Abstract
The concept that invasive cancer is associated with increased levels of reactive oxygen species (ROS) generated by mitochondria is consistent with an ROS-mediated signaling mechanism. As a tumor grows, it encounters adverse microenvironments, one of which is low oxygen (hypoxia), which selects tumor cells with characteristics of increased invasiveness. Hypoxic environments select for tumor cells with stabilized HIF1 apha, a transcription factor that regulates genes coding for pro-tumor cytokines that signal stromal cells such as macrophages and fibroblasts to support an invasive tumor cell phenotype. HIF1 alpha-mediated switches in the energy production of tumor cells from OXPHOS to glycolysis, as well as age-associated decreases in the metabolic rate of the host, enhance invasive qualities of tumor cells. An increase in environmental oxygen in combination with a mitochondrial targeted catalase mimetic and a metabolism booster may be of interest to investigate as a treatment strategy for invasive cancer.
Keywords: Mitochondria, Cancer invasiveness, Redox signaling, Hypoxia, HIF1 alpha
The concept that increased levels of reactive oxygen species (ROS) generated by mitochondria increase cancer invasiveness is consistent with an ROS-mediated signaling mechanism rather than the more popular theory of ROS-mediated cellular stress and molecular damage. We have both published and unpublished data that support this signaling concept using transgenic mice that express mitochondrial targeted catalase (mCAT). We showed that mCAT transgenic mice had increased lifespan compared to wild type littermates (Schriner et al. 2005) and pathological findings revealed that mCAT decreased the incidence of spontaneous epithelial tumors in old mice (Treuting et al. 2008). We validated the anti-tumor effect of mCAT using a transgenic mouse model of invasive breast cancer (PyMT) which showed a robust decrease in invasiveness and metastasis of breast cancer (Goh et al. 2011). In this article, we present evidence and argue for mitochondrial redox signaling as a cellular messaging system for tumor cells to promote their invasiveness.
Hypoxia resistance is mediated by mitochondrial hydrogen peroxide
As a tumor grows, it encounters adverse microenvironments, one of which is low oxygen (hypoxia). These environments select for tumor cells with altered genotypes, which display characteristics of increased invasiveness. For example, tumors developed in hypoxic environments are more likely to metastasize than those that are well oxygenated (Hockel et al. 1996; Brizel et al. 1997), and they are also known to develop high glycolytic activity, believed to be important for tumor progression (Dang and Semenza 1999). Mitochondria are now considered to play a role in oxygen sensing, especially hypoxia (Murphy 2009). It is known that the production of superoxide by the respiratory complex (probably complex 3) increases under conditions of low oxygen levels (Chandel et al. 2000). We compared the ability of mCAT to impair PyMT tumor cell growth under ambient and reduced oxygen conditions. PyMT cells thrived under 2 per cent oxygen compared to 20 per cent oxygen, but PyMT cells expressing mCAT were less adapted to grow under the hypoxic conditions. This observation suggests that cancer cells promote their own hypoxia resistance, which is linked to invasiveness, by inducing the production of super oxide, which is converted to hydrogen peroxide (Fig. 1), and that mCAT attenuates the hydrogen peroxide thereby reducing the hypoxia resistance. Therefore, the presence of mCAT in PyMT tumor cells translates to reduced invasiveness and is consistent with our published data in PyMT transgenic mice with metastatic breast cancer (Goh et al. 2011).
Fig. 1.
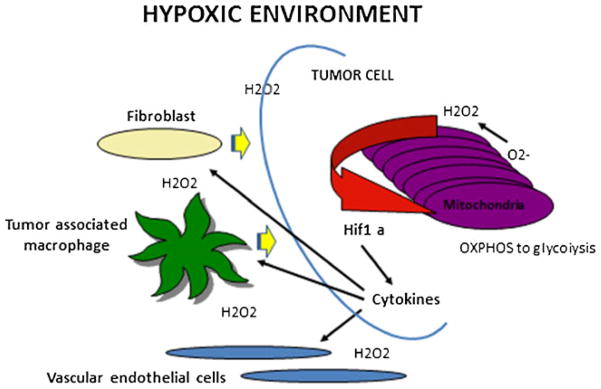
An hypoxic tumor microenvironment drives cancer invasiveness through mitochondrial redox signaling. The production of super oxide by mitochondrial respiratory III complex increases under conditions of low oxygen, ie, hypoxia. Superoxide is not the main signal because most of it is rapidly converted to hydrogen peroxide by ample supplies of mitochondrial SOD2. Hydrogen peroxide can easily pass through mitochondrial and cellular membranes so it can act as a major signal from mitochondria to the cytosol and into the extracellular space to affect other cell types. In addition, hydrogen peroxide stabilizes HIF1 alpha thus leading to transcription of genes that code for pro-tumor cytokines that signal stromal cells such as macrophages and fibroblasts to support an invasive tumor cell phenotype. Hydrogen peroxide-mediated HIF1 alpha helps the tumor cell convert energy production from OXPHOS to glycolysis, a metabolic switch that has been associated with increased metastatic potential
We have shown a down regulation of hypoxia inducible factor 1 alpha (HIF1alpha) in PyMT tumor cells expressing mCAT, substantiating the role of ROS in HIF1alpha-mediated tumor growth (Fig. 1). Hypoxia inducible factor (HIF) is the master regulator for hypoxia-induced gene expression. HIF-1, the first member of the family, is a heterodimeric protein consisting of a constitutively expressed subunit, HIF-1beta, and an oxygen-sensitive inducible subunit, HIF-1alpha. HIF-1alpha protein is usually degraded under normal oxygen concentrations (normoxia), but in hypoxic conditions, HIF-1alpha protein accumulates and associates with HIF-1beta, triggering the transcription of a host of hypoxia-inducible genes (Chi et al. 2006). As a solid tumor grows in size, the central cells begin to experience hypoxia, stabilize HIF1alpha, and turn on downstream genes, including those involved in recruiting blood vessels to growing tumors and those encoding proteins with central roles in glucose transport (glucose transporters), metabolism, differentiation, cell proliferation, apoptosis, extracellular matrix synthesis and other significant biological processes (Fig. 1). Changes in the expression of all of these genes, a signature of the hypoxia response, are a strong predictor of clinical outcomes in breast and ovarian cancers (Chi et al 2006).
In addition to these molecular changes, a number of physiological changes have been shown at the cellular level to occur in hypoxia-challenged cancer cells, and are believed to be important to their adaptation. Hypoxia engages a metabolic switch in cancer cells, orchestrated by HIF-1, whereby there is an induction of glycolysis, a suppression of mitochondrial mass, and an active repression of mitochondrial respiration that reduces oxygen consumption. Interfering with this adapation results in increased oxygen consumption and causes an increased sensitivity to apoptotic cell death under hypoxic conditions (Kim et al 2006; Papandreou et al 2006; Fukuda et al 2007; Zhang et al 2008). We used WST-1, a tetrazolium salt that is cleaved to formazan by cellular mitochondrial dehydrogenases, to measure mitochondrial activity as indicated by oxidative phosphorylation (OXPHOS). We have found that PyMT primary tumor cells, when transfected with the mCAT transgene, show much higher levels of oxidative phosphorylation. When grown under normoxic (20 per cent oxygen) conditions, both PyMT primary tumor cells with and without the mCAT transgene proliferate equally well, but when grown under hypoxic (2 per cent oxygen) conditions, PyMT tumor cells without the mCAT transgene proliferate at more than twice the rate of those with the mCAT transgene. The data suggest that the presence of the mCAT transgene prevents a down regulation of mitochondrial respiration, and attenuates the ability of PyMT tumor cells to adapt to a hypoxic environment.
Age-associated metabolic rate drives cancer progression
In addition to changes in mitochondrial redox signaling in the tumor cells themselves, tumor progression can be effected by host factors, including metabolic activity. We have shown that the resting metabolic rate of the host, measured by indirect calorimetry as the rate of oxygen consumption during the day, is tightly associated with the rate of tumor progression in the PyMT mouse breast cancer model. Mice with higher rates of oxygen consumption show lower rates of tumor progression (manuscript under review). This suggests that higher rates of oxygen consumption lead to reduced hypoxia in the tumor microenvironment, reducing selection for hypoxia-resistant and hence invasive primary tumor cells. Since aging C57BL/6 mice display measurable decreases in oxygen consumption (unpublished observations), lower resting metabolic rates in aging mice should correspond with enhanced tumor progression.
Hyperoxia as a treatment strategy for invasive cancer
Mitochondria respond to hypoxia by inducing resistance and downstream signaling of tumor progression. It is therefore intriguing to consider how mitochondrial redox signaling might be involved in response to increased levels of oxygen, ie., hyperoxia. PyMT primary tumor cells, with and without the mCAT transgene, were allowed to grow in a hypoxic environment for 48 h, upon which time they were transferred to a 20 per cent oxygen environment. We found that PyMT primary tumor cells that had the mCAT transgene and had been maintained in a hypoxic environment, died when put back into ambient oxygen. This cell death did not occur in PyMT cells without mCAT (unpublished results). The cell death of PyMT/mCAT cells corresponded with a significant spike in their rate of mitochondrial respiration. The data suggest that in addition to preventing hypoxia resistance, mCATalso prevents a down regulation of oxidative phosphorylation, resulting in sensitivity to oxygen-induced apoptosis. Immunoblotting of total protein extracts from PyMT primary tumor cells, with and without the mCAT transgene and grown under normoxic, hypoxic, and hypoxic to normoxic conditions, showed that tumor cells with mCAT had protein phosphorylation levels indicative of reduced hypoxia resistance, including reduced phosphorylation of Akt, AMPK and increased phosphorylation of ERK2. AMPK is phosphorylated under hypoxic conditions and drives glycolysis (Luo et al 2005; Hardie 2007). ERK phosphorylation has been linked to small tumors with good prognosis (Svensson et al. 2005). Since the PyMT tumor cells are primary, and contain stromal cells, it is not yet known which cell types are involved in mediating the death signaling associated with the presence of mCAT but it is likely a combination.
In summary and relative to cancer, we suggest that mitochondrial ROS are generated by tumor cells not as a consequence of cellular damage, but as an active process to promote their rapid evolution towards a glycolytic and hypoxia resistant state. We also propose that this evolution of hypoxia resistance and glycolytic metabolism in tumor cells is associated with increased invasiveness, and that it can depend on the metabolic state of the host (Table 1). Hyperoxia in combination with a mitochondrial targeted catalase mimetic and a metabolism booster may be of interest to investigate as a strategy to treat invasive cancer.
Table 1.
A number of processes can affect mitochondrial redox signaling and subsequent alteration of cancer invasiveness. Intra-tumoral hypoxia is a major driving force in orchestrating the invasive attributes of tumor cells. It is thus expected that hyperoxia would have the opposite effect. Hypoxia is associated with more dependence on glycolysis for energy in contrast to OXPHOS. A high metabolic rate by the host suppresses invasiveness by attenuating hypoxia in the tumor microenvironment
| Process | Mittochondrial site | Effect on Invasiveness |
|---|---|---|
| Hypoxia | Tumor cells | Enhancement |
| OXPHOS | Tumor cells | Suppression |
| Glycolisis | Tumor cells | Enhancement |
| High metabolic rate | Host microenvironment | Suppression |
| Hypoxia | Tumor cells and microenvironment | Suppression |
References
- Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275(33):25130–8. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, Salim A, Wang Y, et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CD, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- Fukuda R, Zhang H, Kim JW, Shimoda L, Semenza GL, Dang CV. HIF-1 regulatescytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Goh J, Enns L, Fatemie S, Hopkins H, Morton J, Pettan-Brewer C, Ladiges W. Mitochondrial targeted catalase suppresses invasive breast cancer in mice. BMC Cancer. 2011;11:191. doi: 10.1186/1471-2407-11-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer. 1996 [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Meta. 2006 doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptati to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Svensson S, Jirström K, Rydén L, Roos G, Emdin S, Ostrowski MC, Landberg G. ERK phosphorylation is linked to VEGRF2 expression and Ets-2 phosphorylation in breast cancer a tumours with good prognosis. Oncogene. 2005;24:4370. doi: 10.1038/sj.onc.1208626. [DOI] [PubMed] [Google Scholar]
- Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Ladiges WC. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci. 2008;63:813. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


