Abstract
Background
Cercopithifilaria bainae is a filarioid parasite that infects dogs, being transmitted by Rhipicephalus sanguineus group ticks in many countries of the Mediterranean basin. This study assessed the incidence density rate (IDR) of infection by C. bainae in dogs and the probability of co-infection with other tick-borne pathogens (i.e., Anaplasma platys, Babesia vogeli and Hepatozoon canis), in an area of high endemicity in southern Italy.
Methodology/Principal Findings
From March 2011 to October 2012, a field study involving 58 young dogs naturally exposed to tick infestation was conducted. Skin and blood samples obtained from each dog six times during an 18-month period were tested for C. bainae by parasite detection within skin snip sediments, with subsequent confirmation through PCR and DNA sequencing. Dogs examined monthly for ticks and A. platys, B. vogeli and H. canis were microscopically and/or molecularly diagnosed and after the first and the second summer seasons, the IDR for positive animal-month at risk was 3.8% and 1.7% in November 2011 and October 2012, respectively. All 58 C. bainae-infected dogs were simultaneously infected with at least one other tick-borne pathogen. After the first summer season (assessment in November 2011), a C. bainae-infected dog had a 33% probability of being infected with H. canis or A. platys, whereas after the second tick season (assessment in October 2012) the probability of co-infection was 78%, 22% and 11% for H. canis, A. platys and B. vogeli, respectively.
Conclusions
Our data indicate that tick-infested dogs are at risk of acquiring infection by C. bainae. In addition, the detection of C. bainae microfilariae indicates a prior tick exposure and, should stimulate testing for other tick-borne disease causing pathogens.
Introduction
The genus Cercopithifilaria comprises 28 species of filarial nematodes that live beneath the skin of wild and domestic mammals (e.g., ruminants, cercopithecid primates, carnivores, rodents and marsupials) [1]. Dogs may be infected by Cercopithifilaria grassii, Cercopithifilaria sp. II and Cercopithifilaria bainae [2], [3], [4], with the latter species having a prevalence of infection reaching as high as 45.4% in some dog populations from the Mediterranean area [2], [5]. Indeed, the distribution of C. bainae overlaps that of tick vectors belonging to the Rhipicephalus sanguineus group. In the vector, microfilariae reach the infective larval stage (L3) within approximately 30 days [6], [7].
Although the pathological relevance of this filarioid has not been clearly defined, histological findings suggest that microfilariae may cause mild focal epidermal and subepidermal edematous changes and a perivascular interstitial dermatitis, characterized by the presence of neutrophils, eosinophils and lymphocytes [8]. Moreover, it is not clear whether the infection by C. bainae affects the dog's immune response to other tick-borne pathogens or vice versa, facilitating their acquisition during the tick bite. However, given the paucity of data available about the biological behavior of this nematode under natural conditions, this study aimed to assess, for the first time, the incidence of C. bainae infection in dogs naturally exposed to R. sanguineus group ticks and to examine the probability of co-infections with other tick-borne pathogens (i.e., Anaplasma platys, Babesia vogeli and Hepatozoon canis), in dogs residing in an area of high tick infestation pressure from southern Italy.
Materials and Methods
This study was carried out in a private shelter in the municipality of Putignano (40°51′N, 17°07′E), province of Bari, Apulia region, southern Italy. This area has a high prevalence of canine tick-borne pathogens and dogs maintained in this shelter were constantly exposed to ticks [9], [10].
This research was an extension of studies previously performed [8], [11]. Sampled animals (n = 58) included young dogs (≤6 months old) of both genders, which were dewormed (Drontal plus®; Bayer AG, Germany) and vaccinated (Duramune® DAPPI + LC; Fort Dodge Animal Health, Italy) at their enrolment (March-May 2011). Each dog was clinically examined, searched for ticks, and sampled for skin and blood six times during the study (i.e., at the baseline in March-May 2011, in July, September, November 2011, and April, October 2012), following the procedures previously described [11], [12]. Dogs were examined monthly (from March 2011 to October 2012) for the presence of ticks by thumb counting of the following body regions: head, ears, breast-neck, thorax, abdomen, fore and back limbs, inter-digital areas, armpit, tail and inguinal area [11], and ticks collected were morphologically identified [13], [14].
Cercopithifilaria spp. larvae were detected by microscopic examination of skin snips sampled from the inter-scapular region, soaked in saline solution (pH 7.4) and processed as described elsewhere [8]. Briefly, skin snips were soaked in saline solution for about 6 hours at room temperature, and the sediment (20 µl) was examined microscopically at different magnifications (10 and 40×). The microfilariae species were identified morphologically and confirmed by PCR amplification and DNA sequencing [3], [8]. Infections with other tick-borne pathogens (i.e., A. platys, B. vogeli and H. canis) were confirmed by cytological examination of stained blood smears or by PCR amplification and DNA sequencing of selected amplicons, as described elsewhere [11].
The incidence density rate (IDR) of infection was calculated based on the number of dogs infected with Cercopithifilaria spp. larvae at each sampling time point divided by the number of dog-months of follow-up, as previously described [11]. In addition, co-infections with other pathogens were statistically analyzed at two sampling times following the peak of tick infestation (i.e., November 2011 and October 2012), without considering transient positivity that may have occurred at interim sampling times. The conditional probability of co-infections was calculated by assessing the absolute frequency of positive animals, based on the Bayes' theorem using the statistical software R version 2.15.2 [15].
The study was conducted according to the principles of Good Clinical Practice (VICH GL9 GCP, 2000 http://www.emea.eu.int/pdfs/vet/vich/059598en.pdf) in the guideline for the testing and evaluation of the efficacy of antiparasitic substances for the treatment and prevention of tick and flea infestation in dogs and cats (http://www.emea.eu.int/pdfs/vet/ewp/000500en.pdfl) and the guideline on Statistical Principles for Veterinary Clinical Trials (CVMP/816/00, 2000 www.emea.eu.int/pdfs/vet/ewp/081600en.pdf). The study design and the experimental procedures were approved and authorized by the Italian Ministry of Health (authorization number DGSA n°. 0001997; 04/02/2011).
Results and Discussion
At baseline (March-May 2011), Cercopithifilaria spp. microfilariae were not visualized in skin snips from any of the young dogs enrolled. The highest IDR values for positive animal-month at risk (Table 1), were found in November 2011 and October 2012, (i.e., 3.8% and 1.7%, respectively), after the first and the second summer seasons, which, in turn, correspond to the highest number of ticks found on dogs in the study area [9]. Based on morphological characteristics, PCR amplification and sequencing (data not shown), microfilariae were identified as C. bainae (Fig. 1A). New cases of C. bainae infection in dogs occurred throughout the year in the study area, in accordance with the ongoing presence of the tick vector. The finding of dermal microfilariae at the first sampling time (July 2011) in animals younger than 6 months of age indicates that the pre-patent period of C. bainae is short, at least less than 6 months, as suggested in a previous study [8]. In addition, the highest IDR found in November 2011 (i.e., 3.8%) occurred six months after the time (i.e., June), in which the highest prevalence of ticks harboring infective stages of C. bainae was confirmed by tick dissection [16]. Although requiring confirmation, this period of prepatency is similar to that reported for Cercopithifilaria rugosicauda, which infects roe deer in Europe [17].
Table 1. Incidence density rate (IDR) of positive animal-month at risk for Cercopithifilaria bainae-infected dogs throughout the study period.
| Sampling time | Number of dogs* | Number of new cases | Dog-months of follow-up | IDR |
| Baseline (March-May 2011) | 58 | 0 | – | – |
| July 2011 | 58 | 2 | 174 | 1.1 |
| September 2011 | 53 | 0 | 106 | 0 |
| November 2011 | 52 | 4 | 104 | 3.8 |
| April 2012 | 51 | 4 | 255 | 1.5 |
| October 2012 | 48 | 5 | 288 | 1.7 |
These numbers represent the negative dogs that have been included in the cohort for calculating the IDR at each sampling time.
Figure 1. Tick-borne pathogens identified during the study.
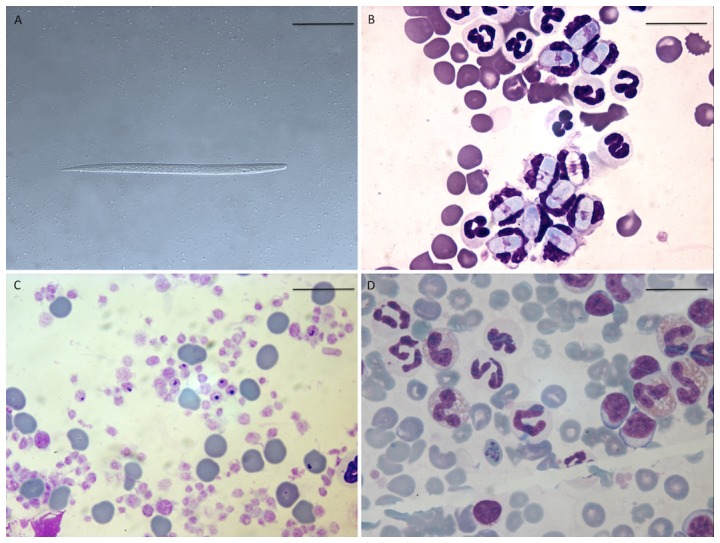
(A) First-stage larvae (L1) of Cercopithifilaria bainae retrieved in a skin snip of a dog (scale-bar = 50 µm); (B) Hepatozoon canis gamont (scale-bar = 20 µm); (C) Anaplasma platys (scale-bar = 20 µm).; (D) Babesia vogeli trophozoites retrieved at blood-smear examination (scale-bar = 20 µm).
All 15 C. bainae-infected dogs were also cytologically and/or PCR-positive for other tick-borne pathogens (i.e., A. platys, B. vogeli and H. canis) at one or more follow-up time points. Of four dogs infected with C. bainae in November 2011, two were infected with H. canis (Fig. 1B) and the other two with A. platys (Fig. 1C). Therefore, based on the Bayes' theorem, a dog positive for C. bainae presented a 33% probability of being infected with H. canis or A. platys. After the second tick season (October 2012), co-infections were documented in nine dogs, four of which were new C. bainae cases. Seven of nine dogs were co-infected with H. canis (77.7%), and two dogs (22.2%) were co-infected with A. platys, of which one was also infected with B. vogeli (11.1%; 1/9) (Fig. 1D). Therefore, the probability that a C. bainae-infected dog was concurrently infected with another tick-borne pathogen was 78%, 22% and 11% for H. canis, A. platys and B. vogeli, respectively. Of 48 and 39 animals negative for C. bainae in November 2011 and October 2012, respectively, 17 (35.4 %) and 21 (53.8 %) were positive for one or more TBD causing pathogen.
Co-infections in dogs with pathogens transmitted by ticks is a frequent occurrence in southern Italy [11], [18] and this study indicates that C. bainae-infected dogs are at risk for prior, current or future co-infections with other pathogens (i.e., A. platys, B. vogeli and H. canis). The high probability that C. bainae-infected dogs were co-infected with H. canis during the first (33%) and second (78%) tick seasons also indicates a high risk of environmental tick infestation. Although all dogs were infested by R. sanguineus group ticks during any time within the study period, the highest prevalence of tick infestation in C. bainae-positive dogs (100%; 8/8) was recorded in April, May and August 2012, whereas the lowest infestation level (25%; 1/4) was in January and February of the same year, when ticks are less prevalent in this area [9].
In spite of erythematous and papular dermatitis reported in infected animals [8], the pathogenic role of C. bainae in dogs remains to be elucidated. Although this filarioid is regarded as minimally pathogenic, the detection of dermal microfilariae provides evidence for a previous tick infestation and should stimulate consideration of infections with other tick-borne pathogens. However, whether C. bainae infection adversely affects the dog's immune response to other TBD causing pathogens, or vice versa, deserves to be studied.
Acknowledgments
This study was conducted under the frame of the EurNegVec COST Action TD1303.
Funding Statement
This work was supported by Bayer Animal Health (Germany). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bain O, Uni S, Takaoka H (2002) A synthetic look at a twenty years old taxon, Cercopithifilaria its probable evolution. In: Proceedings of the 10th International Congress of Parasitology (ICOPA) Vancouver: Monduzzi Editore. pp. 365–368.
- 2. Otranto D, Brianti E, Dantas-Torres F, Weigl S, Latrofa MS, et al. (2011) Morphological and molecular data on the dermal microfilariae of a species of Cercopithifilaria from a dog in Sicily. Vet Parasitol 182: 221–229. [DOI] [PubMed] [Google Scholar]
- 3. Otranto D, Brianti E, Dantas-Torres F, Miró G, Latrofa MS, et al. (2012) Species diversity of dermal microfilariae of the genus Cercopithifilaria infesting dogs in the Mediterranean region. Parasitology 140: 99–108. [DOI] [PubMed] [Google Scholar]
- 4. Otranto D, Varcasia A, Solinas C, Scala A, Brianti E, et al. (2013) Redescription of Cercopithifilaria bainae Almeida & Vicente, 1984 (Spirurida, Onchocercidae) from a dog in Sardinia, Italy. Parasit Vectors 6: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Otranto D, Brianti E, Latrofa MS, Annoscia G, Weigl S, et al. (2012) On a Cercopithifilaria sp. transmitted by Rhipicephalus sanguineus: a neglected, but widespread filarioid of dogs. Parasit Vectors 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brianti E, Otranto D, Dantas-Torres F, Weigl S, Latrofa MS, et al. (2012) Rhipicephalus sanguineus (Ixodida, Ixodidae) as intermediate host of a canine neglected filarial species with dermal microfilariae. Vet Parasitol 10: 330–337. [DOI] [PubMed] [Google Scholar]
- 7. Ramos RA, Giannelli A, Brianti E, Annoscia G, Cantacessi C, et al. (2013) Tick vectors of Cercopithifilaria bainae in dogs: Rhipicephalus sanguineus sensu lato versus Ixodes ricinus . Parasitol Res 112: 3013–3017. [DOI] [PubMed] [Google Scholar]
- 8. Otranto D, Brianti E, Abramo F, Gaglio G, Napoli E, et al. (2012) Cutaneous distribution and localization of Cercopithifilaria sp. microfilariae in dogs. Vet Parasitol 190: 143–150. [DOI] [PubMed] [Google Scholar]
- 9. Lorusso V, Dantas-Torres F, Lia RP, Tarallo VD, Mencke N, et al. (2010) Seasonal dynamics of the brown dog tick, Rhipicephalus sanguineus, on a confined dog population in Italy. Med Vet Entomol 24: 309–315. [DOI] [PubMed] [Google Scholar]
- 10. Dantas-Torres F, Otranto D (2011) Rhipicephalus sanguineus on dogs: relationships between attachment sites and tick developmental stages. Exp Appl Acarol 53: 389–389. [DOI] [PubMed] [Google Scholar]
- 11. Dantas-Torres F, Capelli G, Giannelli A, Ramos RA, Lia RP, et al. (2013) Efficacy of an imidacloprid/flumethrin collar against fleas, ticks and tick-borne pathogens in dogs. Parasit Vectors 6: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Otranto D, Dantas-Torres F, de Caprariis D, Di Paola G, Tarallo VD, et al. (2013) Prevention of canine leishmaniosis in a hyper-endemic area using a combination of 10% imidacloprid/4.5% flumethrin. PLoS One 8: e56374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker JB, Keirans JE, Horak IG (2000) The genus Rhipicephalus (Acari, Ixodidae): a guide to the brown ticks of the world. Cambridge: Cambridge University Press.
- 14. Dantas-Torres F, Latrofa MS, Annoscia G, Giannelli A, Parisi A, et al. (2013) Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasit Vectors 6: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R Core Team (2012) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- 16.Ramos RA, Giannelli A, Carbone D, Baneth G, Dantas-Torres F, et al. (2013) Seasonal occurrence of Hepatozoon canis and Cercopithifilaria bainae in Rhipicephalus sanguineus group ticks from the environment. Ticks Tick-borne Dis “In press”. [DOI] [PubMed]
- 17. Winkhardt HJ (1980) The larval development of Dipetalonema rugosicauda (syn, Wehrdikmansia rugosicauda) in the tick Ixodes ricinus. II. The development of Dipetalonema rugosicauda in Ixodes ricinus and investigations about the occurrence of the microfilariae in the roe deer (C. capreolus). Z Tropenmed Parasitol 31: 21–30. [PubMed] [Google Scholar]
- 18. De Tommasi AS, Otranto D, Dantas-Torres F, Capelli G, Breitschwerdt EB, et al. (2013) Are vector-borne pathogen co-infections complicating the clinical presentation in dogs? Parasit Vectors 6: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]


