Abstract
Increased expression of the aquaporin NtAQP1, which is known to function as a plasmalemma channel for CO2 and water, increases the rate of both photosynthesis and transpiration. In contrast, increased expression of Arabidopsis hexokinase1 (AtHXK1), a dual-function enzyme that mediates sugar sensing, decreases the expression of photosynthetic genes and the rate of transpiration and inhibits growth. Here, we show that AtHXK1 also decreases root and stem hydraulic conductivity and leaf mesophyll CO2 conductance (g m). Due to their opposite effects on plant development and physiology, we examined the relationship between NtAQP1 and AtHXK1 at the whole-plant level using transgenic tomato plants expressing both genes simultaneously. NtAQP1 significantly improved growth and increased the transpiration rates of AtHXK1-expressing plants. Reciprocal grafting experiments indicated that this complementation occurs when both genes are expressed simultaneously in the shoot. Yet, NtAQP1 had only a marginal effect on the hydraulic conductivity of the double-transgenic plants, suggesting that the complementary effect of NtAQP1 is unrelated to shoot water transport. Rather, NtAQP1 significantly increased leaf mesophyll CO2 conductance and enhanced the rate of photosynthesis, suggesting that NtAQP1 facilitated the growth of the double-transgenic plants by enhancing mesophyll conductance of CO2.
Introduction
Aquaporins (AQPs), also known as MIPs (major intrinsic proteins), are integral membrane proteins that increase the permeability of membranes to water, as well as small uncharged molecules [1]. Of all kingdoms, the plant kingdom contains the largest known AQP family consisting over 30 members [2], [3]. There are 35 AQPs in Arabidopsis (Arabidopsis thaliana [4]), 36 in maize (Zea mays [1]) and 37 in tomato (Solanum lycopersicum [5]. Based on sequence similarities, AQPs have been divided into five subgroups: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), NOD26-like intrinsic proteins (NIPs), small basic intrinsic proteins (SIPs) and X intrinsic proteins (XIP) [4], [6]. Plant PIPs can be divided into two major groups, PIP1 and PIP2, on the basis of their sequences and water-channel activity. PIP2 proteins exhibit high levels of water-channel activity in Xenopus oocytes and yeast vesicles; whereas PIP1 proteins often have relatively low permeability to water [7]–[12].
Evidence for the role of PIP1 aquaporin in planta has come from mutant analyses and the manipulation of PIP1 expression in plants. Analysis of Arabidopsis mutants has shown that AtPIP1,2 can account for a significant portion of aquaporin-mediated leaf water transport [13]. The antisense expression of AtPIP1,2 in Arabidopsis has been associated with reductions in the membrane hydraulic conductivity of isolated protoplasts and decreased total root hydraulic conductivity [14], [15]. Antisense suppression of NtAQP1 (a member of the PIP1 subgroup) in tobacco (Nicotiana tabacum) lowered the level of expression of several PIP1 homologues and resulted in a significant decrease in protoplast membrane water permeability, reduced root hydraulic conductivity and decreased transpiration [16],[17].
The results of heterologous expression in Xenopus oocytes suggest that, in addition to functioning as a water channel, NtAQP1 is also a membrane CO2 pore that facilitates the transport of CO2 across membranes [7], [18]. The movement of CO2 between the substomatal cavities and the sites of carboxylation within chloroplasts, through plasma and chloroplast membranes, is generally termed leaf mesophyll conductance (g m) [19]. The ability of NtAQP1 and its Arabidopsis homolog AtPIP1,2 to function as CO2 membrane transport facilitators has been demonstrated in in vivo experiments. Increased expression of NtAQP1 in tobacco plants enhanced CO2 incorporation and stomatal conductance; whereas antisense suppression of NtAQP1 had the opposite effect [18]. In other studies, overexpression of AtPIP1,2 or NtAQP1 in tobacco plants significantly enhanced the rates of growth, transpiration and photosynthesis [20]–[22]; whereas antisense suppression of NtAQP1 in tobacco plants and T-DNA insertion Arabidopsis mutants in AtPIP1,2 reduced g m and led to lower rates of photosynthesis [21], [23], [24].
Unlike NtAQP1, overexpression of Arabidopsis hexokinase (AtHXK1) in Arabidopsis and tomato plants decreased photosynthesis, transpiration and growth [25], [26]. AtHXK1 is a sugar-sensing enzyme that monitors glucose levels, most likely in mesophyll cells of photosynthetic tissues. When glucose levels are sufficiently high, this enzyme inhibits the expression of photosynthetic genes, decreases chlorophyll levels and reduces the rate of photosynthesis [25]–[29]. In addition, AtHXK1 also stimulates stomatal closure and decreases transpiration in response to increasing sugar levels [26], [30]. In light of the opposite effects of AtHXK1 and NtAQP1 on photosynthesis and growth, we examined the relationship between AtHXK1 and NtAQP1 using double-transgenic plants that express AtHXK1 and NtAQP1 simultaneously. We found that NtAQP1 significantly compensated for the growth inhibition imposed by AtHXK1, primarily by enhancing mesophyll CO2 conductance and the rate of photosynthesis, while the hydraulic conductivity in those plants remained unchanged.
Materials and Methods
Construction of transgenic AQP1 plants
Cloning of the full-length cDNA of the tobacco (Nicotiana tabacum) NtAQP1 under the control of the 35S constitutive promoter was performed as described in [22]. MP-1 lines (Solanum lycopersicum cv. MP-1) were transformed using the Agrobacterium tumefaciens transformation method [31]. Plants were assayed for the presence of NtAQP1 by PCR using the following primers: 35Sprom-Fwd: TATCCTTCGCAAGACCCTCC, and NtAQP1-Rev: TGCCTGGTCTGTGTTGTAGAT.
Plant material
All experiments were conducted using wild-type (WT) tomato (Solanum lycopersicum cv. MP-1), isogenic independent transgenic homozygote tomato lines expressing different levels of the Arabidopsis AtHXK1 (HK37, HK4 and HK38 lines), as previously described in Dai [25], and an isogenic NtAQP1-expressing transgenic homozygote line AQP1. Double-transgenic homozygous plants NtAQP1xAtHXK1 (AQP1xHK4) were generated by crossing the AQP1 and HK4 parental lines. After self-pollination of the F1 hybrid plants, screening for F2 plants homozygous for both genes was performed using the highly sensitive Taq-Man DNA quantitative PCR method with specific probes, as described by German et al. [32]. Further validation of homozygosity was carried out by PCR analysis of tens of F3 plants using specific primers for NtAQP1 (35Sprom-Fwd-TATCCTTCGCAAGACCCTCC, NtAQP1-Rev- TGCCTGGTCTGTGTTGTAGAT) and AtHXK1 (Fwd-CGGGAAGCAAGAGCGTGTT, Rev-CTCCTCGGGTTGCTATGATG).
Measurements of root hydraulic conductance
The hydraulic conductance of the tomato root system (L r) was assessed using plants grown hydroponically and was determined by measuring the flow induced in response to 1 bar of applied pressure. De-topped root systems were fitted with a plastic tube filled with deionized water and connected to a beaker located on a balance (Sartorius ±0.01 mg). The root system was sealed in a chamber containing the hydroponic solution in which the plants had been grown. The pressure in the chamber was regulated using a needle valve, which was adjusted to allow a small leak into the chamber, so that the air used to pressurize the chamber also served to aerate the medium. Water flow through the root system was automatically recorded by a computer at 30 s intervals. At the end of each experiment, the roots were dried in an oven for 72 h at 90°C and the dry weight of the root system was then measured.
Measurements of stem hydraulic conductivity
Stem hydraulic conductivity was assessed on five to seven stems of each genotype. Short sections of stems (∼2–3 cm long) were cut under water directly from the intact plants to prevent embolisms caused by air entering into the cut vessels. Stems were connected to a balance (Sartorius ±0.1 mg) by a plastic tube and a filtered 10 mM KCl solution, used as a perfusion solution, was located on the balance in a beaker. Stem segments were first perfused under elevated pressure (0.2 MPa) to remove any embolisms and hydraulic conductivity (K s) was then calculated as the flow rate multiplied by the length of the stem segment and divided by the pressure gradient.
Xylem cross-sectional area was microscopically determined for each stem to allow the calculation of the xylem-specific stem conductivity (K sx, which equals K s divided by total xylem area). Free-hand cross-sections were excised and stained for a few seconds in a diluted Safranin solution. The sections were then rinsed in deionized water for few minutes and photographed under a compound microscope. Xylem area was later determined using the ImageJ software (http://rsbweb.nih.gov/ij/).
Measurements of whole-plant transpiration
Whole-plant transpiration rates and relative daily transpiration (RDT) were determined using lysimeters, as described in detail by Sade et al. [22]. WT, AQP1, HK4, AQP1xHK4 and grafted plants were planted in 3.9-L pots and grown under controlled conditions. Each pot was placed on a temperature-compensated load cell with digital output and was sealed to prevent evaporation from the surface of the growth medium. A wet, vertical wick made of 0.14 m2 cotton fibers partially submerged in a 1-L water tank was placed on a similar load cell and used as a reference for the temporal variations in the potential transpiration rate. The output of the load cells was monitored every 10 s and the average readings over 3-min intervals were logged in a data logger for further analysis. The whole-plant transpiration rate was calculated as a numerical derivative of the load cell output following a data-smoothing process [22]. The plant's daily transpiration rate was normalized to the total leaf area [measured using a LI-COR area meter, model Li-3100; (Lincoln, Nebraska, USA)] or to total plant weight, and to the data for neighboring submerged wick. These figures were averaged for each line and graft type (amount taken up by the wick daily = 100%).
Protein extraction and analysis of hexokinase activity
Protein extraction and hexokinase activity measurements were performed as described by Dai et al. [25].
RNA extraction, cDNA preparation and quantitative real-time PCR
Leaf tissue was harvested from WT, AQP1, HK4 and AQP1xHK4 plants and total RNA was extracted from that tissue using EZ-RNA kit (Biological Industries Co., Beit Haemek, Israel) according to the manufacturer's protocol. The RNA was treated with DNase (Ambion, Austin, TX, USA), according to the manufacturer's instructions, to degrade any residual DNA. The presence of RNA was confirmed by gel electrophoresis and DNA degradation was confirmed by PCR. For cDNA preparation, total RNA (1 µg) was taken for reverse transcription-PCR using MMLV RT (ProMega, Madison, WI, USA) in a 25-µl reaction with 2 µl of random primers (ProMega, Madison, WI, USA) and 1 µl of mixed poly-dT primers. cDNA samples were diluted 1∶7 in RNase-free- DEPC (Diethylpyrocarbonate) water. Quantitative real-time PCR reactions were performed using SYBR Green mix (Thermo-Scientific, Waltham, Massachusetts, USA). Reactions were run in a RotorGene 6000 cycler (Corbett, Mortlake, New South Wales, Australia). Following an initial pre-heating step at 95°C for 15 min, there were 40 cycles of amplification consisting of 10 s at 95°C, 15 s at 55°C, 10 s at 60°C and 20 s at 72°C. Results were analyzed using RotorGene software. Data were normalized using SlCyP (cyclophilin – accession; M55019) as a reference gene. The following primers were used for amplification: SlCAB1 (Fwd-TTGTGTTGATGGGAGCCGT, Rev-AAGGCCTAATGGGTCGAAGCT), SlCyP (Fwd-CGTCGTGTTTGGACAAGTTG, Rev-CCGCAGTCAGCAATAACCA) and TRAMP (Fwd-GTGAAGGGCTTCATGGTAGG, Rev-GGAAGTGGTGCCAAAATAGG). For each line tested, five to six independent samples were examined, with two replicates per sample.
Gas-exchange measurements and estimation of g m based on gas exchange and chlorophyll fluorescence
Gas exchange was measured using a Li-6400 portable gas-exchange system (LI-COR, Lincoln, Nebraska, USA). Analysis was performed on fully expanded leaves (5th–6th leaf from top) of plants growing under favorable conditions. All measurements were conducted between 10:00 AM and 1:00 PM. Photosynthesis was induced in saturating light (1200 µmol m−2 s−1) with 370 µmol mol−1 CO2 surrounding the leaf (C a) and 15% photosynthetically active photon flux density. The flow rate was set to 500 µmol air s−1. The leaf-to-air vapor pressure deficit was kept around 1-2.5 kPa during all measurements. Leaf temperature was ∼28°C (ambient temperature).
Chlorophyll fluorescence was measured using the LI-6400 open gas-exchange system with an integrated fluorescence chamber head (LI-6400-40; LI-COR). The actual photochemical efficiency of photosystem II (Φ PSII) was calculated using Equation 1. Steady-state fluorescence (F s) and maximum fluorescence were measured during a light-saturating pulse of ca. 8000 µmol m−2 s−1 (F m′), following the protocol used by Genty [33]. This procedure was repeated four times with similar results.
| (1) |
The electron transport rate (J) was then calculated using Equation 2, in which PPFD is the photosynthetically active photon flux density, α is leaf absorbance and β reflects the partitioning of absorbed quanta between photosystem II and photosystem I (PSII and PSI). Leaf absorbance (α) was measured between wavelengths of 400–700 nm, using an integrated sphere device (LI-COR, 1800–12s), as described by [34] – [36] .There were six to eight independent biological repeats for each line. A β value of 0.5 was used as described in [37]–[40].
| (2) |
From combined gas-exchange and chlorophyll-fluorescence measurements, the mesophyll conductance for CO2 (g m) was estimated as g m = A N/(C i−(Γ* (J+8·(A N+Rl)))/(J−4·(A N+Rd))), where A N and C i were obtained from gas-exchange measurements, as described by [41]. A value of 49.2 µmol mol−1 for the CO2 compensation point under non-respiratory conditions (Γ*) was used, after [42]. Respiration in the light (Rl) was determined from dark respiration values (Rd) obtained with the Li-6400 instrument at 25°C (−1.4±0.2 µmol CO2 m−2 s−1). A value equal to half of the dark respiration was used as a surrogate for Rl [43].
Results
AtHXK1 decreases root and stem hydraulic conductivity
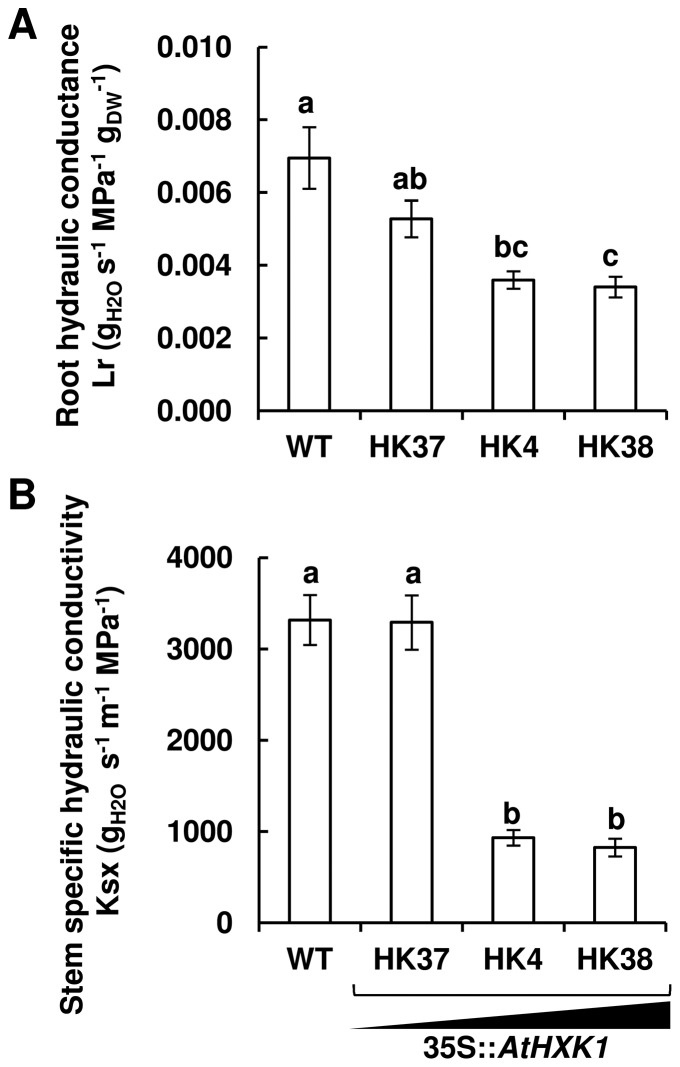
To examine the effects of AtHXK1 on hydraulic proprieties, we measured the root conductance and stem hydraulic conductivity of tomato lines expressing elevated levels of AtHXK1 (Fig. 1). HK37, HK4 and HK38 are very well characterized independent isogenic transgenic tomato lines that express AtHXK1 at different levels [25]. These lines exhibit HXK activity that is about 2, 5 and 6 times higher than that of WT plants, respectively [25]. The root hydraulic conductance (L r) and xylem-specific stem hydraulic conductivity (K sx) of HK4 and HK38 lines with high levels of AtHXK1 expression were significantly lower than those of WT plants (Fig. 1A and 1B).
Figure 1. AtHXK1 decreases root hydraulic conductance and stem hydraulic conductivity.
Root conductance (A) and xylem-specific stem hydraulic conductivity (B) were determined for control (WT) and transgenic plants expressing different levels of AtHXK1 (AtHXK1 expression from moderate to high: HK37<HK4<HK38; [25]). Data are means ± SE (n≥6 for L r; n≥5 for K sx). Different letters indicate a significant difference (t test, P<0.05).
NtAQP1 complements AtHXK1-mediated growth inhibition
While AtHXK1 decreases hydraulic conductivity, photosynthesis and growth [25], [26], NtAQP1 increases hydraulic conductivity and enhances photosynthesis and growth [20], [22]. In light of these opposite effects of AtHXK1 and NtAQP1, we were interested in exploring the relationship between NtAQP1 and AtHXK1 at the whole-plant level. To that end, we developed tomato line expressing NtAQP1 against the same genetic background (MP1 [31]) as that of the HK lines and assigned it AQP1. Expression of the NtAQP1gene and the level of NtAQP1 protein were determined by quantitative PCR and Western blot analysis, respectively (Fig. S1). We then created double-transgenic plants expressing both AtHXK1 and NtAQP1 simultaneously by crossing AQP1 lines with the HK4 line. Plants homozygous for both genes were identified and are referred to as AQP1xHK4.
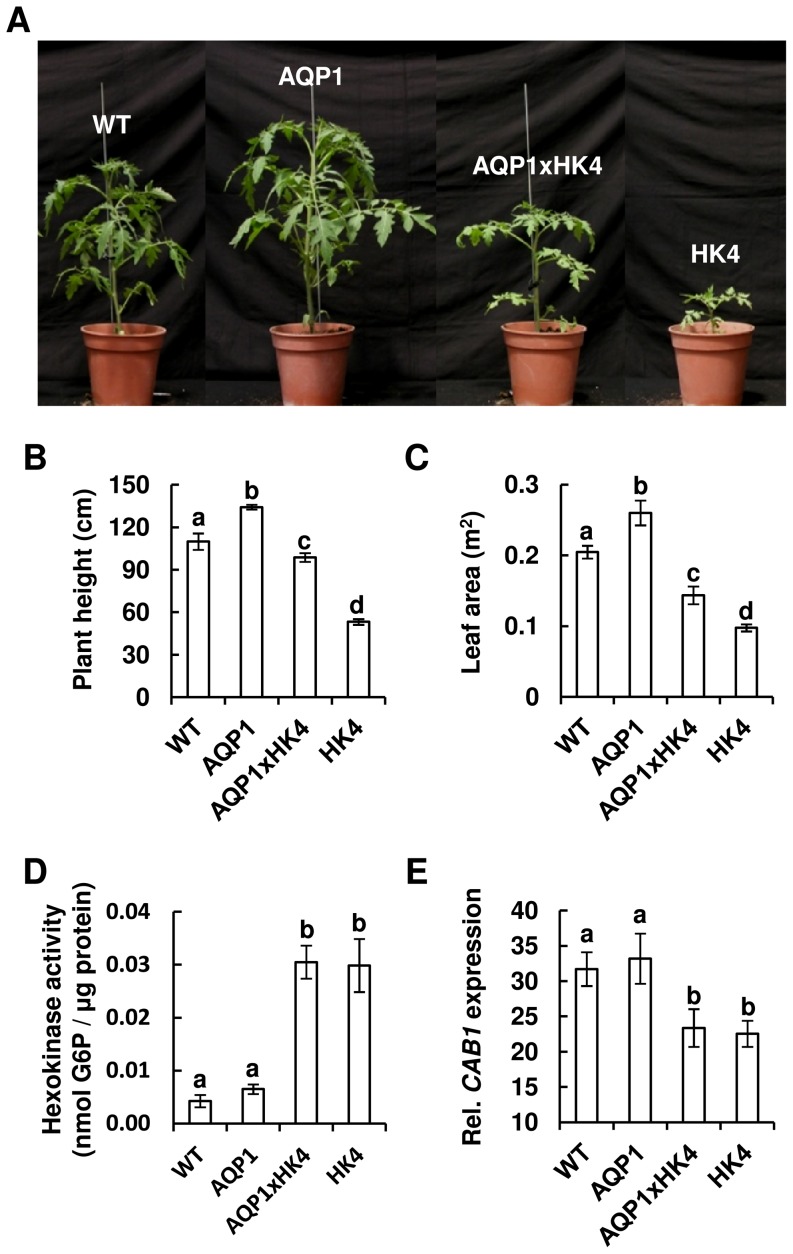
AQP1xHK4 plants were taller and had more leaf area than the HK4 parent line (Fig. 2), suggesting that NtAQP1 complemented the growth-inhibition effects of AtHXK1. To verify that this complementation effect was not the result of lowered expression of AtHXK1, HXK activity and the sugar-sensing effects of HXK were checked. HXK activity in the double-transgenic plants was similar to that of the HK4 parent plants, about 7-fold higher than that of the control WT and the AQP1 (homozygote) parent plants (Fig. 2D). We also examined the effect of HXK on the expression of the well-established sugar-sensing photosynthesis marker gene CAB1, which is known to be repressed by AtHXK1 [26], [27], [29]. CAB1 expression in AQP1xHK4 was repressed to levels similar to those observed in the HK4 plants (Fig. 2E), indicating that AtHXK1 mediated sugar-sensing effects in the double-transgenic plants. These results suggest that the growth complementation effects of NtAQP1 do not stem from suppression of HXK activity, but rather are probably due to epistatic physiological effects of NtAQP1.
Figure 2. NtAQP1 complements growth inhibition of AtHXK1.
(A) Representative images of 5-week-old tomato plants homozygous for NtAQP1 (AQP1), AtHXK1 (HK4) or both genes (AQP1xHK4). (B) Height (n≥8) and (C) leaf area (n≥6) of 9-week-old plants. (D) Hexokinase activity was determined using protein extracted from mature leaves of WT, AQP1, HK4 and AQP1xHK4 plants. Data are means of five independent biological repeats ± SE. (E) Relative expression of SlCAB1 (Solanum lycopersicum a/b binding protein) in WT, AQP1, HK4 and AQP1xHK4 plants. Data are means of five-six independent biological repeats ± SE. (B–E) Different letters indicate a significant difference (t test, P<0.05).
Growth complementation of AQP1xHXK is related to NtAQP1 copy number
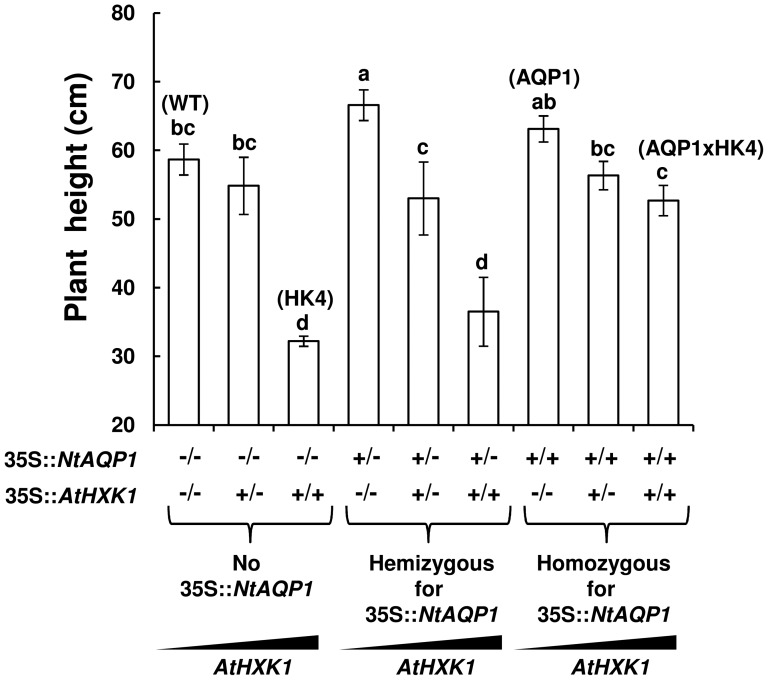
The epistatic effects of NtAQP1 on plant growth were observed primarily in plants homozygous for both genes, NtAQP1 and AtHXK1 (Fig. 3). Crossing AQP1xHK4 with WT, HK4 or AQP1 lines yielded plants that were heterozygous or homozygous for NtAQP1, AtHXK1 or both genes. Only plants that were homozygous for AtHXK1 exhibited significant growth inhibition, and NtAQP1 enhanced the growth of AtHXK1 homozygous plants only when present in the homozygous state (Fig. 3). Plants that were heterozygous for NtAQP1 and lacked AtHXK1 displayed slightly improved growth, but that effect was abolished in the presence of one or two copies of AtHXK1, suggesting a dosage effect in the relationship between NtAQP1 and AtHXK1.
Figure 3. Plant growth is affected by NtAQP1 and AtHXK1 gene dosage.
Height of transgenic plants with one copy (hemizygous, +/−) or two copies (homozygous, +/+) of AtHXK1 and NtAQP1. The zygosity state of each gene is specified on the x-axis. −/− indicates the absence of the specified gene, −/+ indicates hemizygosity and +/+ indicates homozygosity. Three left columns: no NtAQP1; middle three columns: hemizygous for NtAQP1; three right columns: homozygous for NtAQP1. Data are means of at least six independent repeats ± SE. Different letters indicate a significant difference (t test, P<0.05).
NtAQP1 enhances the stomatal conductance and transpiration of AtHXK1 plants
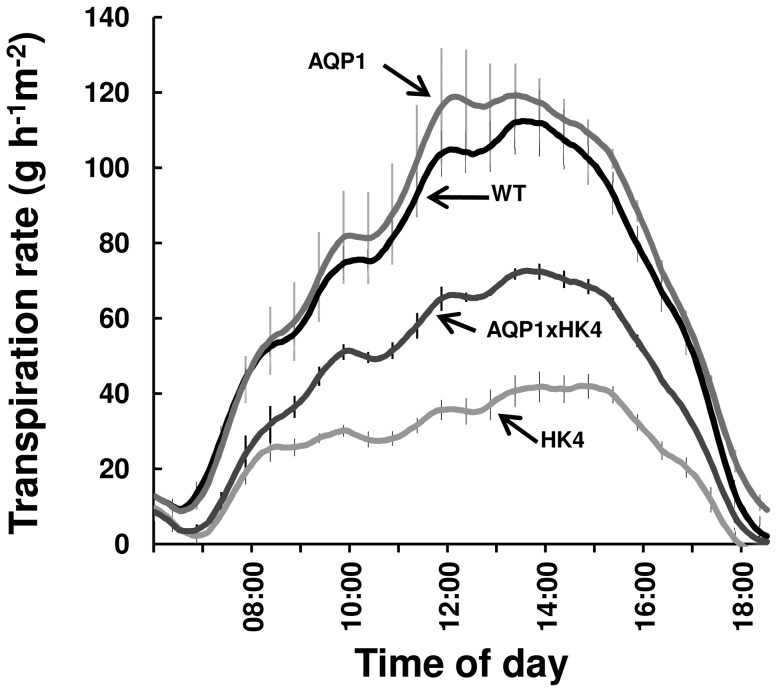
In previous studies, overexpression of AtHXK1 decreased stomatal conductance and transpiration; whereas overexpression of NtAQP1 increased stomatal conductance and transpiration [18], [20]–[22], [26], [30]. Therefore, we tested the combined effects of AtHXK1 and NtAQP1 on stomatal conductance and transpiration. Stomatal conductance (gs) of HK4 plants was significantly lower than that of WT plants (Table 1; [30]). Meanwhile, the gs of the double-transgenic plants was similar to that of the WT plants (Table 1). Continuous measurements of whole-plant transpiration per unit leaf area over the course of the day revealed significantly lower transpiration rates in HK4 plants, as compared to WT and AQP1 plants (Fig. 4). Yet, the double-transgenic plants had intermediate-level transpiration rates that were higher than those of HK4 plants (Fig. 4). These results indicate that NtAQP1 enhanced stomatal conductance and compensated for the limitations imposed on transpiration rates by AtHXK1.
Table 1. Photosynthetic and hydraulic characteristics of WT, AQP1, AQP1xHK4 and HK4 plants.
| WT | AQP1 | AQP1xHK4 | HK4 | |
| L r (gH2O s−1 MPa−1 gDW−1) | 0.00319±0.0003 (7) a | 0.00269±0.0005 (7) ab | 0.00201±0.0004 (10) b | 0.00178±0.00008 (6) b |
| K sx (gH2O s−1 m−1 MPa−1) | 1501.48±167.7 (7) a | 1081.73±196.2 (7) a | 381.61±36.6 (5) b | 274.25±16.8 (6) b |
| A N (µmol CO2 m−2 s−1) | 28.189±0.68 (18) a | 27.580±0.60 (20) a | 28.073±0.49 (15) a | 20.831±1.34 (13) b |
| g s (mol H2O m−2 s−1) | 0.702±0.04 (18) a | 0.637±0.04 (20) ab | 0.697±0.03 (15) a | 0.525±0.06 (13) b |
| g m (mol CO2 m−2 s−1) | 0.248±0.019 (18) a | 0.232±0.014 (20) ab | 0.2004±0.007 (15) b | 0.148±0.015 (13) c |
| C i (µmol CO2 mol−1) | 312.5±2.67 (18) a | 305.1±4.39 (20) a | 313.27±2.39 (15) a | 311.29±5.53 (13) a |
| C c (µmol CO2 mol−1) | 185.6±6.27 (18) a | 183.8±6.34 (20) a | 173.9±3.09 (15) a | 154.9±6.26 (13) b |
| J (µmol m−2 s−1) | 233.3±3.19 (18) b | 238.8±3.5 (20) ab | 247.7±2.16 (15) a | 204.05±6.22 (13) c |
L r, root hydraulic conductance; K sx, xylem-specific stem hydraulic conductivity; A N, net photosynthesis; g s, stomatal conductance; g m, mesophyll CO2 conductance; C i, substomatal CO2 concentration; Cc, Chloroplast CO2 concentration; J, the rate of electron transport. Presented data are means ± SE (n, number of replicates, as indicated in parentheses). Different letters in a row indicate significant differences (t test, P<0.05).
Figure 4. Transpiration rate of AQP1xHK4 plants.
The rate of transpiration was monitored continuously throughout the day for each line (AQP1, HK4, AQP1xHK4 and WT). The presented data are the means ± SE for each 10th sampling point (n = 6). The transpiration data were normalized to the total leaf area and the amount taken up by the neighboring submerged fixed-size wick each day, which was set to 100%.
Growth and transpiration complementation occurs when NtAQP1 and AtHXK1 are simultaneously expressed in the shoot
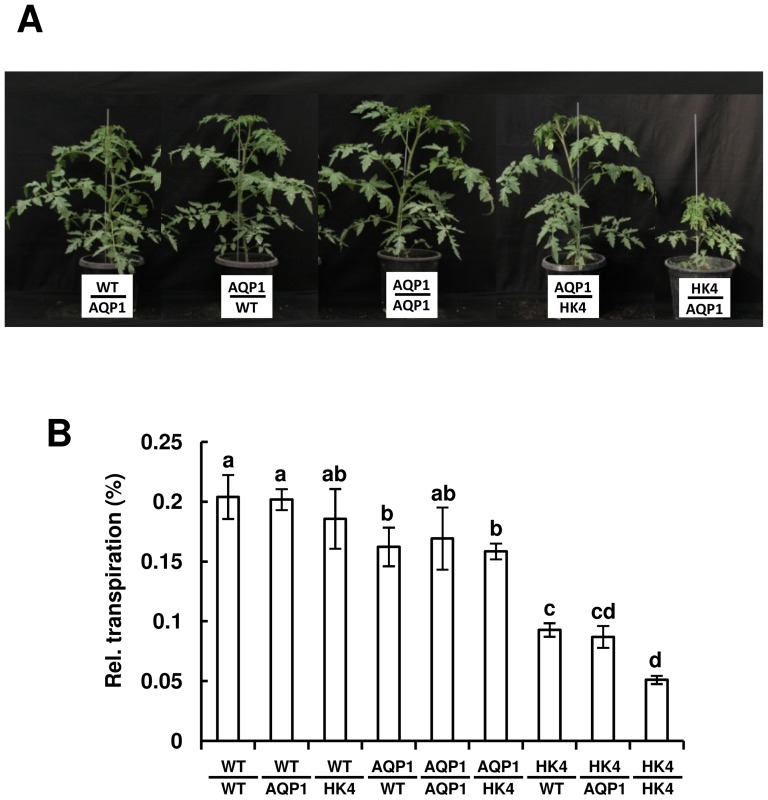
To examine whether the decreased transpiration and growth imposed by AtHXK1 and its complementation by NtAQP1 emanate from distinct effects on roots or shoots, we created reciprocal grafts, in which WT, AQP1 and HK4 shoots were grafted onto WT, AQP1 and HK4 roots, covering all nine possible combinations. (Five combinations involving AQP1 are shown in Figure 5A and the other four combinations are shown in Figure 2A of [30]). AtHXK1 inhibited growth only when expressed in shoots, independent of the root genotype (Fig. 5A and [30]). Similarly, measurements of cumulative whole-plant relative daily transpiration of the grafted plants indicated that AtHXK1 decreased transpiration by about 50% only when expressed in shoots, independent of the root genotype [(Fig. 5B), in line with our recent discovery that AtHXK1 stimulates stomatal closure and reduces transpiration when expressed in shoots [30].(i.e., NtAQP1 in roots had no complementation effect on HK4 shoots) (Fig. 5A). These results show that separate expression of NtAQP1 and AtHXK1 in roots or shoots is insufficient to achieve complementation of AtHXK1 phenotypes by NtAQP1 and that the complementation of AtHXK1effects by NtAQP1 occurs only when both genes are expressed simultaneously in the shoots.
Figure 5. Reciprocal grafting and whole-plant relative daily transpiration.
Reciprocal grafting of WT, AQP1 and HK4 plants was performed at the seedling stage. The plants were photographed (A and Fig. 2A in [30]) and their transpiration was measured about 4 weeks after grafting. (B) Whole-plant relative daily transpiration of reciprocal-grafted plants. Data were normalized to the total plant weight and the amount of water taken up by the neighboring submerged fixed-size wick each day, which was set to 100%. Presented data are means of four independent repeats ± SE. Different letters indicate a significant difference (t test, P<0.05).
NtAQP1 does not improve hydraulic conductance of AtHXK1 plants, but does increase the conductance of CO2 in the leaf mesophyll and the rate of photosynthesis
The enhanced transpiration of the double-transgenic plants relative to HK4 plants might suggest that NtAQP1 could potentially improve the low hydraulic properties of HK4 plants. We, therefore, measured the root and stem hydraulic conductivity of the WT, AQP1, HK4 and double-transgenic plants. NtAQP1 did not improve the root conductance or xylem-specific stem hydraulic conductivity (L r and K sx, respectively) of the double-transgenic plants, which remained low, as in the HK4 plants (Table 1). However, gas-exchange analysis of the double-transgenic plants revealed that NtAQP1 increased photosynthesis rates (A N), CO2 conductance (g m) and stomatal conductance (g s), with no effect on intracellular CO2 concentration (C i), as compared to the low A N, g s and g m values observed in the HK4 plants (Table 1). In addition, NtAQP1 increased both the concentration of CO2 in the chloroplasts (C c) and the electron transport rate (J), as compared to the HK4 plants (Table 1). We, therefore, suggest that the complementation of AtHXK1 effects by NtAQP1 is primarily due to the role of NtAQP1 as a CO2 facilitator, which enhances the conductance of CO2 in the mesophyll thereby elevating the rate of photosynthesis despite the low expression of CAB1 in AQP1xHK4 plants.
Discussion
PIP1-AQPs were shown to enhance cell permeability to both CO2 and water [7], [13], [18]. Overexpression of NtAQP1 in tobacco plants enhanced leaf mesophyll CO2 conductance (g m), hydraulic conductivity, stomatal conductance (g s), transpiration and photosynthesis (A N) [18], [20], [21]. Expression of NtAQP1 in tomato plants also enhanced photosynthesis, stomatal conductance and transpiration [22]. However, in our study, NtAQP1 did not enhance photosynthesis, stomatal conductance or hydraulic conductivity relative to WT plants (Fig. 5, Table 1) and enhanced transpiration only slightly (Fig. 4). These differences may be due to the different tomato genotype used in our study (MP1- [31], an indeterminate variety) or to different expression levels of NtAQP1. Nevertheless, photosynthesis, stomatal conductance and transpiration were elevated by NtAQP1 in the double-transgenic plants (AQP1xHK4), as compared to the HK4 parental (isogenic) line. Yet, the hydraulic conductivity of AQP1xHK4 remained low as in the HK4 plants, implying that the increased transpiration that was observed is not directly related to hydraulic characteristics. Rather, the increased transpiration is most likely due to high g m values in the mesophyll, which opens stomata and increases the influx of CO2 to help maintain constant levels of C i in the substomatal cavity [44], [45]. High levels of A N, g s and g m, accompanied by constant C i, were also reported in previous studies of tobacco plants overexpressing NtAQP1 [18], [21], [42].
AtHXK1 is a sugar-sensing enzyme that inhibits the expression of photosynthetic genes, decreases chlorophyll levels and reduces the rate of photosynthesis in response to increasing sugar levels [25]–[29]. As a result, tomato and Arabidopsis plants with high levels of AtHXK1 expression display severe growth inhibition directly correlated to AtHXK1 expression and activity levels [25], [26]. It is likely that part of the growth inhibition imposed by AtHXK1 is the result of insufficient photosynthesis, since the increased photosynthesis rate observed in AQP1xHK4 plants partially eliminated this growth inhibition.
The increased rate of photosynthesis observed in AQP1xHK4 plants, despite the low level of expression of the photosynthetic gene CAB1 in those plants, can probably be attributed to NtAQP1, which accelerates CO2 mesophyll conductance (g m) [21], [22]. The CO2 mesophyll conductance of HK4 plants is significantly lower than that of WT plants and is enhanced by simultaneous expression of NtAQP1, indicating that CO2 mesophyll conductance significantly affects growth.
It appears that, in addition to its known sugar-sensing effect (reducing expression of photosynthetic genes and reducing the rate of photosynthesis [25]–[27], [29], [46]; Fig. 2E and Table 1), AtHXK1 also reduces g m, perhaps by reducing the expression of TRAMP (Fig. S2), the tomato homolog of NtAQP1 [47]. Indeed, lower g m levels have been observed in tobacco NtAQP1 antisense lines [21] and Arabidopsis pip1;2 mutants (a CO2-facilitating AQP;[24]). In those studies, the decrease in g m was accompanied by lower C c. In agreement with the findings of those studies, the HK4 plants in our study exhibited lower C c than the WT plants and the expression of NtAQP1in the double-transgenic plants (AQP1xHK4) led to full complementation of C c (Table 1). Interestingly, the HK4 plants had lower electron transport rates (J) than the WT plants, while a clear recovery was observed in the AQP1xHK4 plants (Table 1) despite the low level of expression of the photosynthetic gene CAB1 in the AQP1xHK4 plants (Fig. 2E).
It has previously been shown that expression level of NtAQP1 which affects g m levels also affects electron transport rates (J) [21], [24], [48]. Flexas et al. [21] hypothesized that modified intercellular CO2 concentrations may trigger differences in the leaf photosynthetic capacity, so that the photosynthetic machinery can adjust to the change in mesophyll conductance. This would also explain why g m usually scales with photosynthetic capacity, as has been observed in broad comparisons of different species [49], [50].
The effect of AtHXK1 on g m suggests that HXK might coordinate photosynthesis with sugar levels by several mechanisms in different cell types. It inhibits expression of photosynthetic genes [25], [46] and reduces g m most likely in mesophyll photosynthetic cells. In guard cells HXK mediates stomatal closure in response to sugars and reduces stomatal conductance (g s) [26], [30]. These findings support the existence of a multilevel feedback-inhibition mechanism that is mediated by HXK in response to sugars. When sugar levels are high, likely when the rate of photosynthesis exceeds the rate at which the sugar is loaded and carried by the phloem, the surplus of sugar is sensed by HXK in mesophyll and guard cells, which respond in concert to reduce both unnecessary investments in photosynthetic capacity and water loss. This response includes reducing the expression of photosynthetic genes, slowing chlorophyll production, diminishing mesophyll CO2 conductance and closing the stomata.
In addition to these effects in shoots, HXK reduces the hydraulic conductivity of stem and roots via an as yet unknown mechanism. This reduction in hydraulic conductivity occurs independently of stomatal conductance, as it also happens in the double-transgenic plants that have WT levels of stomatal conductance (Table 1). Nevertheless, grafting experiments indicate that neither overexpression of AtHXK1 in roots nor expression of AtHXK1 in the stem has any visible physiological effects. Rather, overexpression of AtHXK1 in shoots is necessary and sufficient to obtain a photosynthesis effect and growth inhibition [25], [30]. The dominant effect of AtHXK1, lowering hydraulic conductance in AQP1xHK4, might be the reason for the intermediate transpiration rate of AQP1xHK4 plants, which is lower than that of WT plants (Fig. 4), despite the increase in stomatal conductance to levels similar to that of WT plants (Table 1). It has been suggested that NtAQP1 might play independent roles in leaves and roots, a hydraulic role in roots and a membrane CO2 permeability role in shoots [22]. The improved g m observed in the double-transgenic plants supports the notion that, in leaves, NtAQP1 functions as a CO2 transmembrane facilitator and that the complementation effect of NtAQP1 may be primarily attributed to its affect on CO2 conductance in leaf mesophyll. The roles of HXK and PIP1 in the regulation of photosynthesis, stomatal conductance and transpiration are well established [18], [20]–[22], [25]–[29]. This study suggests that HXK and PIP1 together may influence these central properties of plant physiology and, eventually, plant growth.
Supporting Information
Expression analysis of NtAQP1 in AQP1 transgenic line: Presence of NtAQP1 DNA, RNA and protein. (A) The presence of NtAQP1 was assayed by PCR using NtAQP1-specific primers; transgenic AQP1 plants yielded the expected 930-bp product. WT is a negative non-transformed wild-type plant. + stands for a positive PCR control with a plasmid containing NtAQP1. Ladder: 100-bp ladder. (B) cDNA of AQP1 was subjected to semi-quantitative PCR using NtAQP1-specific primers; Fwd-CCGGGCAGGTGTACTATCC, Rev-TGCCTGGTCTGTGTTGTAGAT. Amplification was performed using 35 PCR cycles. SlCyP (cyclophilin – accession; M55019) was used as a control. (C) Western blot analysis of protein extracts from AQP1 plants probed with NtAQP1-specific antibody (upper panel); Ponceau red staining of the Western blot indicating equal protein loading (lower panel). Western blot analysis and Ponceau staining were performed exactly as described in Sade et al. [22]).
(TIF)
Expression of the TRAMP is suppressed by AtHXK1 . Expression level of TRAMP (tomato ripening associated membrane protein, accession no. NM_001247210), the tomato NtAQP1 homolog, was determined by quantitative real-time PCR using cDNA extracted from leaves of WT and HK4 plants. Data are means of five independent biological repeats ± SE. Different letters indicate a significant difference (t test, P<0.05). SlCyP (cyclophilin) was used for normalization.
(TIF)
Acknowledgments
We wish to thank Mr. Leonid Mourakhovsky for his dedicated and diligent care of the plants grown for this research and Mr. Gil Lerner for his outstanding technical support. Contribution of the Agriculture Research Organization, The Volcani Center, Bet Dagan, Israel. No. 101/2014.
Funding Statement
This research was supported by the Israel Ministry of Agriculture, Chief Scientist Research Grants 261-0865 and 261-0845 (http://www.moag.gov.il/agri/yhidotmisrad/madanrashi/); and by grant no. IS-4541-12 from BARD, the United States-Israel Binational Agricultural and Development Fund (http://www.bard-isus.com/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Biol Cell 97: 749–764. [DOI] [PubMed] [Google Scholar]
- 2. Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology 59: 595–624. [DOI] [PubMed] [Google Scholar]
- 3. Heinen RB, Ye Q, Chaumont F (2009) Role of aquaporins in leaf physiology. J Exp Bot 60: 2971–2985. [DOI] [PubMed] [Google Scholar]
- 4. Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjovall S, et al. (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126: 1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sade N, Vinocur BJ, Diber A, Shatil A, Ronen G, et al. (2009) Improving plant stress tolerance and yield production: Is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol 181: 651–661. [DOI] [PubMed] [Google Scholar]
- 6. Danielson JA, Johanson U (2008) Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens . BMC Plant Biol 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biela A, Grote K, Otto B, Hoth S, Hedrich R, et al. (1999) The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18: 565–570. [DOI] [PubMed] [Google Scholar]
- 8. Chaumont F, Barrieu F, Jung R, Chrispeels MJ (2000) Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol 122: 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marin-Olivier M, Chevalier T, Fobis-Loisy I, Dumas C, Gaude T (2000) Aquaporin PIP genes are not expressed in the stigma papillae in Brassica oleracea . Plant J 24: 231–240. [DOI] [PubMed] [Google Scholar]
- 10. Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, et al. (2002) Plasma membrane aquaporins in the motor cells of Samanea saman: Diurnal and circadian regulation. Plant Cell 14: 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaspar M, Bousser A, Sissoeff I, Roche O, Hoarau J, et al. (2003) Cloning and characterization of ZmPIP1-5b, an aquaporin transporting water and urea. Plant Sci 165: 21–31. [Google Scholar]
- 12. Suga S, Maeshima M (2004) Water channel activity of radish plasma membrane aquaporins heterologously expressed in yeast and their modification by site-directed mutagenesis. Plant Cell Physiol 45: 823–830. [DOI] [PubMed] [Google Scholar]
- 13. Postaire O, Tournaire-Roux C, Grondin A, Boursiac Y, Morillon R, et al. (2010) A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiol 152: 1418–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaldenhoff R, Grote K, Zhu JJ, Zimmermann U (1998) Significance of plasmalemma aquaporins for water-transport in Arabidopsis thaliana . Plant J 14: 121–128. [DOI] [PubMed] [Google Scholar]
- 15. Martre P, Morillon R, Barrieu F, North GB, Nobel PS, et al. (2002) Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol 130: 2101–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R (2002) PIP1 plasma membrane aquaporins in tobacco: From cellular effects to function in plants. Plant Cell 14: 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siefritz F, Otto B, Bienert GP, van der Krol A, Kaldenhoff R (2004) The plasma membrane aquaporin NtAQP1 is a key component of the leaf unfolding mechanism in tobacco. Plant J 37: 147–155. [DOI] [PubMed] [Google Scholar]
- 18. Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R (2003) The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425: 734–737. [DOI] [PubMed] [Google Scholar]
- 19. Evans JR, Kaldenhoff R, Genty B, Terashima I (2009) Resistances along the CO2 diffusion pathway inside leaves. J Exp Bot 60: 2235–2248. [DOI] [PubMed] [Google Scholar]
- 20. Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, et al. (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flexas J, Ribas-Carbo M, Hanson DT, Bota J, Otto B, et al. (2006) Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J 48: 427–439. [DOI] [PubMed] [Google Scholar]
- 22. Sade N, Gebretsadik M, Seligmann R, Schwartz A, Wallach R, et al. (2010) The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol 152: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uehlein N, Sperling H, Heckwolf M, Kaldenhoff R (2012) The Arabidopsis aquaporin PIP1;2 rules cellular CO(2) uptake. Plant Cell Environ 35: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 24. Heckwolf M, Pater D, Hanson DT, Kaldenhoff R (2011) The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO transport facilitator. Plant J Cell Mol Biol 67: 795–804. [DOI] [PubMed] [Google Scholar]
- 25. Dai N, Schaffer A, Petreikov M, Shahak Y, Giller Y, et al. (1999) Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell 11: 1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelly G, David-Schwartz R, Sade N, Moshelion M, Levi A, et al. (2012) The pitfalls of transgenic selection and new roles of AtHXK1: A high level of AtHXK1 expression uncouples hexokinase1-dependent sugar signaling from exogenous sugar. Plant Physiol 159: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jang JC, Leon P, Zhou L, Sheen J (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao W, Sheen J, Jang JC (2000) The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol 44: 451–461. [DOI] [PubMed] [Google Scholar]
- 29. Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, et al. (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336. [DOI] [PubMed] [Google Scholar]
- 30. Kelly G, Moshelion M, David-Schwartz R, Halperin O, Wallach R, et al. (2013) Hexokinase mediates stomatal closure. Plant J 75: 977–988. [DOI] [PubMed] [Google Scholar]
- 31. Barg R, Pilowsky M, Shabtai S, Carmi N, Szechtman AD, et al. (1997) The TYLCV-tolerant tomato line MP-1 is characterized by superior transformation competence. J Exp Bot 48: 1919–1923. [Google Scholar]
- 32. German MA, Kandel-Kfir M, Swartzberg D, Matsevitz T, Granot D (2003) A rapid method for the analysis of zygosity in transgenic plants. Plant Sci 164: 183–187.33. Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92. [Google Scholar]
- 33. Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92. [Google Scholar]
- 34. Schultz HR (1996) Leaf absorptance of visible radiation in Vitis vinifera L: Estimates of age and shade effects with a simple field method. Sci Hort 66: 93–102. [Google Scholar]
- 35. Hendrickson L, Furbank RT, Chow WS (2004) A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth Res 82: 73–81. [DOI] [PubMed] [Google Scholar]
- 36. Eppel A, Keren N, Salomon E, Volis S, Rachmilevitch S (2013) The response of Hordeum spontaneum desert ecotype to drought and excessive light intensity is characterized by induction of O2-dependent photochemical activity and anthocyanin accumulation. Plant Sci 201–202: 74–80. [DOI] [PubMed] [Google Scholar]
- 37. Krall JP, Edwards GE (1992) Relationship between photosystem-II activity and CO2 fixation in leaves. Physiol Plant 86: 180–187. [Google Scholar]
- 38.Schreiber U, Bilger W, Neubauer C (1995) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze E–D, Caldwell M, editors. Ecophysiology of photosynthesis. Berlin, Heidelberg: Springer. pp. 49–70.
- 39. Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130: 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flexas J, Ortuno MF, Ribas-Carbo M, Diaz-Espejo A, Florez-Sarasa ID, et al. (2007) Mesophyll conductance to CO2 in Arabidopsis thaliana . New Phytol 175: 501–511. [DOI] [PubMed] [Google Scholar]
- 41. Harley PC, Loreto F, Di Marco G, Sharkey TD (1992) Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol 98: 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Flexas J, Diaz-Espejo A, Galmes J, Kaldenhoff R, Medrano H, et al. (2007) Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ 30: 1284–1298. [DOI] [PubMed] [Google Scholar]
- 43. Villar R, Held AA, Merino J (1995) Dark leaf respiration in light and darkness of an evergreen and a deciduous plant species. Plant Physiol 107: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Messinger SM, Buckley TN, Mott KA (2006) Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiol 140: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mott KA, Sibbernsen ED, Shope JC (2008) The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ 31: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 46. Jang JC, Sheen J (1994) Sugar sensing in higher plants. Plant Cell 6: 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fray RG, Wallace A, Grierson D, Lycett GW (1994) Nucleotide sequence and expression of a ripening and water stress-related cDNA from tomato with homology to the MIP class of membrane channel proteins. Plant Mol Biol 24: 539–543. [DOI] [PubMed] [Google Scholar]
- 48.Sade N, Galle A, Flexas J, Lerner S, Peleg G, et al. (2013) Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions. Planta DOI 10.1007/s00425-013-1988-8. [DOI] [PubMed]
- 49. Evans JR, vonCaemmerer S (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110: 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Evans JR, Voncaemmerer S, Setchell BA, Hudson GS (1994) The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Austral J Plant Physiol 21: 475–495. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression analysis of NtAQP1 in AQP1 transgenic line: Presence of NtAQP1 DNA, RNA and protein. (A) The presence of NtAQP1 was assayed by PCR using NtAQP1-specific primers; transgenic AQP1 plants yielded the expected 930-bp product. WT is a negative non-transformed wild-type plant. + stands for a positive PCR control with a plasmid containing NtAQP1. Ladder: 100-bp ladder. (B) cDNA of AQP1 was subjected to semi-quantitative PCR using NtAQP1-specific primers; Fwd-CCGGGCAGGTGTACTATCC, Rev-TGCCTGGTCTGTGTTGTAGAT. Amplification was performed using 35 PCR cycles. SlCyP (cyclophilin – accession; M55019) was used as a control. (C) Western blot analysis of protein extracts from AQP1 plants probed with NtAQP1-specific antibody (upper panel); Ponceau red staining of the Western blot indicating equal protein loading (lower panel). Western blot analysis and Ponceau staining were performed exactly as described in Sade et al. [22]).
(TIF)
Expression of the TRAMP is suppressed by AtHXK1 . Expression level of TRAMP (tomato ripening associated membrane protein, accession no. NM_001247210), the tomato NtAQP1 homolog, was determined by quantitative real-time PCR using cDNA extracted from leaves of WT and HK4 plants. Data are means of five independent biological repeats ± SE. Different letters indicate a significant difference (t test, P<0.05). SlCyP (cyclophilin) was used for normalization.
(TIF)