Abstract
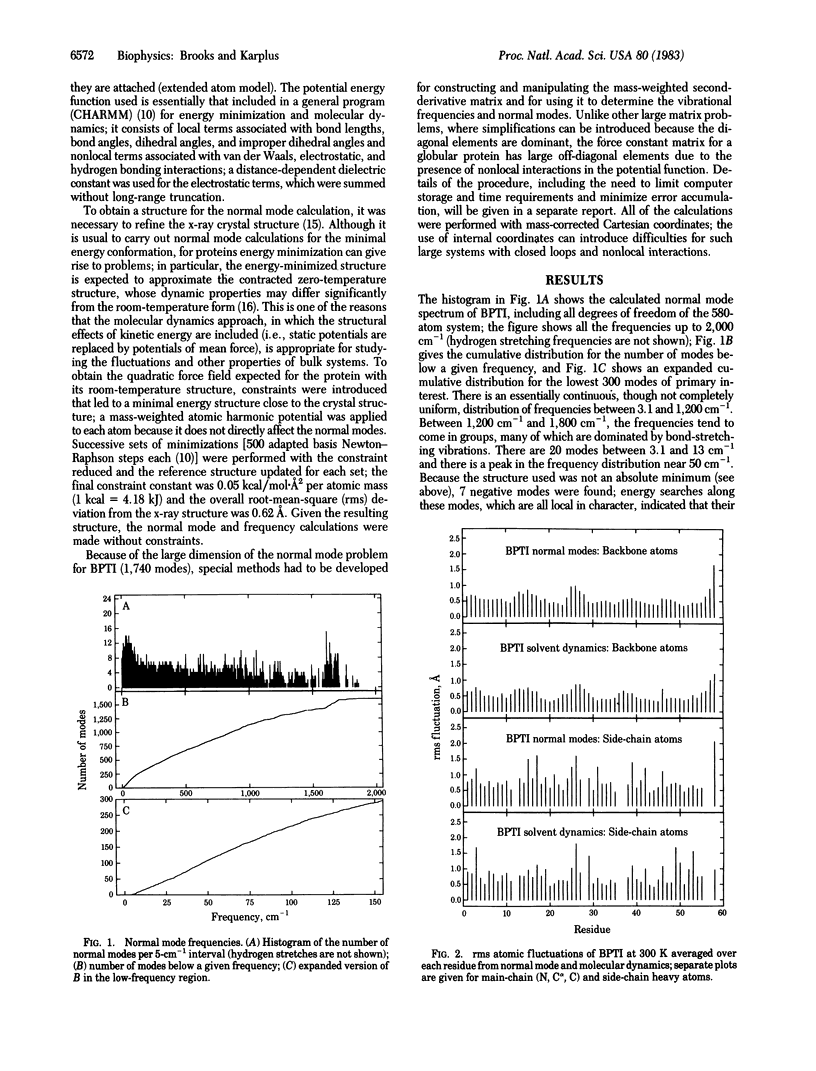
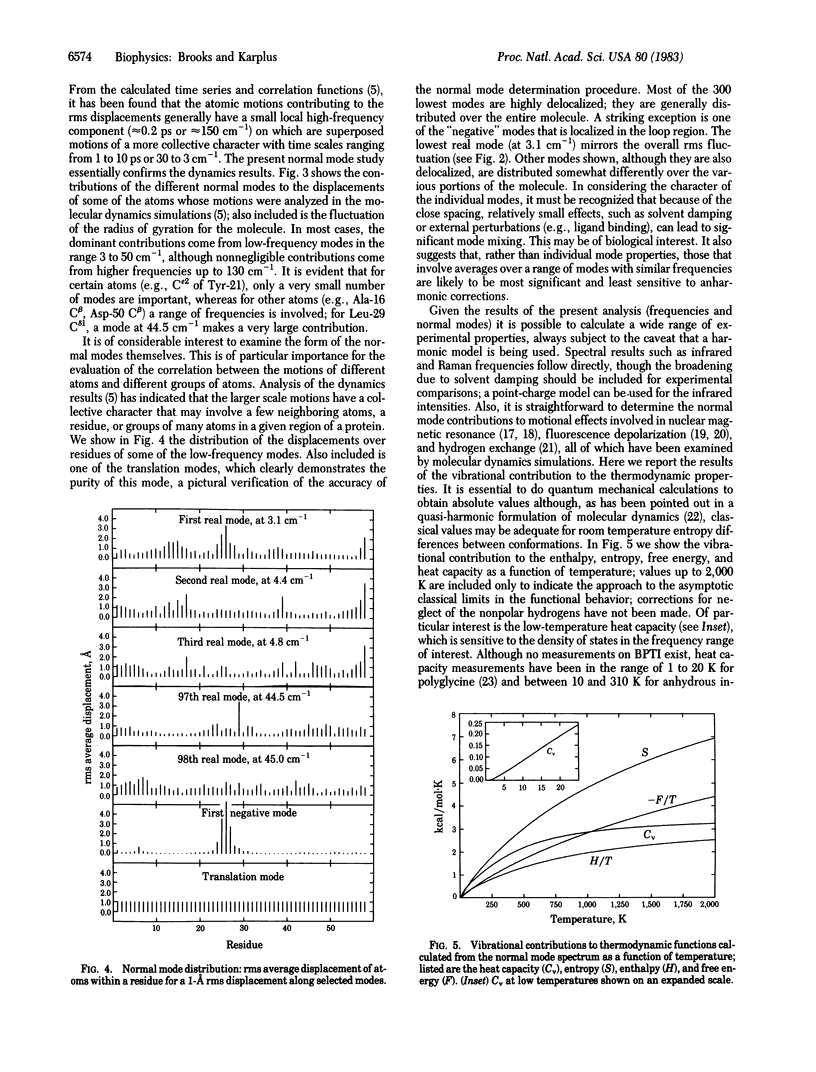
A normal mode analysis making use of an empirical potential function including local and nonlocal (nonbonded) interactions is performed for the bovine pancreatic trypsin inhibitor in the full conformational space of the molecule (1,740 degrees of freedom); that is, all bond lengths and angles, as well as dihedral angles, are included for the 580-atom system consisting of all heavy atoms and polar hydrogens. The heavy-atom frequency spectrum shows a dense distribution between 3 and 1,800 cm-1, with 350 modes below 216 cm-1. Most of the low-frequency modes, of which many have significant anharmonic character, are found to be delocalized over the protein. The root-mean-square amplitudes of the atomic fluctuations are calculated at 300 K from the normal modes and compared with those obtained from a solution molecular dynamics simulation based on the same potential function; very good agreement is obtained for the variation in the main-chain fluctuations as a function of residue number, though larger differences occur for the side chains. The fluctuations are generally, though not always, dominated by frequencies below 30 cm-1, in accord with the results of the dynamics simulation. The vibrational contributions to the thermodynamic properties of the protein are calculated as a function of temperature; the effects of perturbations on the spectrum, suggested for ligand or substrate binding, are examined. The analysis demonstrates that, in spite of the anharmonic contributions to the potential, a normal mode description can provide useful results concerning the internal motions of proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fanconi B., Finegold L. Vibrational states of the biopolymer polyglycine II: theory and experiment. Science. 1975 Oct 31;190(4213):458–460. doi: 10.1126/science.1166312. [DOI] [PubMed] [Google Scholar]
- Gurd F. R., Rothgeb T. M. Motions in proteins. Adv Protein Chem. 1979;33:73–165. doi: 10.1016/s0065-3233(08)60459-3. [DOI] [PubMed] [Google Scholar]
- Hartmann H., Parak F., Steigemann W., Petsko G. A., Ponzi D. R., Frauenfelder H. Conformational substates in a protein: structure and dynamics of metmyoglobin at 80 K. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4967–4971. doi: 10.1073/pnas.79.16.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. C., Dobson C. M., Karplus M. Fluctuations and averaging of proton chemical shifts in the bovine pancreatic trypsin inhibitor. Biochemistry. 1982 Mar 16;21(6):1118–1125. doi: 10.1021/bi00535a002. [DOI] [PubMed] [Google Scholar]
- Hutchens J. O., Cole A. G., Stout J. W. Heat capacities from 11 to 305 degrees K and entropies of hydrated and anhydrous bovine zinc insulin and bovine chymotrypsinogen A. Entropy change for formation of peptide bonds. J Biol Chem. 1969 Jan 10;244(1):26–32. [PubMed] [Google Scholar]
- Ichiye T., Karplus M. Fluorescence depolarization of tryptophan residues in proteins: a molecular dynamics study. Biochemistry. 1983 Jun 7;22(12):2884–2893. doi: 10.1021/bi00281a017. [DOI] [PubMed] [Google Scholar]
- Jacrot B., Cusack S., Dianoux A. J., Engelman D. M. Inelastic neutron scattering analysis of hexokinase dynamics and its modification on binding of glucose. Nature. 1982 Nov 4;300(5887):84–86. doi: 10.1038/300084a0. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. Dynamics of proteins: elements and function. Annu Rev Biochem. 1983;52:263–300. doi: 10.1146/annurev.bi.52.070183.001403. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. The internal dynamics of globular proteins. CRC Crit Rev Biochem. 1981;9(4):293–349. doi: 10.3109/10409238109105437. [DOI] [PubMed] [Google Scholar]
- Levitt M. Molecular dynamics of hydrogen bonds in bovine pancreatic trypsin inhibitor protein. Nature. 1981 Nov 26;294(5839):379–380. doi: 10.1038/294379a0. [DOI] [PubMed] [Google Scholar]
- Levy R. M., Perahia D., Karplus M. Molecular dynamics of an alpha-helical polypeptide: Temperature dependence and deviation from harmonic behavior. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1346–1350. doi: 10.1073/pnas.79.4.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B., Pear M. R., McCammon J. A., Northrup S. H. Molecular dynamics of ferrocytochrome c: anharmonicity of atomic displacements. Biopolymers. 1982 Oct;21(10):1979–1989. doi: 10.1002/bip.360211005. [DOI] [PubMed] [Google Scholar]
- Noguti T., Go N. Collective variable description of small-amplitude conformational fluctuations in a globular protein. Nature. 1982 Apr 22;296(5859):776–778. doi: 10.1038/296776a0. [DOI] [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. Activation of the complement system by antibody-antigen complexes: the classical pathway. Adv Protein Chem. 1979;33:1–71. doi: 10.1016/s0065-3233(08)60458-1. [DOI] [PubMed] [Google Scholar]
- Sturtevant J. M. Heat capacity and entropy changes in processes involving proteins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2236–2240. doi: 10.1073/pnas.74.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S., Ichiye T., van Gunsteren W., Karplus M. Time dependence of atomic fluctuations in proteins: analysis of local and collective motions in bovine pancreatic trypsin inhibitor. Biochemistry. 1982 Oct 12;21(21):5230–5241. doi: 10.1021/bi00264a019. [DOI] [PubMed] [Google Scholar]
- Tasumi M., Takeuchi H., Ataka S., Dwivedi A. M., Krimm S. Normal vibrations of proteins: glucagon. Biopolymers. 1982 Mar;21(3):711–714. doi: 10.1002/bip.360210318. [DOI] [PubMed] [Google Scholar]
- Williams R. J. The conformation properties of proteins in solution. Biol Rev Camb Philos Soc. 1979 Nov;54(4):389–437. doi: 10.1111/j.1469-185x.1979.tb00843.x. [DOI] [PubMed] [Google Scholar]
- van Gunsteren W. F., Karplus M. Protein dynamics in solution and in a crystalline environment: a molecular dynamics study. Biochemistry. 1982 May 11;21(10):2259–2274. doi: 10.1021/bi00539a001. [DOI] [PubMed] [Google Scholar]


