Abstract
Water soluble carbon nanotubes have been found to be one of the most promising nanomaterials in biological and biomedical based applications. However, there have been major concerns on their ability to cause cellular and DNA damages upon exposure. In this work, we explore the toxic effects of three multi-wall carbon nanotubes (MWCNTs: non-purified, purified, and carboxylate-functionalized) to human skin keratinocyte cells (HaCaT). Cytotoxicity tests using the conventional MTT and the water-soluble tetrazolium (WST-1) assays for 0.5 or 24 hrs exposure to 20 μg/mL of MWCNTs show that all three caused minimum cytotoxicity that is generally not statistically significant. Assessment of direct and oxidative DNA damages using both alkaline Comet assay and formamidopyridine-DNA glycosylase modified Comet assay reveals that treatment with 20 μg/mL of MWCNTs does not cause significant direct DNA damages, but causes great amount of oxidative DNA damages in HaCaT cells. The oxidative DNA damage reaches the maximum amount at 4 hrs of incubation in DMEM, but decreases to the minimum at 8 and 24 hrs of incubation, indicating repair of the oxidative damages by the intrinsic DNA repair mechanism of the cells.
Keywords: Carbon nanotubes, cytotoxcity, DNA damage, oxidative DNA damage
Introduction
Single or multiple sheets of graphite roll to form seamless cylinders are called carbon nanotubes (CNTs). A single sheet forms a single-walled carbon nanotube (SWCNT), while multiple sheets form a multi-walled carbon nanotube (MWCNT). Since the discovery of CNTs, researchers have been exploring their physical properties to understand the potential to improve existing products and enable new ones, especially in biomedical and materials research (Bianco et al. 2005; Martin and Kohli 2003; Pantarotto et al. 2004; Prato and Kostarelos 2008; Singh et al. 2006). For biomedical applications, the lack of solubility of CNTs in aqueous media has been a major barrier (Bandyopadhyaya et al. 2002; Sayes et al. 2006; Smart et al. 2006). CNTs are difficult to dissolve due to their tendency to form aggregates by strong Van der Waals interactions (Soto et al. 2007). Recent exploration to chemically modified CNTs has made it possible to solubilize or disperse them into aqueous media, thus opening the path for facile manipulation and processing under physiological conditions (Bandyopadhyaya et al. 2002; Singh et al. 2006).
However, the emphasis on benefits has been offset by debates over safety of CNTs (Jia et al. 2005; Johnston et al. 2010; Mocan et al. 2010; Ray et al. 2009). Humans may be subject to CNTs exposure by ingestion, dermal contact, and inhalation. Because of their small size, extremely large surface area, and modifiability, CNTs may induce greater chemical reactivity, permeability, and conductivity in biological systems (Jia et al. 2005; Monteiro-Riviere and Inman 2006; Murray et al. 2009; Pacurari et al. 2008). Previous investigations reported conflicting results on toxicity of CNTs (Cui et al. 2010; Hu et al. 2010; Johnston et al. 2010; Monteiro-Riviere and Inman 2006; Pulskamp et al. 2007; Vankoningsloo et al. 2010; Wick et al. 2007). It was reported that raw CNTs has pulmonary toxicity on lung tissue of mice (Chou et al. 2008; Mitchell et al. 2007; Shvedova et al. 2005; Warheit et al. 2004). Another study suggests that CNTs may be toxic to immortalized HaCaT human keratinocyte cells. Coated and water soluble SWCNTs are not toxic (Bardi et al. 2009), but some of the oxidative carbon materials contained in the mixture was toxic (Wang et al. 2011a). However, other studies showed that MWCNTs elicit DNA damage and inflammatory response relative to their size and shape (Yamashita et al. 2010), inducing DNA damage in embryonic stem cells (Zhu et al. 2007), RAW 264.7 cells (Migliore et al. 2010), normal human dermal fibroblasts (Ding et al. 2005; Patlolla et al. 2010; Shvedova et al. 2005; Tian et al. 2006), and mesothelial cells (Pacurari et al. 2008). Although, these studies have reported findings concerning MWCNT’s toxicity and their ability to induce DNA damage, there are no reports on toxicity study of carboxylate-functionalized MWCNTs. The purpose of this study is to explore toxic effects of carboxylate-modified, water-soluble MWCNTs to human skin keratinocyte cells.
Experiment methods
Materials
MWCNTs (Non-Purified, Purified, and Functionalized with COOH) were provided as a gift by Dr. Somenath Mitra (New Jersey Institute of Technology). HaCaT keratinocytes, a transformed human epidermal cell line, were obtained from Dr. Norbert Fusenig of the German Cancer Research Centre (Heidelberg, Germany). Trypsin/EDTA solutions were purchased from Cambrex Bio Science (Walkersville, MD). Fetal Bovine Serum (FBS), Dulbecco’s minimum essential medium (DMEM), Penicillin/streptomyocin, dimethyl sulfoxide (DMSO) and phosphate buffered saline (PBS) were from Fisher Scientific (Houston, TX). Thiazolyl blue tetrazolium bromide (MTT) was obtained from Sigma-Aldrich. Standard Comet assay, Flare Comet assay, and E. coli formamidopyridine-DNA glycosylase (FPG) kits were obtained from Trevigen™. WST-1, 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate, was purchased from Roche.
HaCaT Cell Culture
Culture of HaCaT cells followed previously published procedure with modifications (Wang et al. 2007). Prior to culturing, frozen passage 42–50 keratinocyte cells were removed from liquid nitrogen (−196 °C) cryo-preserve chamber. Each vial was placed in warm water (37°C) to undergo rapid thawing for high cell survival. Cells were placed in 25 cm2 flasks and grown in culture media containing DMEM, 10% FBS, 1% penicillin at 37°C in a tissue culture incubator (5% CO2) until an 85–90% confluent monolayer was formed. Cells were then trypsinized with 0.25% trysin/EDTA. HaCaT cell suspensions (2 × 105 cells/mL) were added to each well of a 96-well or 6 well plates and incubated for 24 hrs before treatments to insure cell adhesion.
HaCaT Cell Treatment with MWCNTs
Stock solutions of MWCNTs at approximately 1 mg/mL were prepared in 1 × PBS by sonication for 30 min to ensure proper dispersion. It was diluted with 1x PBS to achieve a concentration of 20 μg/mL. Control samples and MWCNT suspensions were loaded into 96 well plates where HaCaT cells were attached. Each plate was divided into four treatment regions with one control group and three treatment groups. The control cells were treated with 100 μL of 1x PBS buffer while others with 100 μL of 20 μg/mL suspensions of non-purified, purified, or COOH functionalized MWCNTs for 75 min. Following treatment, the wells were washed 3 times with 1 × PBS to remove most of the MWCNT particles. Thereafter, 200 μL of DMEM medium without FBS was added to each well and incubated for 30 min or 24 hrs before cytotoxicity testing.
MTT Assay
The viability of HaCaT cells subsequent to MWCNT treatments was determined using the MTT assay as described (Wang et al. 2011b). Briefly, a 50 μL MTT solution (5 mg/mL in PBS buffer) was added to each well and incubated for 30 min at 37°C with 5% CO2, allowing viable cells to convert the pale yellow MTT to the insoluble purple dye (formazan). After incubation, all MTT and DMEM solution was aspirated from the wells and 200 μL of DMSO was added to the plate and incubated for 10 min to dissolve the formazan salt, which is proportional to the number of viable cells. The plate was read using a Biosystem Fluoroskan II Microplate Reader (Helsinki, Finland) at 550 nm.
Since MWCNT also absorbs light at 550 nm (Gandra et al. 2009), and some MWCNT debris remain on the skin cell surface, this may interfere with measurement for the absorption of the formazan at 550 nm. To eliminate this, supernatants were transferred to a separate plate and absorbance values of these supernatants were read. These results were compared to the direct readings in the presence of the cells.
WST-1 Assay
For WST-1 assay, Roche Applied Sciences standard protocol was used. Cells were treated the same way as the MTT assay. Following treatment, 90 μL of DMEM and 10 μL of WST-1 reagent was added to each well and incubated for 2 hrs at 37°C in a 5% CO2 incubator. The absorbance of formazan dye is read using a Biosystem Fluoroskan II Microplate Reader at 450 nm.
Alkaline Comet Assay
HaCaT cells were plated in 6 well plates (culture area 9.6 cm2/well, culture medium 2 mL/well) one day before exposure. Semi-confluent cultures were exposed for 30 min or 24 hrs with 20 μg/mL of MWCNTs. Untreated controls and positive controls treated with 25 μM KMnO4 were included in all series. The Comet assay was performed under alkaline conditions following the Trevigen protocol. Briefly, after exposure to MWCNTs, the cells were allowed to incubate for 0, 4, 8, and 24 hrs in DMEM. The incubation should allow cells to repair damaged DNA, thus providing information for time-dependent DNA damage and repair. Then the cells were trypsinized by 0.25% trysin/EDTA. Culture medium (0.5 mL) was added to avoid over-trypsinizing and centrifugation at 1000 rpm for 5 min to collect cell pellets. HaCaT cells were resuspended in 1x PBS and added to 75 μL of molten (37 °C) 0.5% low-melting-point agarose gel to achieve a cell concentration of 1×105 cells/mL. The agarose was pipetted onto the Comet slides. Slides were stored in the dark at 4°C for 10 min before adding pre-chilled lysis buffer for 45 min. The slides were immersed in freshly prepared alkaline solution (0.25 M NaOH containing 0.1 μM EDTA, pH 12.6) for 30 min at room temperature. Slides were then removed and washed twice with alkaline solution for 5 min. Gel electrophoresis was performed at 1 V/cm for 30 min (running amperage 3–5 mA with the distance between the two electrodes of 25 cm). The Comet slides were washed with 70% ethanol for 5 min and air-dried for 2.5 h at room temperature. A 50 μL of diluted SYBR Green solution was placed onto each dried agarose circle. The slides were then read with a fluorescence microscope equipped with the Lotus DNA Damage Analysis Software. A total of 75 cells/sample were scored to determine the average percentage of DNA damaged.
FPG Comet Assay
The standard alkaline Comet assay described in the previous section was carried out with addition of the E. coli formamido-pyrimidine-DNA glycosylase (FPG) for detection of oxidative DNA damages. After lyses, Comet slides were immersed in freshly prepared 1 x flare buffer at room temperature to equilibrate the slides. The flare buffer was changed 3 times over a 30 min period. Slides were removed from the flare buffer and 75 μL of working FPG enzyme solution (1:100 dilution of FPG activity:4 U/uL) was added to each sample and incubated at 37 °C for 45 min. Afterwards, slides were immersed in freshly prepared alkaline solution (0.25 M NaOH containing 0.1 μM EDTA, pH 12.6) for 30 min at room temperature identical to the standard Comet assay. Percent of DNA damage was scored the same way as the alkaline Comet assay.
Statistical Analysis
The MTT and WST-1 assays were replicates of three experiments with the same parameters. The Comet assays were replicated at four independent time intervals. All data were presented as mean ± SEM. Tukey’s test and Student’s t-test for independent samples were used. These tests were performed using statistical Analysis Software SAS version 9.0. Differences were considered statistically significant when the P-value was ≤ 0.01.
Results
Dispersion of MWCNTs in HaCaT Cell Culture
Non-purified, purified, and carboxylate-functionalized MWCNTs (20 μg/mL) were added to HaCaT cells in DMEM cell culture medium and incubated at room temperature for 75 min. After treatment, all samples were washed 3 times with 1× PBS buffer as an attempt to remove all MWCNTs in solution. Figure 1 shows the microscopy images of control and treated HaCaT cells. As shown, cells adhere to the bottom of the wells, and the ones without washing (Figure 1a) contain large amounts of MWCNTs, while washings with 1x PBS remove most of the attached MWCNTs (Figure 1b). This is important since MWCNTs have absorption at the wavelength where MTT formazan salt absorbs light and may interfere with the cell viability test.
Figure 1.
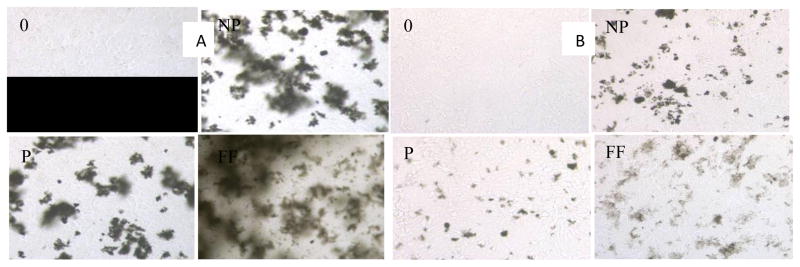
A). Microscopic images of dispersed MWCNTs in HaCaT cells in culture medium. 0: negative control; NP: non-purified; P: purified; FF: Carboxylate-functionalized MWCNTs. B). After 3 washings with 1x PBS.
Cell Viability
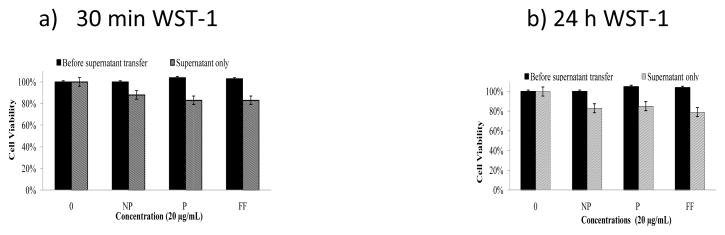
Treatment of HaCaT cells with MWCNTs did not decrease cell viability for both 30 min and 24 h exposure times based on both the MTT and WST-1 assays (Figures 2 & 3). All MWCNT treatments, except for the purified MWCNTs, showed similar responses, with the number of viable cells remain more than 90% of the respective control group. As determined by microscopy, MWCNTs are present in the bottom of the wells as well as on the cell membrane (Figure 1). To remove MWCNTs and minimize interference, the supernatants of all samples were transferred to a clean 96-well plate followed by measurement of the absorbance of the formazan salt. It shows that only the viability of HaCaT cells treated with purified MWCNTs decreases significantly compared to the control with a P value of 0.001 for the 30 min treatment. A similar observation was seen for the 24 hr treatment. A slight decrease (not statistically significant) was seen for all the other treated groups. WST-1 test results were similar to those of the MTT assay (Figure 3a and b).
Figure 2.

Viability of HaCaT cells after exposure to non-purified (NP), purified (P) and carboxylate functionalized (FF) MWCNTs for 30 min (a) or 24 hrs (b) using the MTT assay. “Supernatant only” means that the supernatant was transferred to a clean 96 well plates before absorbance measurement.
Figure 3.
Viability of HaCaT cells after exposure to non-purified (NP), purified (P) and carboxylate functionalized (FF) MWCNTs for 30 min (a) or 24 hrs (b) using the WST-1 assay. “Supernatant only” means that the supernatant was transferred to a clean 96 well plates before absorbance measurement.
Time Dependent DNA Damage
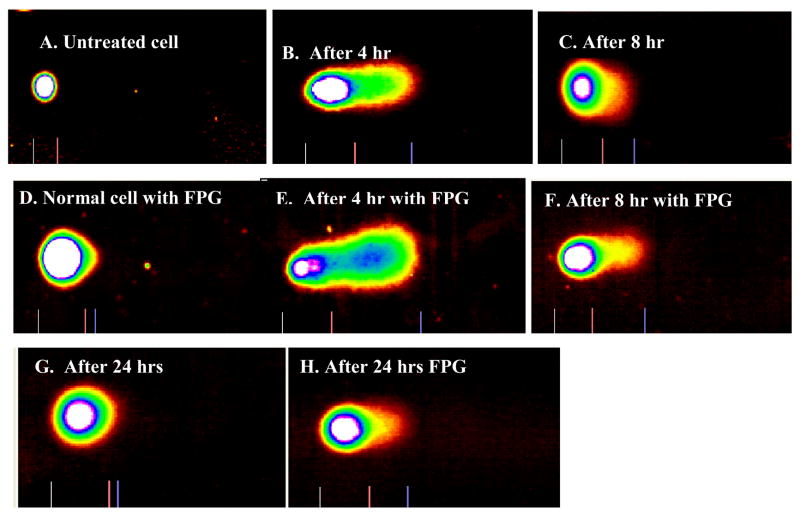
Damages to cellular DNA of the HaCaT keratinocyte cells due to exposure to MWCNTs were assessed using Comet assay (Figure 4). The normal untreated HaCaT cell is round-shaped (Figure 4A), while the ones treated with FPG showed a slight tail (Figure 4D), with about 2% DNA damage. Figure 4B shows direct DNA damage of cells 4 hrs after treatment with MWCNTs while Figure 4C represents that of 8 hrs after treatment. Figures 4-D, E, and F represent the same results corresponding to A, B, and C, respectively, but with FPG treatment to cleave the oxidative damaged DNA.
Figure 4.
Comet assay images of HaCaT cells: A) Normal untreated HaCaT cell; B). HaCaT cells exposed to purified MWCNTs followed by 4 hrs incubation before Comet Assay; C) HaCaT cells exposed to MWCNTs followed by 8 hrs incubation before Comet Assay; D) Normal HaCaT cell treated with FPG; E). HaCaT cells exposed to MWCNTs followed by treatment with FPG and 4 hrs incubation before Comet Assay; F) HaCaT cells exposed to MWCNTs followed by treatment with FPG and 8 hrs of incubation before Comet Assay; G) HaCaT cells exposed to MWCNTs followed by 24 hr incubation prior to Comet Assay; H) HaCaT cell exposed to MWCNTs followed by FPG treatment and 24 hrs of incubation before Comet Assay.
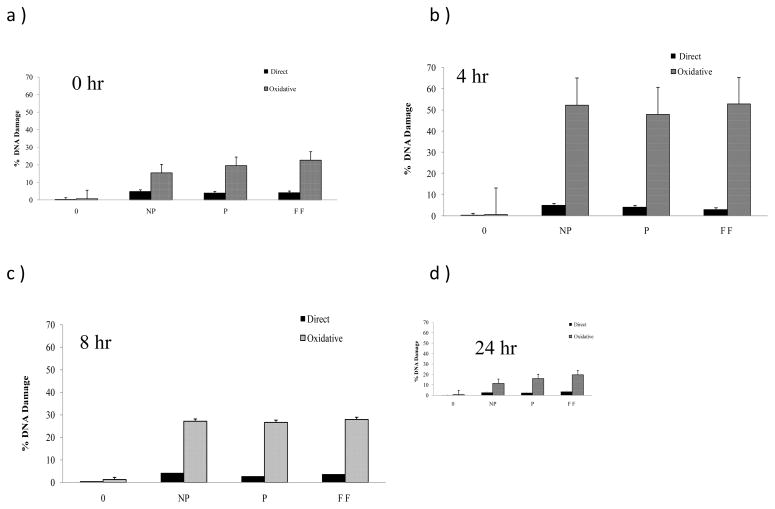
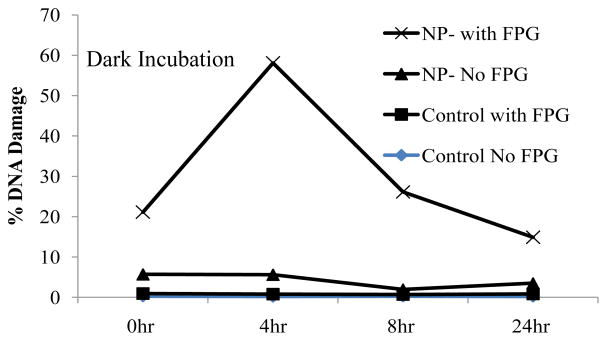
Increased DNA damage of HaCaT cells with respective treatments was detected. Figure 5 depicts the amount of DNA damages via tail moment as a result of exposure to MWCNTs with different incubation times, 0, 4, 8, and 24 hrs. Exposure of different MWCNTs to cells immediately following treatment caused a significant increase in DNA damage (5 to 18% for non-purified, 3 to 22% for purified, and 3 to 25% for functionalized) in HaCaT cells upon addition of FPG enzyme for examination of oxidative DNA damage (Figure 5a). After 4 hr incubation, an even greater amount of oxidative DNA damage is seen for all MWCNT treatments (Figure 5b), while no increase is observed for direct DNA damage (Figure 5b). At 8 hr incubation (figure 5c), the oxidative DNA damage is less compared to the 4 hr incubation, but still slightly higher than the DNA damage without incubation. After 24 hr incubation (Figure 5d), the oxidative DNA damage is similar to that without incubation. The direct DNA damage is minimum at all four time points. This time dependent oxidative DNA damage is captured in Figure 6. It clearly indicates that MWCNTs do not cause significant direct DNA damage, but does cause oxidative damage. The oxidative damage reaches the maximum at 4 hrs and decreases significantly at 8 hrs, and continues to decrease to a minimum level at 24 hrs. This suggests that the oxidative DNA damages are corrected by natural repair mechanisms after incubation of more than 4 hrs.
Figure 5.
Direct and oxidative (with FPG) HaCaT cell DNA damage assessment after treatment of non-purified (NP), purified (P), and functionalized (FF) MWCNTs expressed as mean DNA damage of 75 cells. a) Immediately following MWCNT treatment, b) 4hr incubation, c) 8hr incubation, d) 24 hr incubation prior to Comet assay. FPG treated was significant in all cases (P≤0.01).
Figure 6.
Incubation time-dependent DNA damage for HaCaT cells treated with non-purified (NP) MWCNTs following with incubation of different times. The greatest increase in DNA damage is shown after 4 hr of incubation.
Discussions
Three different MWCNTs, regular non-purified, purified by acid/oxidation, and COOH-functionalized, were evaluated regarding their physical properties, cytotoxicity, and ability to induce oxidative DNA damages. The purification process should remove the catalysts used and the carboxylate-functionalization provides the water solubility for the MWCNTs (Wang and Mitra 2006). In fact, the carboxylate functionalized MWCNTs were highly dispersible in water and ethanol and showed no precipitation after prolonged standing under ambient conditions.
The cytotoxicity tests reveal that the MWCNTs are generally not significantly cytotoxic at the dose used (20 μg/mL) to skin keratinocytes, the most likely cell type the MWCNTs would interact with upon skin exposure. Possible interference for the cytotoxicity test by MWCNTs’ absorbance at 550/450 nm for both the MTT and WST-1 test were dealt with by measuring the formazan absorbance in the supernatant after removal of the cells and the MWCNTs residues on the cells. These tests also did not show significant toxicity. These results are similar to those of Monteiro-Riviere et al using human epidermal keratinocytes in two different studies that MWCNTs caused minimal cytotoxicity (Monteiro-Riviere and Inman 2006; Monteiro-Riviere et al. 2005). Monteiro-Riviere also found MWCNTs to be present inside of the cytoplasmic vacuoles of the human keratinoctye cells (Monteiro-Riviere et al. 2005). They also found that MWCNTs are capable of entering human keratinocytes and produce a biological effect manifested by IL-8 release. MWCNTs also alter proteins in human keratinocytes associated with metabolism, cell signaling, and stress (Witzmann and Monteiro-Riviere 2006). Evidence of dermal irritation by Eedy (Eedy 1996), and the toxicity to keratinocytes studies by Shvedova (Shvedova et al. 2003; Shvedova et al. 2005), suggest that MWCNTs not optimized for intracellular delivery may enter cells and adversely affect cellular function. It has also been suggested that MWCNTs dosed in medium caused an interaction with medium supplements which reduced the availability of ingredients to cells. This results in toxicity by medium depletion. In this study, samples were dosed in 1X PBS buffer to eliminate medium depletion. In addition to MTT assay, WST-1 assay was used to confirm the results, and similar results from WST-1 were obtained. This was in contrast to previous studies by Belyanskaya which studied the cytotoxic effect of well-characterized SWCNT on human mesothelioma cell lines (Belyanskaya et al. 2007). The discrepancies in cytotoxicity by CNTs may be attributed to other known or unknown materials present in the CNTs during the process of manufacturing as evidenced by the recent study by Wang et al (Wang et al. 2011a). They suggested that the existence of oxidized carbon fragments is responsible for the two CNTs exhibiting cytotoxicity.
Our results clearly indicate that MWCNTs can cause oxidative DNA damage in keratinocyte cells, but minimum direct DNA damages. Previous studies show that DNA damages and oxidative stresses caused by single and multi-walled CNTs and it is suggested to be due to impurities or metal catalysts contained in the formation of fibrous carbon materials (Gandra et al. 2009; Herzog et al. 2009; Migliore et al. 2010; Murray et al. 2009; Singh et al. 2009). This study explored not only non-purified, but also purified and functionalized MWCNTS. Our tests show that DNA oxidative damages caused by MWCNTs may not be related to metal catalysts. It may also be attributed to the internalization of the MWCNTs as it was reported by Monteiro-Riviere (2005). In a recent report, He et al(He et al. 2011) showed that MWCNTs induced substantial ROS production and mitochondrial damage in lung cells, and they also activated the NF-κB signaling pathway in macrophages.
Comet assay has been developed as a useful assay for detection of DNA damages by various agents including UV light and their repair (Collins 2009; Lacoste et al. 2007; Laffon et al. 2002; Myllyperkio et al. 2000). The alkaline Comet assays show that direct DNA damage caused by MWCNTs is minimal. The oxidative DNA damage caused by MWCNTs, revealed by applying the FPG enzyme to cleave oxidative sites, is dependent on the incubation time of HaCaT cells in DMEM after exposure to MWCNTs. It reaches the highest percent of damages at about 4 hrs of incubation and decreases to minimum damages at 24 hrs of incubation. This suggests that the oxidative DNA damages caused by MWCNTs can be repaired by the intrinsic cellular repair system after prolonged incubation in DMEM without addition of antibiotics and protein. Oxidative DNA damages, if properly repaired, do not result in harm to the cell, but severe damages beyond repair will result in cell death and mismatch repairs will result in mutation and possibly carcinogenesis (D’Errico et al. 2003; Krystona et al. 2011). Our findings suggest that the oxidative damages to DNA caused by MWCNTs are not severe enough to cause cell death (30 min or 24 hr).
Acknowledgments
The authors wish to thank Dr. Alice Walker and Mr. Christian Rogers for aiding in core laboratory equipments; Dr. Ruomei Gao for discussions and the use of the MWCNTs; NSF for the fellowships: Louis Stokes Alliance for Minority Participation Bridges to the Doctorate (HRD-0115807) and Partnership for Research and Education in Materials (DMR-0611539). Core research facilities were supported by grants from the NSF (CHE-0840450) and NIH (NCRR 2G12RR013459-11).
References
- Bandyopadhyaya R, Nativ-Roth E, Regev O, Yerushalmi-Rozen R. Stabilization of individual carbon nanotubes in aqueous solutions. Nano Lett. 2002;2:25–28. [Google Scholar]
- Bardi G, Vittorio O, Maffei M, Pizzorusso T, MC Adipocytes differentiation in the presence of Pluronic F127-coated carbon nanotubes. Nanomedicine. 2009;5(4):378–81. doi: 10.1016/j.nano.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Belyanskaya L, Manser P, Spohn P, Bruinink A, Wick P. The reliability and limits of the MTT reduction assay for carbon nanotubes-cell interaction. Carbon. 2007;45(13):2643–2648. [Google Scholar]
- Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Chou C, Hsiao H, Hong Q, Chen C, Peng Y, Chen H, Yang P. Single-Walled Carbon Nanotubes Can Induce Pulmonary Injury In Mouse Model. Nano Lett. 2008;8(2):437–445. doi: 10.1021/nl0723634. [DOI] [PubMed] [Google Scholar]
- Collins AR. Investigating oxidative DNA damage and its repair using the comet assay. Mutat Res. 2009;681:24–32. doi: 10.1016/j.mrrev.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Cui HF, Vashist S, Al-Rubeaan K, Luong J, Sheu FS. Interfacing Carbon Nanotubes with Living Mammalian Cells and Cytotoxicity Issues. Chem Res Toxicol. 2010;23:1131–1147. doi: 10.1021/tx100050h. [DOI] [PubMed] [Google Scholar]
- D’Errico M, Teson M, Calcagnile A, Proietti De Santis L, Nikaido O, Botta E, Zambruno G, Stefanini M, Dogliotti E. Apoptosis and efficient repair of DNA damage protect human keratinocytes against UVB. Cell Death Differentiation. 2003;10:754–756. doi: 10.1038/sj.cdd.4401224. [DOI] [PubMed] [Google Scholar]
- Ding L, Stilwell J, Zhang T, Elboudwarej O, Jiang H, Selegue JP, Cooke PA, Gray JW, Chen FF. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. NanoLett. 2005;5(12):2448–2464. doi: 10.1021/nl051748o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eedy DJ. Carbon-fiber-induced airborne irritant contact dermatitis. Cont Dermat. 1996;35:362–363. doi: 10.1111/j.1600-0536.1996.tb02418.x. [DOI] [PubMed] [Google Scholar]
- Gandra N, Chiu P, Li W, Anderson Y, Mitra S, He H, Gao R. Photosensitized singlet oxygen production upon two-photon excitation of single-walled carbon nanotubes and their functionalized analogues. J Phys Chem. 2009;113(13):5182–5185. doi: 10.1021/jp809268q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Young S-H, Schwegler-Berry D, Chisholm WP, Fernback JE, Ma Q. Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-κB signaling, and promoting fibroblast-to-myofibroblast transformation. Chem Res Toxicol. 2011;24(12):2237–2248. doi: 10.1021/tx200351d. [DOI] [PubMed] [Google Scholar]
- Herzog E, Byrne H, Davoren M, Casey A, Duschl A, Oostingh G. Dispersion medium modulates oxidative stress response of human lung epithelial cells upon exposure to carbon nanomaterial samples. Toxicol Appl Pharmacol. 2009;236:276–281. doi: 10.1016/j.taap.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Hu X, Cook S, Wang P, Hwang H-M, Liu X, Williams QL. In vitro evaluation of cytotoxicity of engineered carbon nanotubes in selected human cell lines. Sci Tot Environ. 2010;408(8):1812–1817. doi: 10.1016/j.scitotenv.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39:1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- Johnston HJ, Hutchison GR, Christensen FM, Peters S, Hankin S, Aschberger K, Stone V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology. 2010;4(2):207–246. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- Krystona TB, Georgieva AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Lacoste S, Castonguay Ae, Drouin Re. Repair kinetics of specific types of nitroso-induced DNA damage using the comet assay in human cells. Mutat Res. 2007;624:18–30. doi: 10.1016/j.mrfmmm.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Laffon B, Pásaro E, Méndez J. DNA damage and repair in human leukocytes exposed to styrene-7,8-oxide measured by the comet assay. Toxicol Lett. 2002;126:61–68. doi: 10.1016/s0378-4274(01)00432-5. [DOI] [PubMed] [Google Scholar]
- Martin C, Kohli P. The emerging field of nanotube biotechnology. Nat Rev Drug Disc. 2003;2(1):29–37. doi: 10.1038/nrd988. [DOI] [PubMed] [Google Scholar]
- Migliore L, Saracino D, Bonelli A, Colognato R, D’Errico M, Magrini A, Bergamaschi A, Bergamaschi E. Carbon nanotubes induce oxidative DNA damage in RAW 264.7 cells. Environ Mol Mutagen. 2010;51(4):294–303. doi: 10.1002/em.20545. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Gao J, Vander Wal R, Gigliotti A, Burchiel SW, McDonald JD. Pulmonary And Systemic Immune Response To Inhaled Multiwalled Carbon Nanotubes. Toxicol Sci. 2007;100(1):203–214. doi: 10.1093/toxsci/kfm196. [DOI] [PubMed] [Google Scholar]
- Mocan T, Clichici S, Agoşton-Coldea L, Mocan L, Şimon Ş, Ilie IR, Biriş AR, Mureşan A. Implications of oxidative stress mechanisms in toxicity of nanoparticles. Acta Physiol Hung. 2010;97 (3):247–255. doi: 10.1556/APhysiol.97.2010.3.1. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere N, Inman A. Challenges for assessing carbon nanomaterial toxicity to the skin. Carbon. 2006;44:1070–78. [Google Scholar]
- Monteiro-Riviere N, Nemanich R, Inman A, Wang Y, Riviere J. Multi-walled carbon nanotubes interactions with human epidermal keratinocytes. Toxicol Lett. 2005;155:377–84. doi: 10.1016/j.toxlet.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Murray AR, Kisin E, Leonard SS, Young SH, Kommineni C, Kagan VE, Castranova V, Shvedova AA. Oxidative stress and inflammatory response in dermal toxicity of single-walled carbon nanotubes. Toxicology. 2009;257(3):161–171. doi: 10.1016/j.tox.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Myllyperkio MH, Koski TRA, Vilpo LM, Vilpo JA. Kinetics of excision repair of UV-induced DNA damage, measured using the comet assay. Mutat Res. 2000;448:1–9. doi: 10.1016/s0027-5107(99)00224-9. [DOI] [PubMed] [Google Scholar]
- Pacurari M, Yin XJ, Zhao J, Ding M, Leonard SS, Schwegler-Berry D, Ducatman BS, Sbarra D, Hoover MD, Castranova V, et al. Raw Single-Wall Carbon Nanotubes Induce Oxidative Stress and Activate Mapks, AP-1, NF-κb, and Akt In Normal and Malignant Human Mesothelial Cells. Environ Health Perspect. 2008;116(9):1211–1217. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantarotto D, Singh R, McCarthy D, Erhardt M, Briand J-P, Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew Chem Int Ed. 2004;43:5242–5246. doi: 10.1002/anie.200460437. [DOI] [PubMed] [Google Scholar]
- Patlolla A, Knighten B, Tchounwou P. Multi-walled carbon nanotubes induce cytotoxicity, genotoxicity and apoptosis in normal human dermal fibroblast cells. Ethnic Disease. 2010;20(Suppl 1):65–72. [PMC free article] [PubMed] [Google Scholar]
- Prato M, Kostarelos K. Functionalized Carbon Nanotubes In Drug Design and Discovery. Acc Chem Res. 2008;41(1):60–68. doi: 10.1021/ar700089b. [DOI] [PubMed] [Google Scholar]
- Pulskamp K, Diabate S, Krug H. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168:58–74. doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J Environ Sci Health C: Environ Carcinogen Ecotoxicol Rev. 2009;27(1):1–35. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayes C, Liang F, Hudson J, Mendez J, Guo W, Beach J, Moore V, Doyle C, West J, Billups W, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161:135–142. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Shvedova A, Castranova V, Kisin E, Schwegler-Berry D, Murray AR, Gandelsman VZ, Maynard A, Baron P. Exposure to carbon nanotubes material: Assessment of nanotubes cytotoxicity using human keratinocyte cells. J Toxicol Environ Health, Part A. 2003;66:1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- Shvedova A, Kisin E, Mercer R, Murray A, Johnson V, Potapovich A, Tyurina Y, Gorelik O, Arepalli S, Schwegler-Berry D, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Singh K, Pandey R, Wang X, Lake R, Ozkan C, Wang K, Ozkan O. Covalent functionalization of single walled carbon nanotubes with peptide nucleic acid: Nanocomponents for molecular level electronics. Carbon. 2006;44:1730–39. [Google Scholar]
- Singh N, Manshian B, Jenkins G, Griffiths S, Williams P, Maffeis T, Wright C, Doak S. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;1:1–24. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Smart S, Cassady A, Lu G, Martin D. The biocompatibility of carbon nanotubes. Carbon. 2006;44:1034–1047. [Google Scholar]
- Soto K, Garza K, Murr L. Cytotoxic effects of aggregated nanomaterials. Acta Biomat. 2007;3(3):351–358. doi: 10.1016/j.actbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Tian F, Cui D, Schwarz H, Estrada G, Kobayashi H. Cytotoxicity of single-wall carbon nanotubes on human fibroblasts. Toxicol in vitro. 2006;20(7):1202–1212. doi: 10.1016/j.tiv.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Vankoningsloo S, Piret J-P, Saout C, Noel F, Mejia J, Zouboulis CC, Delhalle J, Lucas S, Toussaint O. Cytotoxicity of multi-walled carbon nanotubes in three skin cellular models: Effects of sonication, dispersive agents and corneous layer of reconstructed epidermis. Nanotoxicology. 2010;4(1):84–97. doi: 10.3109/17435390903428869. [DOI] [PubMed] [Google Scholar]
- Wang R, Mikoryak C, Li S, Bushdiecker D, Musselman IH, Pantano P, Draper RK. Cytotoxicity screening of single-walled carbon nanotubes: Detection and removal of cytotoxic contaminants from carboxylated carbon nanotubes. Mol Pharmaceut. 2011a;8(4):1351–1361. doi: 10.1021/mp2001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Sheng YH, Feng M, Leszczynski J, Wang L, Tachikawa H, Yu H. Light-induced cytotoxicity of 16 polycyclic aromatic hydrocarbons on the US EPA Priority Pollutant List in human skin HaCaT keratinocytes: Relationship between phototoxicity and excited state properties. Environ Toxicol. 2007;22(3):318–327. doi: 10.1002/tox.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yu H, Wickliffe JK. Limitation of the MTT and XTT assays for measuring cell viability due to superoxide formation induced by nano-scale TiO2. Toxicol in Vitro. 2011b;25(8):2147–2151. doi: 10.1016/j.tiv.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mitra S. Rapid functionalized, water dispensed carbon nanotubes at high concentration. J Am Chem Soc. 2006;128:95–99. doi: 10.1021/ja053003q. [DOI] [PubMed] [Google Scholar]
- Warheit D, Laurence B, Reed K, Roach D, Reynolds G, Webb T. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- Wick P, Manser P, Limbach L, Dettlaff-Weglikowska U, Krumeich F, Roth S, Stark W, Bruinink A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168:121–131. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Witzmann FA, Monteiro-Riviere NA. Multi-walled carbon nanotube exposure alters protein expression in human keratinocytes. Nanomed Nanotech Biol Med. 2006;2(3):158–168. doi: 10.1016/j.nano.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Yoshioka Y, Higashisaka K, Morishita Y, Yoshida T, Fujimura M, Kayamuro H, Nabeshi H, Yamashita T, Nagano K, et al. Carbon Nanotubes Elicit DNA Damage and Inflammatory Response Relative to Their Size and Shape. Inflammation. 2010;33(4):276–280. doi: 10.1007/s10753-010-9182-7. [DOI] [PubMed] [Google Scholar]
- Zhu L, Chang D, Dai L, Hong Y. DNA damage induced by multi-walled carbon nanotubes in mouse embryonic stem cells. Nano Lett. 2007;7(12):3592–3597. doi: 10.1021/nl071303v. [DOI] [PubMed] [Google Scholar]