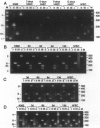
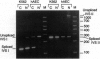
Abstract
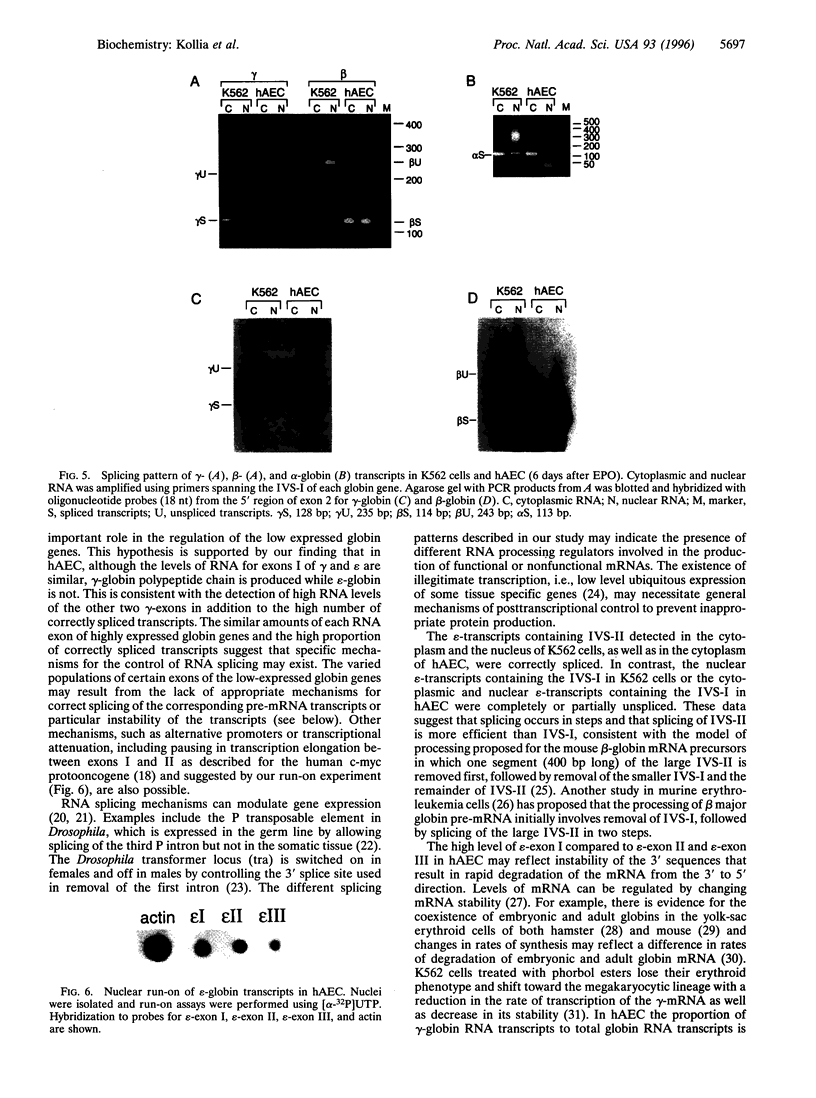
The developmental changes in hemoglobin gene expression known as "switching" involve both the sequential activation and silencing of the individual globin genes. We postulated that in addition to changes in transcription, posttranscriptional mechanisms may be involved in modulating globin gene expression. We studied globin RNA transcripts in human adult erythroid cells (hAEC to analyze the mechanism of silencing of the embryonic epsilon-globin gene in the adult stage and in K562 erythroleukemic cells to analyze the inactive state of their adult beta-globin genes. In hAEC, which express primarily the beta-globin gene, quantitative PCR analysis shows that beta-mRNA exon levels are high and comparable among the three exons; the RNA transcripts corresponding to exons of the gamma-globin gene are low, with slight differences among the three exons. Although epsilon-globin is not expressed, epsilon-globin RNA transcripts are detected, with exon I levels comparable to that of gamma-globin exon I and much higher than epsilon-exons II and III. As expected, in K562 cells that express high levels of epsilon- and gamma-globin, epsilon- and gamma-mRNA levels are high, with comparable levels of exons I, II, and III. In K562 cells beta-mRNA levels are very low but beta-exon I levels are much higher than that of exons II or III. Moreover, all or most of the globin transcripts for the highly expressed globin genes in both cell types (gamma and beta in hAEC, epsilon and gamma in K562 cells) found in the cytoplasm or nucleus are correctly processed. The globin transcripts that are detected both in the cytoplasm and nucleus of cells without expression of the corresponding protein are largely unspliced (containing one or two intervening sequences). These studies suggest that in addition to changes in transcription rates, changes in completion or processing of globin RNA transcripts may contribute to the developmental regulation of the hemoglobin phenotype.
Full text
PDF
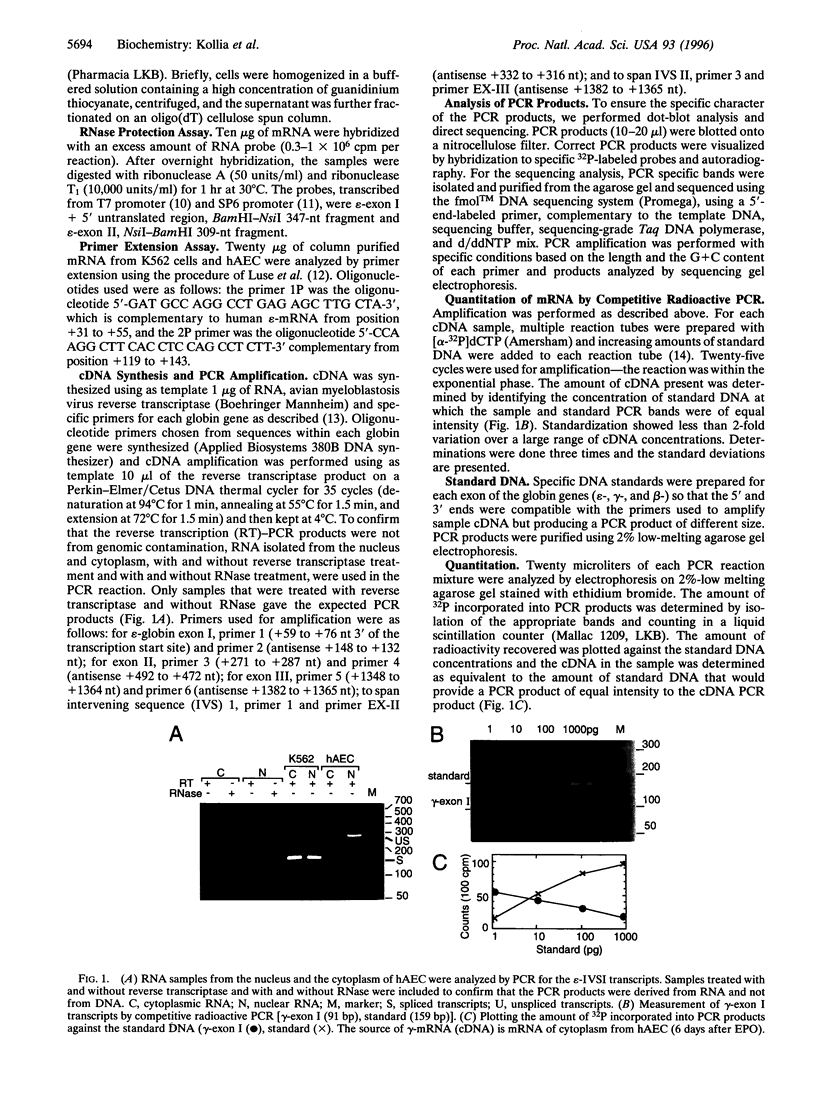

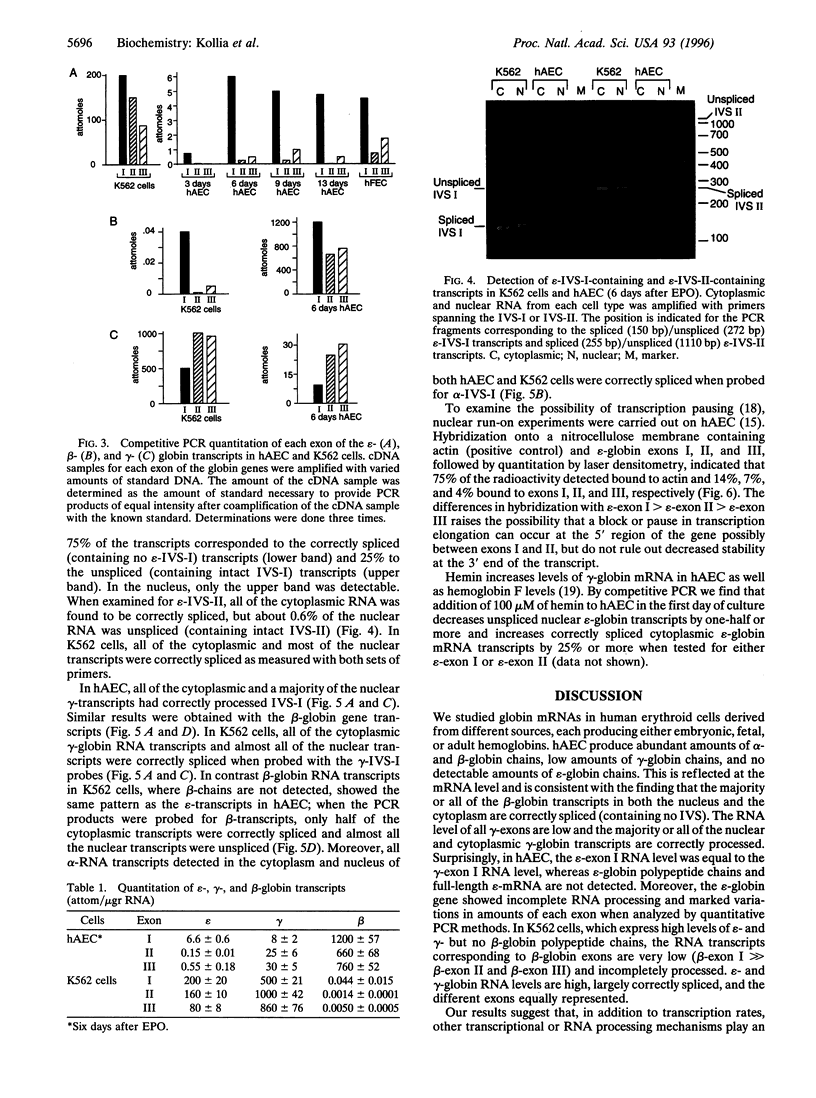

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albitar M., Peschle C., Liebhaber S. A. Theta, zeta, and epsilon globin messenger RNAs are expressed in adults. Blood. 1989 Aug 1;74(2):629–637. [PubMed] [Google Scholar]
- Allan M., Lanyon W. G., Paul J. Multiple origins of transcription in the 4.5 Kb upstream of the epsilon-globin gene. Cell. 1983 Nov;35(1):187–197. doi: 10.1016/0092-8674(83)90221-0. [DOI] [PubMed] [Google Scholar]
- Berg P. E., Schechter A. N. The impact of molecular biology on the diagnosis and treatment of hemoglobin disorders. Mol Genet Med. 1992;2:1–38. doi: 10.1016/b978-0-12-462002-5.50006-6. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Chou T. B., Mims I., Zachar Z. On/off regulation of gene expression at the level of splicing. Trends Genet. 1988 May;4(5):134–138. doi: 10.1016/0168-9525(88)90136-9. [DOI] [PubMed] [Google Scholar]
- Boggs R. T., Gregor P., Idriss S., Belote J. M., McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987 Aug 28;50(5):739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- Boussios T., Bertles J. F. The globin gene expression program in the hamster embryo. Exp Hematol. 1988 Jan;16(1):1–4. [PubMed] [Google Scholar]
- Boussios T., Condon M. R., Bertles J. F. Ontogeny of hamster hemoglobins in yolk-sac erythroid cells in vivo and in culture. Proc Natl Acad Sci U S A. 1985 May;82(9):2794–2798. doi: 10.1073/pnas.82.9.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton T. W., Chui D. H., Gauldie J., Patterson M. Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2853–2857. doi: 10.1073/pnas.76.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S. X., Gutman P. D., Dave H. P., Schechter A. N. Identification of a transcriptional silencer in the 5'-flanking region of the human epsilon-globin gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5306–5309. doi: 10.1073/pnas.86.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Maniatis T. Transcriptional regulation of globin gene expression in the human erythroid cell line K562. Science. 1983 Jun 17;220(4603):1281–1283. doi: 10.1126/science.6574602. [DOI] [PubMed] [Google Scholar]
- Dalyot N., Fibach E., Rachmilewitz E. A., Oppenheim A. Adult and neonatal patterns of human globin gene expression are recapitulated in liquid cultures. Exp Hematol. 1992 Oct;20(9):1141–1145. [PubMed] [Google Scholar]
- Dean A., Wu Y. J., Ley T., Fordis C. M., Schechter A. N. Augmentation of hemoglobin synthesis by S-phase specific drugs in the K562 cell line. Prog Clin Biol Res. 1985;191:205–216. [PubMed] [Google Scholar]
- Donaldson D. S., McNab A. R., Rovera G., Curtis P. J. Nuclear precursor molecules of the two beta-globin mRNAs in Friend erythroleukemia cells. J Biol Chem. 1982 Aug 10;257(15):8655–8660. [PubMed] [Google Scholar]
- Fibach E., Kollia P., Schechter A. N., Noguchi C. T., Rodgers G. P. Hemin-induced acceleration of hemoglobin production in immature cultured erythroid cells: preferential enhancement of fetal hemoglobin. Blood. 1995 May 15;85(10):2967–2974. [PubMed] [Google Scholar]
- Fibach E., Manor D., Oppenheim A., Rachmilewitz E. A. Proliferation and maturation of human erythroid progenitors in liquid culture. Blood. 1989 Jan;73(1):100–103. [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. Z., Rodgers G. P., Zeng F. Y., Zeng Y. T., Schechter A. N. Diagnosis of thalassemia using cDNA amplification of circulating erythroid cell mRNA with the polymerase chain reaction. Blood. 1991 Nov 1;78(9):2433–2437. [PubMed] [Google Scholar]
- Kabnick K. S., Housman D. E. Determinants that contribute to cytoplasmic stability of human c-fos and beta-globin mRNAs are located at several sites in each mRNA. Mol Cell Biol. 1988 Aug;8(8):3244–3250. doi: 10.1128/mcb.8.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. C., Kahn A., Chelly J. Illegitimate transcription: its use in the study of inherited disease. Hum Mutat. 1992;1(5):357–360. doi: 10.1002/humu.1380010502. [DOI] [PubMed] [Google Scholar]
- Kinniburgh A. J., Ross J. Processing of the mouse beta-globin mRNA precursor: at least two cleavage-ligation reactions are necessary to excise the larger intervening sequence. Cell. 1979 Aug;17(4):915–921. doi: 10.1016/0092-8674(79)90331-3. [DOI] [PubMed] [Google Scholar]
- Krumm A., Meulia T., Brunvand M., Groudine M. The block to transcriptional elongation within the human c-myc gene is determined in the promoter-proximal region. Genes Dev. 1992 Nov;6(11):2201–2213. doi: 10.1101/gad.6.11.2201. [DOI] [PubMed] [Google Scholar]
- Laski F. A., Rio D. C., Rubin G. M. Tissue specificity of Drosophila P element transposition is regulated at the level of mRNA splicing. Cell. 1986 Jan 17;44(1):7–19. doi: 10.1016/0092-8674(86)90480-0. [DOI] [PubMed] [Google Scholar]
- Lowe W. L., Jr, Roberts C. T., Jr, Lasky S. R., LeRoith D. Differential expression of alternative 5' untranslated regions in mRNAs encoding rat insulin-like growth factor I. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8946–8950. doi: 10.1073/pnas.84.24.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumelsky N. L., Forget B. G. Negative regulation of globin gene expression during megakaryocytic differentiation of a human erythroleukemic cell line. Mol Cell Biol. 1991 Jul;11(7):3528–3536. doi: 10.1128/mcb.11.7.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luse D. S., Haynes J. R., VanLeeuwen D., Schon E. A., Cleary M. L., Shapiro S. G., Lingrel J. B., Roeder R. G. Transcription of the beta-like globin genes and pseudogenes of the goat in a cell-free system. Nucleic Acids Res. 1981 Sep 11;9(17):4339–4354. doi: 10.1093/nar/9.17.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattox W., Ryner L., Baker B. S. Autoregulation and multifunctionality among trans-acting factors that regulate alternative pre-mRNA processing. J Biol Chem. 1992 Sep 25;267(27):19023–19026. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei R., Calame K. Differential stability of c-myc mRNAS in a cell-free system. Mol Cell Biol. 1988 Jul;8(7):2860–2868. doi: 10.1128/mcb.8.7.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz S. W., Brewer G., Kobs G., Ross J. Substrate specificity of the exonuclease activity that degrades H4 histone mRNA. J Biol Chem. 1987 Jul 5;262(19):9382–9388. [PubMed] [Google Scholar]
- Petersen D. D., Koch S. R., Granner D. K. 3' noncoding region of phosphoenolpyruvate carboxykinase mRNA contains a glucocorticoid-responsive mRNA-stabilizing element. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7800–7804. doi: 10.1073/pnas.86.20.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich N., Papayannopoulou T., Stamatoyannopoulos G., Enver T. Demonstration of a human epsilon-globin gene silencer with studies in transgenic mice. Blood. 1992 Feb 15;79(4):861–864. [PubMed] [Google Scholar]
- Rutherford T., Clegg J. B., Higgs D. R., Jones R. W., Thompson J., Weatherall D. J. Embryonic erythroid differentiation in the human leukemic cell line K562. Proc Natl Acad Sci U S A. 1981 Jan;78(1):348–352. doi: 10.1073/pnas.78.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A. B. Messenger RNA degradation in eukaryotes. Cell. 1993 Aug 13;74(3):413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- Scarpati E. M., Sadler J. E. Regulation of endothelial cell coagulant properties. Modulation of tissue factor, plasminogen activator inhibitors, and thrombomodulin by phorbol 12-myristate 13-acetate and tumor necrosis factor. J Biol Chem. 1989 Dec 5;264(34):20705–20713. [PubMed] [Google Scholar]
- Wada-Kiyama Y., Peters B., Noguchi C. T. The epsilon-globin gene silencer. Characterization by in vitro transcription. J Biol Chem. 1992 Jun 5;267(16):11532–11538. [PubMed] [Google Scholar]