoint the exact roles of sterols in LeEix2-mediated signaling.
MATERIALS AND METHODS
Plant Material
Tobacco (Nicotiana tabacum ‘Xanthi’) and Nicotiana benthamiana plants were grown under greenhouse conditions at 24°C under a 16/8-h light/dark regimen. All experiments were carried out on 4- to 6-week-old plants on leaves 5 to 7.
Elicitor Application
Eix was purified from Fluka crude xylanase extract as reported previously (Dean and Anderson, 1991) and applied to cell cultures or leaf discs at a final concentration of 2.5 µg mL−1 or applied to the petiole of detached leaves at a final concentration of 2.5 µg g−1. Alternatively, Eix (2 µg mL−1) was injected into leaf tissue and HR development was analyzed.
Yeast Two-Hybrid Interaction and cDNA Library Screening
The plasmids (pEG202 and pJG4-5) and yeast strain EGY48 (ura3, his3, trp1, lexApo-leu2) were kindly provided by Roger Brent (Massachusetts General Hospital). The basic procedure for the two-hybrid system was according to Gyuris et al. (1993). To create the in-frame LexA-LeEix2 fusion construct (bait), we cloned the LeEix2 receptor (amino acids 110–913) fused in frame to the C terminus of the DNA-binding domain of LexA in the “bait” plasmid pEG202. The bait vector construct was confirmed by DNA sequencing. A tomato cDNA prey library in pJG4-5 (Zhou et al., 1995) was used for interaction screening. Screening was done as described previously (Hanania et al., 1999).
BiFC Analysis
The LeEix2 receptor (YN-LeEix2) was cloned as described previously (Bar et al., 2010). SlCPI (forward primer, 5′-CTACTAGTATGAAAGGCAATAAAGTGAATAGTGCAGCACCTAGTC-3′; reverse primer, 5′-GCACTAGTTCAAGATGTCATGGCAAACC-3′) was subcloned into the SpeI site of both pSY751 and pSY752. The resulting plasmids, pSY751-LeEix2 (YN-LeEix2) and pSY752-CYC (YC-SlCPI), were used for transient expression assays in N. benthamiana.
Confocal Microscopy
Cells were analyzed using a 510 Zeiss confocal laser scanning microscope with the following configuration: 30-mW argon and helium-neon lasers, and 488 and 568 maximum lines. All images depict single sections. Contrast and intensity for each image were manipulated uniformly using Adobe Photoshop and/or ImageJ software.
Endosome-to-Membrane Ratio Quantification
Each cell quantified was imaged in approximately 2-μm z-plane slices. For endosomes, the fluorescence of at least 10 endosomes in five to 10 cells was quantified; for membranes in these cells, the fluorescence of each cell membrane was sampled in at least five different areas and then averaged. The quantification is presented in the green-to-red ratio (mean arbitrary fluorescence units in the green channel divided by mean arbitrary fluorescence units in the red channel; all quantified endosomes were averaged). Endosomal green-to-red ratio was either normalized to membrane fluorescence (Fig. 5) or presented alongside the membranal data (Fig. 3). Quantification was carried out using ImageJ software. All images for quantification were captured using similar conditions.
Construction of Expression Plasmids
FYVE-DsRed (Voigt et al., 2005) was obtained from Dr. Jozef Samaj. GFP-LeEix2 was cloned as described (Bar and Avni, 2009). SlCPI cDNAs were cloned in the sense orientation upstream of the GFP gene into the binary vector pBINPLUS between the 35S-Ω promoter containing the translation enhancer signal and the Nos terminator, generating Pro35S:SlCPI-GFP. The construct was electroporated into Agrobacterium tumefaciens GV3101, and the bacteria were used for transient expression assays.
Ethylene and Ion Leakage Measurements
Ethylene biosynthesis was measured in a gas chromatograph 4 h post Eix application as described previously (Bar and Avni, 2009).
Ion leakage was measured 18 h post Eix application. Six leaf discs (1 cm diameter) of tobacco cv Samsun were sampled from treated areas for each plant floated in 15 mL of double distilled water with gentle shaking. Conductivity was measured with an autoranging EC/Temp Meter TH-2400 (El-Hamma Instruments; http://www.elhamma.com).
Reverse Transcription-PCR Analysis
Total RNA was extracted from leaves of 4- to 6-week-old N. benthamiana silenced plants using the SV total RNA isolation kit (Promega) according to the manufacturer’s instructions. RNA (0.5–2 μg) was converted to cDNA using Moloney murine leukemia virus reverse transcriptase (Promega). Two microliters of each reverse transcriptase reaction was used as a template in a PCR containing the following specific primer pairs: PRb-1b forward primer, 5′-ATGGGATACTCCACAACATTAGTTGC-3′; PRb-1b reverse primer, 5′-CTAGACATCAGTTGGAAGTTCCAACTTG-3. Quantification of the resultant PCRs was performed using ImageJ software.
VIGS Assay
VIGS assays were performed as described (Liu et al., 2002b). pTRV1, pTRV2, and pTRV-tPDS VIGS vectors were obtained from Dr. Savithramma Dinesh-Kumar. An EST segment (SGN-E1234135) possessing 88% identity to SlCPI was amplified using primers NbCPI forward primer (5′-GTTGAATTCCAGGGTTGACGAGAAACCAGGTGATTC-3′) and NbCPI reverse primer (5′-CTTCTCGAGTCAAGATGTCACTGCAAACC-3′) and cloned into the pTRV2 vector in the EcoRI and XhoI sites (Liu et al., 2002b) to generate pTRV2-NbCPI. Five weeks after TRV infection, silenced plants were challenged with Pro35S:tvEix and Pro35S:LeEix2 (Ron and Avni, 2004).
Ethylene Biosynthesis Measurement
Ethylene biosynthesis was measured as described previously (Avni et al., 1994). Six leaf discs per plant were taken from transiently expressing N. benthamiana or tobacco cv Samsun plants (1 cm diameter; average weight was measured for each plant). Discs were sealed in 1 mL of assay medium (10 mm MES, pH 6.0, and 250 mm sorbitol with or without 2.5 µg mL−1 Eix) in a 25-mL flask for 4 h at room temperature. Ethylene production was measured using gas chromatography.
Ion Leakage Measurements
Six leaf discs (1 cm diameter) of tobacco cv Xanthi were sampled from treated, infiltrated areas for each plant and floated in 3 mL of DDW with gentle shaking. Conductivity was measured 18 h post Eix application with a Eutech Con510 instrument.
ROS Measurement
ROS were measured 0 to 40 min post Eix application as indicated. ROS quantification was conducted by adding 100 μm 5-amino salicylic acid to 1 mL of treated tobacco cv Xanthi cell culture. Changes in the color of the culture result from a chemical reaction indicating the presence of ROS. Pixels were quantified with ImageJ software.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NM_001247310 (SlCPI).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. SICPI overexpression stimulates LeEix2-induced expression of PR1-b
Supplemental Figure S2. Effect of the overexpression of SICPI on the induction of the HR.
Supplemental Figure S3. Relative expression of NbCPI in control and NbCPI-silenced plants.
Acknowledgments
We thank Dr. Jozef Samaj for FYVE-DsRed, Dr. Pierre de Wit for Cf9 and Avr9 constructs, and Dr. Guido Sessa for the Pto-AvrPto construct.
Glossary
- LRR-RLP
leucine-rich repeat receptor protein
- ROS
reactive oxygen species
- HR
hypersensitive response
- cDNA
complementary DNA
- LRR
leucine-rich repeat
- BiFC
bimolecular fluorescence complementation
- VIGS
virus-induced gene silencing
References
- Altenbach D, Robatzek S. (2007) Pattern recognition receptors: from the cell surface to intracellular dynamics. Mol Plant Microbe Interact 20: 1031–1039 [DOI] [PubMed] [Google Scholar]
- Anderson RG. (1993) Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci USA 90: 10909–10913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Robinson DG. (2005) Testing for endocytosis in plants. Protoplasma 226: 3–11 [DOI] [PubMed] [Google Scholar]
- Avni A, Bailey BA, Mattoo AK, Anderson JD. (1994) Induction of ethylene biosynthesis in Nicotiana tabacum by a Trichoderma viride xylanase is correlated to the accumulation of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase transcripts. Plant Physiol 106: 1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey BA, Dean JFD, Anderson JD. (1990) An ethylene biosynthesis-inducing endoxylanase elicits electrolyte leakage and necrosis in Nicotiana tabacum cv Xanthi leaves. Plant Physiol 94: 1849–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Avni A. (2009) EHD2 inhibits ligand-induced endocytosis and signaling of the leucine-rich repeat receptor-like protein LeEix2. Plant J 59: 600–611 [DOI] [PubMed] [Google Scholar]
- Bar M, Sharfman M, Ron M, Avni A. (2010) BAK1 is required for the attenuation of ethylene-inducing xylanase (Eix)-induced defense responses by the decoy receptor LeEix1. Plant J 63: 791–800 [DOI] [PubMed] [Google Scholar]
- Benveniste P. (2004) Biosynthesis and accumulation of sterols. Annu Rev Plant Biol 55: 429–457 [DOI] [PubMed] [Google Scholar]
- Bishop GJ, Yokota T. (2001) Plants steroid hormones, brassinosteroids: current highlights of molecular aspects on their synthesis/metabolism, transport, perception and response. Plant Cell Physiol 42: 114–120 [DOI] [PubMed] [Google Scholar]
- Bittel P, Robatzek S. (2007) Microbe-associated molecular patterns (MAMPs) probe plant immunity. Curr Opin Plant Biol 10: 335–341 [DOI] [PubMed] [Google Scholar]
- Boutté Y, Grebe M. (2009) Cellular processes relying on sterol function in plants. Curr Opin Plant Biol 12: 705–713 [DOI] [PubMed] [Google Scholar]
- Boutté Y, Men S, Grebe M. (2011) Fluorescent in situ visualization of sterols in Arabidopsis roots. Nat Protoc 6: 446–456 [DOI] [PubMed] [Google Scholar]
- Bracha-Drori K, Shichrur K, Katz A, Oliva M, Angelovici R, Yalovsky S, Ohad N. (2004) Detection of protein-protein interactions in plants using bimolecular fluorescence complementation. Plant J 40: 419–427 [DOI] [PubMed] [Google Scholar]
- Cattel L, Anding C, Benveniste P. (1976) Cyclization of 1-trans-1′-norsqualene-2,3-epoxide and 1-cis-1′-norsqualene-2,3-epoxide by a cell free system of corn embryos. Phytochemistry 15: 931–935 [Google Scholar]
- Conner SD, Schmid SL. (2003) Regulated portals of entry into the cell. Nature 422: 37–44 [DOI] [PubMed] [Google Scholar]
- Dean JF, Anderson JD. (1991) Ethylene biosynthesis-inducing xylanase. II. Purification and physical characterization of the enzyme produced by Trichoderma viride. Plant Physiol 95: 316–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JFD, Gamble HR, Anderson JD. (1989) The ethylene biosynthesis-inducing xylanase: its induction in Trichoderma viride and certain plant pathogens. Phytopathology 79: 1071–1078 [Google Scholar]
- Geldner N. (2004) The plant endosomal system: its structure and role in signal transduction and plant development. Planta 219: 547–560 [DOI] [PubMed] [Google Scholar]
- Geldner N, Robatzek S. (2008) Plant receptors go endosomal: a moving view on signal transduction. Plant Physiol 147: 1565–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe M, Xu J, Möbius W, Ueda T, Nakano A, Geuze HJ, Rook MB, Scheres B. (2003) Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr Biol 13: 1378–1387 [DOI] [PubMed] [Google Scholar]
- Gyuris J, Golemis E, Chertkov H, Brent R. (1993) Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell 75: 791–803 [DOI] [PubMed] [Google Scholar]
- Hanania U, Avni A. (1997) High-affinity binding site for ethylene-inducing xylanase elicitor on Nicotiana tabacum membranes. Plant Journal 12: 113–120 [Google Scholar]
- Hanania U, Furman-Matarasso N, Ron M, Avni A. (1999) Isolation of a novel SUMO protein from tomato that suppresses EIX-induced cell death. Plant J 19: 533–541 [DOI] [PubMed] [Google Scholar]
- Hansen CG, Nichols BJ. (2010) Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol 20: 177–186 [DOI] [PubMed] [Google Scholar]
- Jones DA, Thomas CM, Hammond-Kosack KE, Balint-Kurti PJ, Jones JD. (1994) Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 266: 789–793 [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Keller P, Simons K. (1998) Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol 140: 1357–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J. (2006) Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell 18: 3171–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxalt AM, Raho N, Have AT, Lamattina L. (2007) Nitric oxide is critical for inducing phosphatidic acid accumulation in xylanase-elicited tomato cells. J Biol Chem 282: 21160–21168 [DOI] [PubMed] [Google Scholar]
- Liu P, Li RL, Zhang L, Wang QL, Niehaus K, Baluska F, Samaj J, Lin JX. (2009) Lipid microdomain polarization is required for NADPH oxidase-dependent ROS signaling in Picea meyeri pollen tube tip growth. Plant J 60: 303–313 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP. (2002a) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Marathe R, Dinesh-Kumar SP. (2002b) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J 30: 415–429 [DOI] [PubMed] [Google Scholar]
- Lovato MA, Hart EA, Segura MJ, Giner JL, Matsuda SP. (2000) Functional cloning of an Arabidopsis thaliana cDNA encoding cycloeucalenol cycloisomerase. J Biol Chem 275: 13394–13397 [DOI] [PubMed] [Google Scholar]
- Marella M, Lehmann S, Grassi J, Chabry J. (2002) Filipin prevents pathological prion protein accumulation by reducing endocytosis and inducing cellular PrP release. J Biol Chem 277: 25457–25464 [DOI] [PubMed] [Google Scholar]
- Martin GB, Brommonschenkel SH, Chunwongse J, Frary A, Ganal MW, Spivey R, Wu TY, Earle ED, Tanksley SD. (1993) Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 262: 1432–1436 [DOI] [PubMed] [Google Scholar]
- Men S, Boutté Y, Ikeda Y, Li X, Palme K, Stierhof YD, Hartmann MA, Moritz T, Grebe M. (2008) Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat Cell Biol 10: 237–244 [DOI] [PubMed] [Google Scholar]
- Miller RG. (1984) The use and abuse of filipin to localize cholesterol in membranes. Cell Biol Int Rep 8: 519–535 [DOI] [PubMed] [Google Scholar]
- Milovanova T, Chatterjee S, Hawkins BJ, Hong N, Sorokina EM, Debolt K, Moore JS, Madesh M, Fisher AB. (2008) Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta 1783: 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongrand S, Stanislas T, Bayer EM, Lherminier J, Simon-Plas F. (2010) Membrane rafts in plant cells. Trends Plant Sci 15: 656–663 [DOI] [PubMed] [Google Scholar]
- Murata M, Peränen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. (1995) VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA 92: 10339–10343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BJ, Kenworthy AK, Polishchuk RS, Lodge R, Roberts TH, Hirschberg K, Phair RD, Lippincott-Schwartz J. (2001) Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J Cell Biol 153: 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH. (1998) Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol 141: 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovecka M, Berson T, Beck M, Derksen J, Samaj J, Baluska F, Lichtscheidl IK. (2010) Structural sterols are involved in both the initiation and tip growth of root hairs in Arabidopsis thaliana. Plant Cell 22: 2999–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HH, Murray F, Insel PA. (2008) Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48: 359–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler H, Riezman H. (2004) Where sterols are required for endocytosis. Biochim Biophys Acta 1666: 51–61 [DOI] [PubMed] [Google Scholar]
- Robatzek S, Bittel P, Chinchilla D, Köchner P, Felix G, Shiu SH, Boller T. (2007) Molecular identification and characterization of the tomato flagellin receptor LeFLS2, an orthologue of Arabidopsis FLS2 exhibiting characteristically different perception specificities. Plant Mol Biol 64: 539–547 [DOI] [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. (1999) Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell 10: 961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron M, Avni A. (2004) The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 16: 1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron M, Kantety R, Martin GB, Avidan N, Eshed Y, Zamir D, Avni A. (2000) High-resolution linkage analysis and physical characterization of the EIX-responding locus in tomato. Theor Appl Genet 100: 184–189 [Google Scholar]
- Sharfman M, Bar M, Ehrlich M, Schuster S, Melech-Bonfil S, Ezer R, Sessa G, Avni A. (2011) Endosomal signaling of the tomato leucine-rich repeat receptor-like protein LeEix2. Plant J 68: 413–423 [DOI] [PubMed] [Google Scholar]
- Simon-Plas F, Perraki A, Bayer E, Gerbeau-Pissot P, Mongrand S. (2011) An update on plant membrane rafts. Curr Opin Plant Biol 14: 642–649 [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. (1997) Functional rafts in cell membranes. Nature 387: 569–572 [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39 [DOI] [PubMed] [Google Scholar]
- Soldati T, Schliwa M. (2006) Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol 7: 897–908 [DOI] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. (1999) Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA 96: 6775–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Muranaka T. (2007) Molecular genetics of plant sterol backbone synthesis. Lipids 42: 47–54 [DOI] [PubMed] [Google Scholar]
- Thomma BP, Nürnberger T, Joosten MH. (2011) Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23: 4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn RA, Wulff BB, Rivas S, Durrant MC, van der Ploeg A, de Wit PJ, Jones JD. (2005) Structure-function analysis of cf-9, a receptor-like protein with extracytoplasmic leucine-rich repeats. Plant Cell 17: 1000–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt B, Timmers AC, Samaj J, Hlavacka A, Ueda T, Preuss M, Nielsen E, Mathur J, Emans N, Stenmark H, et al. (2005) Actin-based motility of endosomes is linked to the polar tip growth of root hairs. Eur J Cell Biol 84: 609–621 [DOI] [PubMed] [Google Scholar]
- Wachtler V, Rajagopalan S, Balasubramanian MK. (2003) Sterol-rich plasma membrane domains in the fission yeast Schizosaccharomyces pombe. J Cell Sci 116: 867–874 [DOI] [PubMed] [Google Scholar]
- Yang H, Richter GL, Wang X, Młodzińska E, Carraro N, Ma G, Jenness M, Chao DY, Peer WA, Murphy AS. (2013) Sterols and sphingolipids differentially function in trafficking of the Arabidopsis ABCB19 auxin transporter. Plant J 74: 37–47 [DOI] [PubMed] [Google Scholar]
- Yordanova ZP, Kapchina-Toteva VM, Woltering EJ, Batchvarova RB, Yakimova ET. (2009) Xylanse-induced cell death events in detached tobacco leaves. Biotechnol Biotechnol Equip 23: 1199–1204 [Google Scholar]
- Zhou J, Loh YT, Bressan RA, Martin GB. (1995) The tomato gene Pti1 encodes a serine/threonine kinase that is phosphorylated by Pto and is involved in the hypersensitive response. Cell 83: 925–935 [DOI] [PubMed] [Google Scholar]