Abstract
Background
Antiviral resistance among influenza A viruses is associated with high morbidity and mortality in immunocompromised hosts. However, treatment strategies for drug-resistant influenza A are not established. A triple-combination antiviral drug (TCAD) regimen consisting of amantadine (AMT), oseltamivir (OSL) and ribavirin (RBV) demonstrated good efficacy in an animal model.
Methods
We first analysed the pharmacokinetics (PKs) of TCAD therapy in healthy volunteers. We then performed a pilot study of TCAD therapy in patients undergoing chemotherapy or haematopoietic cell transplantation. AMT (75 mg), OSL (50 mg) and RBV (200 mg) were administered three times a day for 10 days. The safety and PKs of TCAD therapy were monitored.
Results
The PKs of TCAD therapy in healthy volunteers was shown to be similar to the PKs of each drug individually from a single dose. In the pilot study, six immunocompromised patients received TCAD therapy and one patient received OSL monotherapy. All but one patient completed 10 days of TCAD therapy without side effects; one patient receiving TCAD was withdrawn from the study because of respiratory failure and ultimately recovered. Viral load was decreased after TCAD therapy, despite the presence of either AMT- or OSL-resistant virus in two cases. One patient with 2009 influenza A/H1N1 receiving OSL monotherapy developed confirmed OSL resistance during treatment.
Conclusions
TCAD therapy had similar PKs to each individual antiviral during monotherapy following a single dose and can be administered safely in immunocompromised patients.
Introduction
Patients who are seriously immunocompromised as a result of haematological malignancies or haematopoietic cell transplantation (HCT) have an increased risk of infection with influenza and, once infected, may have serious disease with substantial mortality [1–3]. Among patients undergoing HCT, mortality rates up to 25% have been reported during influenza A outbreaks [4]. Lymphopenia, age and lack of antiviral therapy (AVT) have been related to poor outcome [2,3,5–7]. Although vaccination is an important strategy advocated worldwide for the prevention of influenza, patients undergoing HCT or receiving chemotherapy are not likely to be effectively protected because of poor immune responses to the vaccine and, when infected, may suffer serious disease. Therefore, effective AVT plays a critical role in these severely immunocompromised patients.
Three classes of anti-influenza drugs with non-overlapping mechanisms of action have been clinically utilized, including inhibitors of the viral M2 channel (amantadine hydrochloride [AMT HCl] and rimantadine hydrochloride), the viral neuraminidase (NA; oseltamivir phosphate [OSL] and zanamivir) and the viral RNA polymerase ribavirin (RBV). Although AMT, the first approved drug for influenza, has been used widely in the past, widespread resistance in circulating viruses has substantially limited its utility in recent years [1,8]. OSL has become the most widely used antiviral for influenza as a result of ease of administration, favourable safety and efficacy profiles and general susceptibility in recent circulating strains [9,10]. Although OSL-resistant seasonal influenza A/H1N1 viruses emerged and spread worldwide between 2007 and 2009, these were replaced by the OSL-susceptible pandemic 2009 A/H1N1 strain [11]. However, OSL-resistant virus variants may emerge during treatment, especially in immunocompromised hosts in whom influenza is characterized by high levels of viral replication for prolonged periods of time [12,13]. By 2012, 447 cases of OSL-resistant 2009 pandemic influenza A/H1N1 had been reported, all of which were also resistant to AMT [14–16]. Resistance to OSL is most commonly associated with a single NA H275Y amino acid substitution (N1 numbering) in H1N1 viruses [14,17]. Rates of OSL resistance conferred by this mutation occurred in 8/33 (24%) of HCT recipients with prolonged shedding of influenza viruses [18].
In patients with haematological malignancies or undergoing HCT, early and appropriate treatment for influenza virus is critical. Although some combination antiviral therapies have been utilized, the efficacy of this approach has not been established. A triple-combination antiviral drug (TCAD) regimen consisting of AMT, OSL and RBV has been reported to have synergistic activity against influenza A viruses in vitro and in vivo, including against AMT- and OSL-resistant viruses [19–22]. This paper reports the first prospective studies of TCAD therapy in humans, and the safety and pharmacokinetics (PKs) of TCAD therapy were shown in healthy volunteers and patients with haematological malignancies or undergoing HCT who were infected with influenza, providing pilot data for triple AVT in the patient care setting.
Methods
Study design
This report is based on two separate clinical studies. The first study was a controlled PK study performed in two sites as a randomized, open-label, crossover, single-dose study in healthy volunteers. In total, three groups of 14 healthy adults each were enrolled to compare PKs following single oral doses of AMT, OSL and RBV given alone and as a triple combination. Each group received two treatments in a crossover fashion, with a 7-day washout period between each treatment: group 1 received a single dose of AMT 100 mg (Symmetrel® , Endo Pharmaceuticals, PA, USA) alone and then in combination; group 2 received OSL 75 mg (Tamiflu® , Roche, Basel, Switzerland) alone and then in combination; group 3 received RBV 600 mg (Rebetol® , Schering Corporation, Merck & Co., NJ, USA) alone and then in combination. Study drugs were administered as separate tablets (AMT) and/or capsules (OSL and RBV). Following each dose, serial blood samples were collected over 168 h for PK analysis. The protocol was approved by the institutional review committees at Mahidol-Oxford Tropical Medicine Research Unit in Bangkok and at the National University Hospital in Singapore, and informed consent was obtained prior to study enrolment.
The second study was a pilot study in immunocompromised patients undergoing chemotherapy or HCT at the Fred Hutchinson Cancer Research Center and Seattle Children’s Hospital between February and September 2009. This trial was registered with ClinicalTrials.gov (NCT00867139). The study was approved by each institutional review board, and informed consent was obtained prior to study enrolment. The pilot study consisted of two substudies: the first substudy was a randomized study comparing TCAD therapy and OSL mono therapy in immunocompromised patients with upper respiratory tract infection caused by influenza A, who were over 7 years of age and who were not asthmatic; the second substudy consisted of an open-label study of TCAD in patients with new infiltrate on chest X-ray, O2 saturation ≤92% on room air, age-adjusted severe tachypnea or those excluded from the randomized clinical study as a result of a history of asthma or age <7 years. Intended sample sizes were 20 in the randomized study and 15 in the open-label study. Patients were recruited from individuals who had undergone HCT within 2 years, or combination chemotherapy within 3 months, had chronic graft-versus-host disease (GVHD) requiring systemic treatment after 2 years post-HCT or had GVHD and were taking at least two immunosuppressive drugs. A positive laboratory test for influenza A and onset of illness no more than 5 days prior to diagnosis were requirements to participate. After confirmation of influenza infection by rapid antigen test or PCR, patients in the first substudy were randomized 1:1 to receive a 10-day course of TCAD therapy 75 mg AMT (Symmetrel® , Endo Pharmaceuticals) three times daily, 50 mg OSL (Tamiflu® , Roche) three times daily and 200 mg RBV (Rebetol® , Schering Corporation) three times daily or a 10-day course of 50 mg OSL monotherapy (Tamiflu® , Roche) three times daily. Study drugs were delivered as separate oral solutions. Although the doses for the healthy volunteer PK study were based on the labelled doses for each drug for influenza (AMT and OSL) and hepatitis C (RBV), the doses used in the pilot treatment study were chosen to maximize efficacy while sustaining acceptable tolerability. Based on the PK data, simulations were conducted to determine the steady-state plasma concentrations for each drug from repeated dosing. The treatment trial dose of AMT was intended to provide minimum plasma concentrations needed for maximum efficacy based on in vitro data, whereas the dose of RBV was the minimum dose that provided efficacious plasma levels based on in vitro data [19,22]. In addition, as a result of the relatively short half-lives of AMT and OSL, each drug was administered three times daily in order to maintain trough plasma concentrations (minimum concentration [Cmin]) at or above the in vitro half-maximal effective concentration (EC50) of each drug. The dose of AMT was decreased by 50% in the patients with creatinine clearance between 30 and 50 ml/min. In paediatric patients, drug doses were adjusted for the patient’s body weight. Blood samples were collected prior to therapy and at 1,2 and 4 h following the morning dose of study drug(s) on day 3 or 4 to assess drug concentrations. In order to monitor the safety and adverse events of the study, physical examination and laboratory tests were performed on days 1, 2 (if feasible), 3, 5, 7, 9, 15, 20 and 28 using a check-off list for the study, and a final assessment was performed 30 days after the final dose of study drug. In addition, spontaneous adverse event reporting was used between and after the monitoring.
PK analysis and statistical methods
Plasma concentrations of AMT, OSL carboxylate (the active metabolite of OSL) and RBV were quantified using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). PK parameters, Cmin, maximum concentration (Cmax), time to observed maximum concentration (Tmax), area-under-the-time-concentration curve up to the last measured time point (AUC0–last) and to infinity (AUC0–inf) and terminal elimination half-life (T1/2), were calculated from the plasma concentration–time profiles by non-compartmental analysis using Win-Nonlin Professional Software version 5.0.1 (Pharsight Corporation, CA, USA). An analysis of variance model, including treatment, sequence, period and subject within sequence factors, was used to compare TCAD with monotherapy. The point estimate of the geometric mean (combination/monotherapy) for Cmax, AUC0–last, AUC0–inf and the residual variability from the ANOVA were used to calculate the 90% CIs around the mean to assess bioequivalence [23]. For treatment comparison, CIs of mean ratios with 80–125% were considered to be bio-equivalent [24].
Virological testing
Nasopharyngeal washes or swabs were obtained for initial screening of influenza infection (positive rapid antigen test, direct fluorescent antigen detection or qualitative PCR test) and for measurement of viral load in all patients participating in the pilot study. Continuous analyses using nasopharyngeal swab samples were performed for influenza A subtype and viral load by quantitative PCR (qPCR) at multiple time points during the study period (Additional file 1). The limit of viral detection in the qPCR assay was 3 log10 RNA copies/ml, and the limit of quantification was 4 log10 RNA copies/ml. Subtyping of influenza A viruses into seasonal H1N1, H3N2 or 2009 H1N1 was performed by means of a multiplex real-time PCR (Additional file 1). The presence of resistance-conferring mutations in NA or M2 genes was determined by the Sanger sequencing method (Additional file 1) [25].
Results
PKs of monotherapy and TCAD therapy in healthy adults
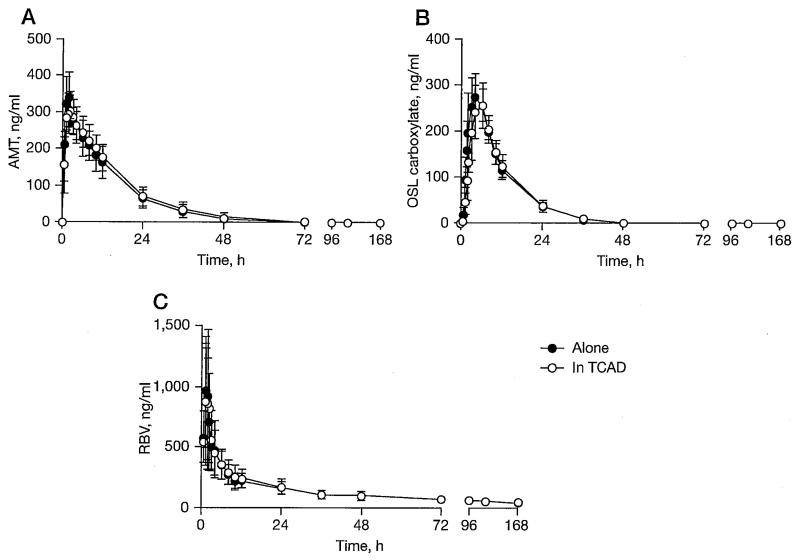
A Phase I controlled PK study was conducted in 42 healthy volunteers to compare the PKs of single oral doses of AMT (100 mg), OSL (75 mg) and RBV (600 mg) given alone and in combination. All 42 individuals received at least one dose of study drug and were included in the safety analysis. Of the participants, 37 (88%) were male and 5 (12%) were female. The mean age was 28 years (range 21–42) and the mean body mass index was 21.4 kg/m2 (range 18–23). All participants were Asian. In total, 41 (98%) of the individuals completed the PK assessment in the study and were included in the PK analysis. One individual was withdrawn prior to study completion as a result of an unrelated medical illness. As shown in Figure 1, the single dose PKs of AMT, OSL carboxylate and RBV were not altered when the three drugs were administered together in the first 24 h. PK parameters in each group were also similar when comparing combination treatment to that when given alone, as shown in Table 1. Moreover, CIs around the geometric mean ratios (combination/monotherapy) of all three drugs were calculated for Cmax, AUC0–last and AUC0–inf. All CIs were within 80–125%, indicating bioequivalence between monotherapy and combination therapy (Table 2). Thus, the PK profiles from a single dose of each drug in TCAD did not differ from those following monotherapy in healthy adults. There were no serious adverse events reported in the PK study in healthy volunteers.
Figure 1.
Plasma concentration of each drug in healthy volunteers
Mean and standard deviation of plasma concentration in each drug is shown. Individuals were randomized to one of three treatment groups: (A) a single dose of amantadine (AMT) hydrochloride 100 mg alone and in a triple combination (AMT 100 mg, oseltamivir [OSL] 75 mg and ribavirin [RBV] 600 mg), (B) a single dose of OSL 75 mg alone and in a triple combination and (C) a single dose of RBV 600 mg alone and in a triple combination. TCAD, triple combination antiviral drug.
Table 1.
Pharmacokinetic parameters for AMT, OSL carboxylate and RBV administered alone or in combinationa in healthy volunteers
| Group 1 |
Group 2 |
Group 3 |
||||
|---|---|---|---|---|---|---|
| Parameter | AMT alone 100 mg (n=14) |
AMT TCAD 100 mg (n=14) |
OSL carboxylate alone 75 mg (n=13) |
OSL carboxylate TCAD 75 mg (n=14) |
RBV alone 600 mg (n= 14) |
RBV TCAD 600 mg (n=14) |
| Cmax, ng/ml | 361 (268–482) |
349 (264–402) |
271 (208–386) |
274 (151–375) |
971 (496–2,550) |
945 (364–2,220) |
| Tmax, h | 1.50 (0.500–4.00) |
1.75 (1.00–6.00) |
4.00 (2.00–6.00) |
6.00 (4.00–6.00) |
1.00 (0.500–1.50) |
1.25 (0.500–3.00) |
| CL/F,I/h | 19.4 (13.3–37.7) |
19.1 (11.0–34.6) |
20.8 (16.0–25.5) |
20.5 (15.9–25.4) |
26.8 (13.4–37.0) |
22.0 (12.9–53.8) |
| V/F, I | 265 (181–439) |
266 (214–361) |
186 (141–253) |
193 (145–312) |
4,050 (2,820–7,260) |
3,450 (2,500–8,060) |
| T1/2, h | 8.87 (6.24–22.9) |
9.04 (6.47–22.9) |
6.26 (5.00–8.99) |
6.57 (5.09–8.59) |
111 (90.3–281) |
105 (84.4–183) |
| AUC0–last, hxng/ml | 5,060 (2,630–7,330) |
5,140 (2,830–8,890) |
3,160 (2,580–4,130) |
3,220 (2,520–4,190) |
16,400 (11,800–27,000) |
19,800 (8,460–25,800) |
| AUC0–inf, hxng/ml | 5,150 (2,650–7,530) |
5,240 (2,900–9,140) |
3,270 (2,670–4,250) |
3,330 (2,680–4,280) |
22,300 (16,200–44,700) |
27,200 (11,200–46,600) |
Values are reported as median (range).
Triple combination antiviral drug (TCAD). AMT, amantadine; AUC0–inf, predicted area under the plasma concentration-time curve after the last dose from zero time to infinity; AUC0–last, total exposure up to the last measured concentration; CL/F, oral clearance; Cmax, maximum observed plasma concentration; OSL, oseltamivir; RBV, ribavirin; Tmax, observed time to reach Cmax; T1/2, terminal elimination half-life; V/F, apparent volume of distribution.
Table 2.
Geometric mean ratiosa in healthy volunteers
| Parameter | Group 1 amantadine (n=14) geometric mean ratio (90% CI) |
Group 2 oseltamivir carboxylate (n=13) geometric mean ratio (90% CI) |
Group 3 ribavirin (n=14) geometric mean ratio (90% CI) |
|---|---|---|---|
| Cmax, ng/ml | 93.8 (87.0, 101) | 94.2 (87.1, 102) | 97.8 (81.2, 118) |
| AUC0–last, hxng/ml | 107 (97.7, 118) | 98.5 (94.2, 103) | 102 (93.3, 110) |
| AUC0–inf, hxng/ml | 107 (97.8, 118) | 98.3 (94.8, 102) | 96.6 (82.0, 114) |
Values are reported as median percentages (90% CI).
Alone/combination. AUC0–inf, predicted area under the plasma concentration-time curve after the last dose from zero time to infinity; AUC0–last, total exposure up to the last measured concentration; Cmax, maximum observed plasma concentration.
Pilot study of immunocompromised patients with influenza
To assess the safety and tolerability of TCAD therapy, we conducted a pilot study in patients who either had haematological malignancies or were undergoing HCT with laboratory-confirmed influenza. A total of seven patients were enrolled in the study; three patients were randomized (one to OSL monotherapy and two to TCAD therapy), and the remaining four patients received open-label TCAD therapy as a result of age, abnormal pulmonary function or severe influenza disease (Table 3). The study was discontinued early by the sponsor as a result of lack of funding. Influenza A infection was confirmed in all patients by PCR. Of the six patients treated with TCAD, five completed a 10-day treatment course and achieved a clinical response defined as cessation of symptoms by day 10, whereas one patient was withdrawn from the study. Her disease started immediately after the transplantation and was suspected to be lower respiratory tract disease. She was withdrawn from the study after 3 days of receiving TCAD as a result of increasing respiratory failure, although she ultimately recovered after the engraftment using high-dose OSL and peramavir, an experimental NA inhibitor [26]. One patient treated with OSL symptomatically improved by day 10. However, he required a second 10-day course of OSL monotherapy because of the rebound of viral load, which did not respond to the second course [27]. Details of patient demographics, underlying conditions, treatment assignment, dosing and outcomes are shown in Table 3.
Table 3.
Subject demographics and clinical outcome
| Subjecta (n=7) |
Age (gender) |
Body weight, kg |
Underlying condition |
Lymphocyte count at enrolment, ×103 cells/μl |
Treatment | Dose, every 8 h |
CrCl, ml/minb |
Virus subtype (resistant drug) |
Viral load at baseline, log10 RNA copies/mlc |
Viral load reduction, log10 RNA copies/ml |
Duration of viral shedding, days |
Clinical outcome |
Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | 17 (M) | 58 | CBT (day 24) | 0.2 | OSL monotherapy |
50 mg OSL+ 75 mg AMT |
109 | 2009 H1N1 (AMT) |
5.7 | 1.6 with rebound at day 7 |
72 | Required second course of treatment |
Acute GVHD (gut) |
| 1-2 | 72 (M) | 91 | T-cell lymphoma |
0.7 | TCAD | 200 mg RBV+ 50 mg OSL+ 75 mg AMT |
121 | H3N2 (AMT) | BLODd | – | – | Response by day 10 |
None |
| 1-3 | 47 (F) | 67 | Unrelated BMT (day 17) |
0.05 | TCAD | 200 mg RBV+ 50 mg OSL+ 37.5 mg AMT |
148 | NDe | BLOD | – | – | Response by day 10 |
Acute GVHD (gut, skin), tachycardia, increased ALP and GGT |
| 2-1 | 58 (M) | 107 | Unrelated PBSCT (day 1,859) |
2.7 | TCAD | 200 mg RBV+ 50 mg OSL+ 75 mg AMT |
47 | ND | BLOD | – | – | Response by day 10 |
None |
| 2-2 | 45 (F) | 88 | Autologous PBSCT (day 3) |
BLOD | TCAD | 200 mg RBV+ 50 mg OSL+ 50 mg AMT |
157 | 2009 H1N1 (AMT) |
5.2 | Withdrew on day 5, no reduction |
Withdrew with viral detection |
Withdrew on day 5, no response |
Acute RDS |
| 2-3 | 9 (F) | 27 | CBT (day 2,084) |
0.4 | TCAD | 150 mg RBV+ 40 mg OSL+ 50 mg AMT |
117 | H3N2 (AMT) | 6.9 | >3.9 | >9–<27f | Response by day 10 |
None |
| 2-4 | 6 (M) | 26 | ALL | 1.2 | TCAD | 150 mg RBV+ 40 mg OSL |
166 | H1N1 (OSL) | 4.6 | >1.6 | 8 | Response by day 10 |
None |
Study 1 is a randomized study and study 2 is an open-label study of triple-combination antiviral drug (TCAD).
Paediatric renal clearance calculated using Schwartz equation; Cockcroft-Gault calculation used for adults.
Determined by quantitative PCR.
3 log10 RNA copies/ml for viral load.
Not determined (ND) as a result of low viral load.
No tests were done between day 9 and day 27, and day 28 was negative. ALL, acute lymphocytic leukaemia; ALP, alkaline phosphatase; AMT, amantadine; BLOD, below limit of detection; BMT, bone marrow transplantation; CBT, cord blood transplantation; CrCl, creatine clearance; F, female; GGT, γ-glutamyl transferase; GVHD, graft-versus-host disease; M, male; OSL, oseltamivir; PBSCT, peripheral blood stem cell transplant; RBV, ribavirin; RDS, respiratory distress syndrome.
PK of TCAD in immunocompromised hosts
Drug concentrations for each agent on day 3 are shown in Table 4. PK analysis was not performed on two patients because of insufficient volume. PK analyses confirmed the presence of therapeutic concentrations of each drug, although a high variability in values was observed.
Table 4.
Pharmacokinetics of each drug in patients receiving OSL monotherapy or TCAD therapy
| OSL |
AMT |
RBV |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age | Dosea, mg |
Cmax, ng/ml |
Cmin, ng/ml |
AUC0–last, hxng/ml |
Dosea, mg |
Cmax, ng/ml |
Cmin, ng/ml |
AUC0–last, hxng/ml |
Dosea, mg |
Cmax, ng/ml |
Cmin, ng/ml |
AUC0–last, hxng/ml |
| 1-1b | 17 | 50 | 467.7 | 226.3 | 1,497.1 | – | – | – | – | – | – | – | – |
| 1-2c | 72 | 50 | 165.6 | 112.1 | 304.0 | 75 | 788.6 | 615.9 | 1,456.5 | – | – | – | – |
| 2-1d | 58 | 50 | 1,564.9 | 1,377.4 | 5,683.1 | 37.5 | 560.2 | 303.0 | 1,662.9 | 200 | 670.8 | 288.6 | 2,161.6 |
| 2-3 | 9 | 40 | 324.8 | 184.6 | 1,010.5 | 50 | 1,161.5 | 915.7 | 4,405.9 | 150 | 1,466.7 | 1,146.0 | 5,369.0 |
| 2-4 | 6 | 40 | 260.6 | 133.6 | 768.8 | 50 | 626.7 | 398.3 | 2,215.5 | 150 | 1,643.0 | 708.2 | 5,397.1 |
lndicated dose was administered every 8 h.
Subject 1-1 received oseltamivir (OSL) monotherapy.
Subject 1-2 had insufficient sample for ribavirin (RBV) level analysis.
Subject 2-1 had renal impairment. AMT, amantadine; AUC0–last, total exposure up to the last measured concentration; Cmax, maximum observed plasma concentration; Cmin, minimum observed plasma concentration; TCAD, triple combination antiviral drug.
Safety of TCAD therapy
A total of six adverse events in three patients were reported in the pilot study. One patient receiving TCAD therapy who withdrew from the study had severe acute respiratory distress syndrome, progressing from the time of enrolment. Another patient receiving TCAD therapy had multiple events, including GVHD, tachycardia and increased alkaline phosphatase and γ-glutamyl transferase. The single patient receiving OSL monotherapy also had confirmed GVHD documented during therapy. These adverse events were judged not to be related to the study drugs. Potentially anticipated adverse effects, such as anaemia or gastrointestinal or neurological symptoms, were not reported.
Virological studies
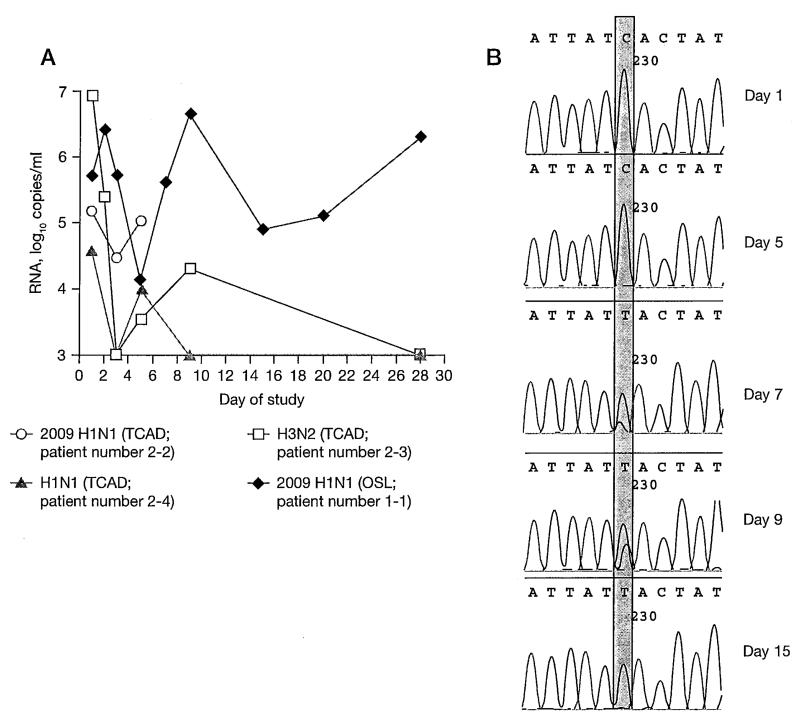
Patients in this trial were infected with OSL-resistant seasonal H1N1 (n=1), AMT-resistant H3N2 (n=2) or AMT-resistant 2009 H1N1 (n=2) influenza viruses (Table 3). In two patients, the subtype of the infecting virus could not be determined as a result of insufficient quantity of viral RNA. In four patients, viral RNA was quantifiable at baseline and follow-up, enabling analysis of viral load variations. Viral load reductions of 1.6–3.9 log10 RNA copies/ml were seen in the three patients receiving TCAD therapy (Figure 2A). The TCAD-treated patient who withdrew from the study as a result of disease progression showed little reduction in viral load. The single patient who received OSL monotherapy showed an initial viral load reduction of 1.6 log10 RNA copies/ml followed by a rebound at day 7 and sustained high viral load until the end of follow-up at 28 days (Figure 2A).
Figure 2.
Virological outcome in immunocompromised patients
(A) The change of viral load after triple combination antiviral drug (TCAD) therapy or oseltamivir (OSL) monotherapy in the four patients with detectable viral load before treatment. The open circle indicates the patient who withdrew from the study on day 5 before receiving the full course of treatment. Patient number in Table 3 is indicated in parentheses. (B) Emergence of mutation during OSL monotherapy. A mutation from C (a blue peak) to T (a red peak) at nucleotide 230 (shaded region) in the neuraminidase gene denoting an H275Y substitution appeared at day 7 in the patient with OSL monotherapy.
Sequence analysis of the NA and M2 genes detected no de novo resistance-associated mutation at any time point in samples with quantifiable viral load from the three patients receiving TCAD, including the patient who withdrew from the study. The OSL resistance mutation conferring H275Y substitution in NA emerged in the patient who received OSL monotherapy by day 7 (Figure 2B). Nasal wash samples from day 9 were sent to the Centers for Disease Control and Prevention where resistance to OSL was confirmed by pyrosequencing and enzyme inhibition assay [27].
Discussion
This study reports the single-dose PKs of single and triple anti-influenza therapy in healthy volunteers and the safety of TCAD therapy in a small pilot study in immunocompromised patients. Importantly, the PK study in healthy adults showed no drug–drug interactions among the three antiviral agents following a single dose, and the pilot study in highly immunocompromised patients demonstrated safety of TCAD therapy in the small number of patients evaluated.
Several different combination antiviral approaches to the treatment of influenza have been previously studied in animals and humans in efforts to improve efficacy and prevent resistance emergence. A clinical trial of combined oral OSL and inhaled zanamivir in adults in the outpatient setting demonstrated inferior outcomes of combined treatment when compared with OSL monotherapy alone [28]. These observations might be explained by possible antagonistic effects of combined NA inhibitor treatment, which have been suggested by in vitro experiments [19]. Other anti-influenza virus compounds, including AMT and RBV, have been studied in combination with OSL in animal models, with a synergistic effect noted in mice [29–31]. TCAD therapy consisting of OSL, AMT and RBV was subsequently shown to have greater antiviral activity compared with double combination therapy in vitro and in the mouse model [20–22]. However, the antiviral effects of TCAD have not been previously evaluated in a prospective study in humans. Kim et al. [32] conducted a retrospective study of critically ill patients on mechanical ventilation who were infected with 2009 A/H1N1 and treated with TCAD therapy or OSL monotherapy. Although the retrospective nature and the absence of virological outcome data precludes reliable evaluation of the potential benefit of TCAD therapy over OSL mono therapy, the authors reported that TCAD was well-tolerated, and no toxicity attributable to AVT was reported for either group.
Drug interactions are an important limiting factor when administering combined antivirals. Although TCAD therapy had shown synergy and efficacy in vitro and in an animal model, it was not known previously how these drugs would interact in humans [20,22]. Our controlled PK study in healthy volunteers demonstrated similar PK in individuals receiving TCAD therapy and those receiving monotherapy, indicating the absence of PK interactions between these antiviral agents. It should be noted that the PK data were derived from a single dose of TCAD and monotherapy, and thus we cannot discount the possibility that drug accumulation from repeated doses may result in drug–drug interactions that were not detected in our study. Morrison et al. [33] have evaluated the PK of AMT and OSL given alone and in combination for 5 days and reported no significant interactions. Moreover, we analysed PK following TCAD therapy after several days of therapy in immunocompromised influenza patients for the first time, although the values were not at steady state, and we were able to document therapeutic drug levels of OSL, AMT and RBV in patients with haematological malignancies or undergoing HCT [34]. Differences between the PK parameters (Cmax and AUC0–last) between healthy volunteers and immunocompromised patients may be attributable to the accumulation of drugs and/or differences in sampling, although the small number of immunocompromised patients enrolled in the treatment study precludes any substantive conclusions regarding the differences in PK parameters between the two study populations.
The consideration of safety is important when delivering multiple therapeutic agents to patients with complex medical conditions who are already receiving multiple drugs. Immunocompromised hosts may require a longer duration of therapy than previously healthy patients because they may not respond promptly to therapy as a result of underlying disease or immunodeficiency because of concern for disease progression or because of prolonged viral shedding [1,8]. We used TCAD for 10 days in the immunocompromised patient population, a period twice as long as typical anti-influenza therapy in a previously healthy individual. All patients except one completed at least 10 days of TCAD therapy, with no apparent treatment-related adverse effects reported. Specifically, we did not see haematological toxicity with oral RBV.
In our study, only one of six patients who received TCAD had no clinical improvement during treatment. This case was lower respiratory tract disease in a severely immunocompromised host [26], which suggests that efficacy of TCAD therapy may be limited in advanced influenza disease in the absence of functioning host immunity.
Although the control group included only one patient who received OSL monotherapy, TCAD therapy may be associated with earlier viral load reduction. A study in paediatric HCT recipients infected with 2009 H1N1 influenza showed prolonged viral shedding for a median of 46 days despite OSL use in most cases [35]. Such prolonged viral shedding was also observed in our OSL-treated patient, from whom OSL-resistant virus was subsequently isolated. This is in-line with a recent experience at our institution demonstrating that 8/33 (24%) of HCT patients infected with 2009 influenza A/H1N1 developed laboratory-confirmed OSL resistance, with a median shedding duration of 60 days [18]. The infecting viruses in the immunocompromised patients reported in this paper were all either AMT- or OSL-resistant at baseline, suggesting that TCAD therapy may provide a virological benefit regardless of the susceptibility of the infecting virus strain. The contribution of RBV could not be formally evaluated in this study.
In this study, the three patients receiving TCAD therapy who continued to have detectable virus did not develop resistance. Similar to the experience with other pathogens associated with high replication and mutation rates facilitating rapid emergence of resistance against anti-infective agents (for example, HIV and HCV), treatment with multiple anti-influenza agents might be warranted in the treatment of influenza in immunocompromised hosts. This preliminary finding in immunocompromised patients may indicate the effect of TCAD therapy on the emergence of drug-resistant viruses, consistent with a probabilistic model in a recent report [36]. Further evaluation of TCAD therapy in immunocompromised patients is needed to determine its antiviral efficacy, effects on the emergence of resistance and cost–benefit ratio, comparing with either monotherapy or double combination therapy.
This study has several limitations. The small sample size of this pilot study precluded comprehensive antiviral efficacy analyses. The number of patients with underlying malignancies or undergoing transplantation who become infected with influenza and required hospitalization in any given year is not high [4], and we were unable to enrol the projected number of patients for full analysis before the study was terminated because of the lack of funding. As a result, only one patient was randomized to the OSL monotherapy control group. Moreover, because the enrolled patients were very diverse in age, underlying disease status and severity of infections, we could not make definitive conclusions on the antiviral efficacy and safety of TCAD therapy from this pilot study. The lack of blinding of the randomized pilot study is also a potential limiting factor. Nonetheless, the results suggest the safety and potential efficacy of TCAD in immunocompromised patients infected with a range of influenza virus subtypes. These promising observations invite further evaluation in larger studies that are currently ongoing (NCT01227967). Furthermore, the open-label portion of the study is limited by the fact that patients recruited in this arm were more heterogeneous in baseline and clinical characteristics.
We conclude that TCAD therapy showed comparable PKs to monotherapy with each drug from a single dose and appeared to be safe in a small pilot study in immunocompromised patients. Further studies with a larger sample size are needed to fully evaluate the safety and efficacy of TCAD therapy in immunocompromised patients.
Supplementary Material
Acknowledgements
We thank Charles Davis for statistical analyses, Nicolas Day for assistance in the design and data analysis and Gayatri Sathyan for data analysis in the controlled PK study. Mahidol-Oxford Tropical Medicine Research Unit is supported by the Wellcome Trust of Great Britain.
Disclosure statement This work was sponsored and supported by Adamas Pharmaceuticals. JAE received research funding from Sanofi Pasteur Vaccines, Novartis, MedImmune, Inc. and Adamas Pharmaceuticals, and served as a consultant for GlaxoSmithKline. PAT received research support from GlaxoSmithKline, Sanofi, Inviragen and Baxter, as well as speaker honoraria from Astra-Zeneca, MSD and Novartis. MJB received research funding from Roche Pharmaceuticals, GlaxoSmithKline and Adamas Pharmaceuticals, served as a consultant for Novartis, GlaxoSmithKline, Gilead Sciences and Roche/Genentech. JTN and GTW are employees of Adamas Pharmaceuticals.
Footnotes
All other authors declare no competing interests.
References
- 1.Casper C, Englund J, Boeckh M. How I treat influenza in patients with hematologic malignancies. Blood. 2010;115:1331–1342. doi: 10.1182/blood-2009-11-255455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39:1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 3.Chemaly RF, Ghosh S, Bodey GP, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85:278–287. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 4.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9:493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljungman P, de la Camara R, Perez-Bercoff L, et al. Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica. 2011;96:1231–1235. doi: 10.3324/haematol.2011.041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SM, Boudreault AA, Xie H, et al. Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood. 2011;117:5050–5056. doi: 10.1182/blood-2010-11-319186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinosa-Aguilar L, Green JS, Forrest GN, et al. Novel H1N1 influenza in hematopoietic stem cell transplantation recipients: two centers’ experiences. Biol Blood Marrow Transplant. 2011;17:566–573. doi: 10.1016/j.bbmt.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 10.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 11.Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med. 2009;360:953–956. doi: 10.1056/NEJMp0900648. [DOI] [PubMed] [Google Scholar]
- 12.van der Vries E, Schutten M, Boucher CA. The potential for multidrug-resistant influenza. Curr Opin Infect Dis. 2011;24:599–604. doi: 10.1097/QCO.0b013e32834cfb43. [DOI] [PubMed] [Google Scholar]
- 13.Storms AD, Gubareva LV, Su S, et al. Oseltamivir-resistant pandemic (H1N1) 2009 virus infections, United States, 2010-11. Emerg Infect Dis. 2012;18:308–311. doi: 10.3201/eid1802.111466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurt AC, Chotpitayasunondh T, Cox NJ, et al. Antiviral resistance during the 2009 influenza A H1N1 pandemic: public health, laboratory, and clinical perspectives. Lancet Infect Dis. 2012;12:240–248. doi: 10.1016/S1473-3099(11)70318-8. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen HT, Fry AM, Loveless PA, Klimov AI, Gubareva LV. Recovery of a multidrug-resistant strain of pandemic influenza A 2009 (H1N1) virus carrying a dual H275Y/I223R mutation from a child after prolonged treatment with oseltamivir. Clin Infect Dis. 2010;51:983–984. doi: 10.1086/656439. [DOI] [PubMed] [Google Scholar]
- 16.van der Vries E, Stelma FF, Boucher CA. Emergence of a multidrug-resistant pandemic influenza A (H1N1) virus. N Engl J Med. 2010;363:1381–1382. doi: 10.1056/NEJMc1003749. [DOI] [PubMed] [Google Scholar]
- 17.Kiso M, Mitamura K, Sakai-Tagawa Y, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364:759–765. doi: 10.1016/S0140-6736(04)16934-1. [DOI] [PubMed] [Google Scholar]
- 18.Renaud C, Boudreault AA, Kuypers J, et al. H275Y mutant pandemic (H1N1) 2009 virus in immunocompromised patients. Emerg Infect Dis. 2011;17:653–660. doi: 10.3201/eid1704.101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen JT, Hoopes JD, Le MH, et al. Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro. PLoS ONE. 2010;5:e9332. doi: 10.1371/journal.pone.0009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen JT, Smee DF, Barnard DL, et al. Efficacy of combined therapy with amantadine, oseltamivir, and ribavirin in vivo against susceptible and amantadine-resistant influenza A viruses. PLoS ONE. 2012;7:e31006. doi: 10.1371/journal.pone.0031006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoopes JD, Driebe EM, Kelley E, et al. Triple combination antiviral drug (TCAD) composed of amantadine, oseltamivir, and ribavirin impedes the selection of drug-resistant influenza A virus. PLoS ONE. 2011;6:e29778. doi: 10.1371/journal.pone.0029778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen JT, Hoopes JD, Smee DF, et al. Triple combination of oseltamivir, amantadine, and ribavirin displays synergistic activity against multiple influenza virus strains in vitro. Antimicrob Agents Chemother. 2009;53:4115–4126. doi: 10.1128/AAC.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services FDA. Center for Drug Evaluation and Research (CDER) [Updated January 2001. Accessed 10 March 2013];Guidance for industry; statistical approaches to establishing bioequivalence. Available from http://www.fda.gov/downloads/Drugs/Guidances/ucm070244.pdf.
- 24.US Department of Health and Human Services FDA. Center for Drug Evaluation and Research (CDER) [Updated March 2003. Accessed 10 March 2013];Guidance for industry; bioavailability and bioequivalence studies for orally adimistered products – general considerations. Available from http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070124.pdf.
- 25.Duwe S, Schweiger B. A new and rapid genotypic assay for the detection of neuraminidase inhibitor resistant influenza A viruses of subtype H1N1, H3N2, and H5N1. J Virol Methods. 2008;153:134–141. doi: 10.1016/j.jviromet.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Campbell AP, Jacob ST, Kuypers J, et al. Respiratory failure caused by 2009 novel influenza A/H1N1 in a hematopoietic stem-cell transplant recipient: detection of extrapulmonary H1N1 RNA and use of intravenous peramivir. Ann Intern Med. 2010;152:619–620. doi: 10.1059/0003-4819-152-9-201005040-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CDC . Oseltamivir-resistant novel influenza A. (H1N1) virus infection in two immunosuppressed patients. Seattle, Washington: [Updated 14 August 2009. Accessed 10 March 2013]. 2009. Available from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm58d0814a1.htm. [PubMed] [Google Scholar]
- 28.Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med. 2010;7:e1000362. doi: 10.1371/journal.pmed.1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilyushina NA, Hoffmann E, Salomon R, Webster RG, Govorkova EA. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir Ther. 2007;12:363–370. [PubMed] [Google Scholar]
- 30.Smee DF, Wong MH, Bailey KW, Sidwell RW. Activities of oseltamivir and ribavirin used alone and in combination against infections in mice with recent isolates of influenza A (H1N1) and B viruses. Antivir Chem Chemother. 2006;17:185–192. doi: 10.1177/095632020601700403. [DOI] [PubMed] [Google Scholar]
- 31.Simeonova L, Gegova G, Galabov AS. Prophylactic and therapeutic combination effects of rimantadine and oseltamivir against influenza virus A (H3N2) infection in mice. Antiviral Res. 2012;95:172–81. doi: 10.1016/j.antiviral.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Kim WY, Young Suh G, Huh JW, et al. Triple-combination antiviral drug for pandemic H1N1 influenza virus infection in critically ill patients on mechanical ventilation. Antimicrob Agents Chemother. 2011;55:5703–5709. doi: 10.1128/AAC.05529-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison D, Roy S, Rayner C, et al. A randomized, crossover study to evaluate the pharmacokinetics of amantadine and oseltamivir administered alone and in combination. PLoS ONE. 2007;2:e1305. doi: 10.1371/journal.pone.0001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chairat K, Tarning J, White NT, Lindegardh N. Pharmacokinetic properties of anti-influenza neuraminidase inhibitors. J Clin Pharmacol. 2013;53:119–139. doi: 10.1177/0091270012440280. [DOI] [PubMed] [Google Scholar]
- 35.Tran D, Science M, Dix D, et al. Pandemic (H1N1) 2009 influenza in Canadian pediatric cancer and hematopoietic stem cell transplant patients. Influenza Other Respi Viruses. 2012;6:e105–e113. doi: 10.1111/j.1750-2659.2012.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perelson AS, Rong L, Hayden FG. Combination antiviral for influenza: predictions from modeling of human infections. J Infect Dis. 2012;205:1642–1645. doi: 10.1093/infdis/jis265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.