Abstract
Cancer stroma has a profound influence on tumor development and progression. The conversion of fibroblasts to activated myofibroblasts is a hallmark of reactive tumor stroma. Among a number of factors involved in this conversion, TGF-β has emerged as a major regulator. CLIC4, an integral protein in TGF-β signaling, is highly upregulated in stroma of multiple human cancers, and overexpression of CLIC4 in stromal cells enhances the growth of cancer xenografts. In this study we show that conditioned media from tumor cell lines induces expression of both CLIC4 and the myofibroblast marker alpha smooth muscle actin (α-SMA) in stromal fibroblasts via TGF-β signaling. Genetic ablation of CLIC4 in primary fibroblasts prevents or reduces constitutive or TGF-β induced expression of α-SMA and extracellular matrix components that are markers of myofibroblasts. CLIC4 is required for the activation of p38 Map Kinase by TGF-β, a pathway that signals myofibroblast conversion in stromal cells. This requirement involves the interaction of CLIC4 with PPM1a, the selective phosphatase of activated p-38. Conditioned media from fibroblasts overexpressing CLIC4 increases tumor cell migration and invasion in a TGF-β dependent manner and promotes epithelial to mesenchymal transition indicating that high stromal CLIC4 serves to enhance tumor invasiveness and progression. Thus, CLIC4 is significantly involved in the development of a nurturing tumor microenvironment by enhancing TGF-β signaling in a positive feedback loop. Targeting CLIC4 in tumor stroma should be considered as a strategy to mitigate some of the tumor enhancing effects of the cancer stroma.
Keywords: CLIC, α-SMA, p38, microenvironment, PPM1a
Introduction
CLIC4 (Chloride Intracellular Channel 4) is a p53 and TGF-β regulated, 28kD member of a family of seven CLIC proteins that are ubiquitously expressed in various tissue types and participate in diverse biological functions (1; 2). CLICs are structural homologues of the Glutathione-S-transferase superfamily of proteins and are redox regulated. CLIC4 is dimorphic, found both in soluble form in the cytosol as well as in organellar membranes (3–5). CLIC4 has putative chloride ion selective channel activity with single channel conductance in biological and artificial membranes (6). In soluble form, CLIC4 contributes to vasculogenesis by promoting endothelial cell proliferation and lumen formation (7; 8). Notably, CLIC4 KO mice have defective angiogenesis (8; 9) and poor skin wound healing (10). Expression of CLIC4 is markedly upregulated in the adjacent stroma of multiple human cancers, co-localizing with alpha smooth muscle actin (α-SMA), a marker of cancer stroma (11; 12). Exogenous expression of CLIC4 in stromal fibroblasts enhances the growth of human breast carcinoma cells in xenografts (12). These properties of CLIC4 in human cancers suggest the protein participates in tumor growth through a microenvironmental function that has not been elucidated. CLIC4 is upregulated by TGF-β and is an integral component of TGF-β signaling through its action in preventing the dephosphorylation of phospho-Smad 2 and 3 in the nucleus (13; 14). Previous studies have shown that CLIC4 is the most upregulated gene during TGF-β mediated myofibroblast conversion of primary human breast fibroblasts and contributes to their stationary phenotype (11). Furthermore, overexpression of CLIC4 in human fibroblasts induces α-SMA expression (12). Located vicinal to the neoplastic epithelial cells, myofibroblasts are specialized fibroblasts that secrete growth factors, cytokines and matrix metalloproteinases. They actively promote tumor growth through their interactions with carcinoma cells changing the latter phenotypically and biochemically (15; 16). Since both expression and xenograft studies in vivo provide compelling evidence that CLIC4 participates in the promotion of a human cancer stroma, we used an in vitro model system to study the functional contributions of CLIC4 in the cancer stroma and to elucidate the mechanisms involved.
Results
Tumor cells induce CLIC4 and α-SMA in surrounding fibroblasts via TGF-β signaling
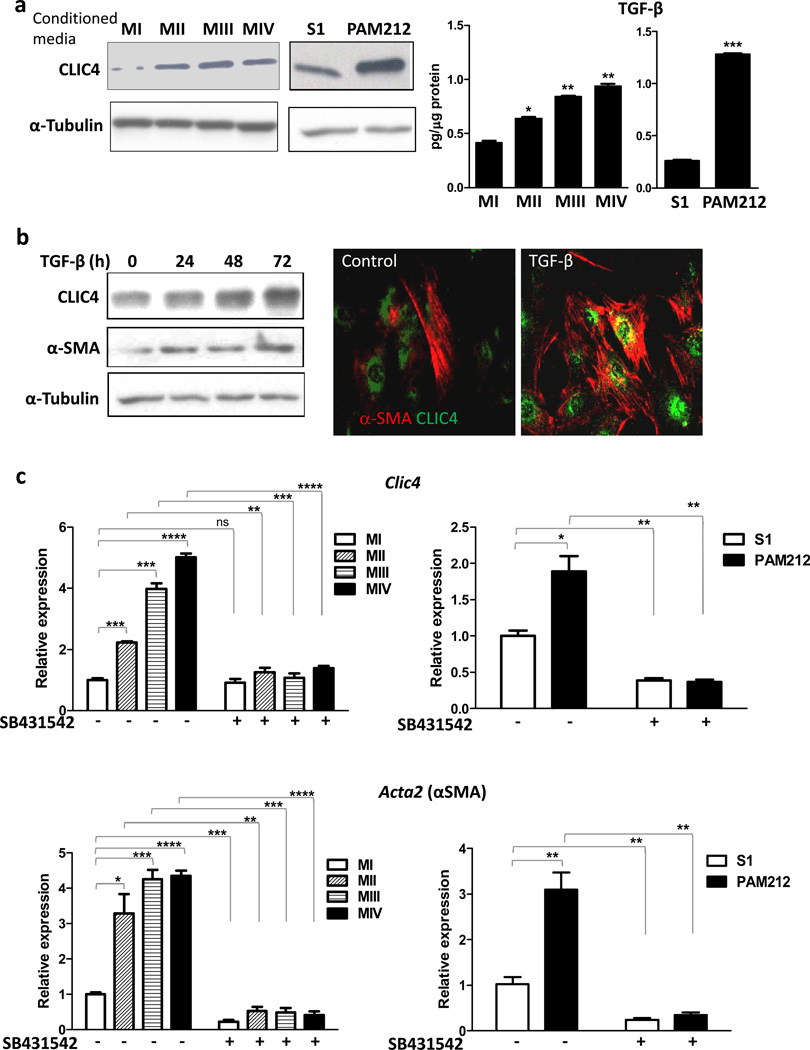
We used several in vitro models to investigate the underlying mechanisms that mediate the upregulation of CLIC4 in cancer stroma, the biological consequences of upregulation and its relation to α-SMA expression. To test whether the increase in stromal CLIC4 seen in multiple cancers could be a tumor cell driven process, we treated primary dermal fibroblasts with conditioned media from mouse and human cancer cell lines. Conditioned media from human mammary cancer (MII, III, IV) (17; 18) and mouse squamous cancer PAM212 cell lines increased the expression of CLIC4 protein in fibroblasts compared to non-tumorigenic cell lines (S1 and MI) indicating that these tumor cells acted on the stroma in a paracrine way to upregulate CLIC4 (Figure 1a, left panel). CLIC4 expression is TGF-β regulated (13). ELISA analysis of conditioned media shows that these tumor cells secrete TGF-β (Figure 1a, right panel) and the amount increases with increasing invasiveness indicating that increased TGF-β might contribute to the progressively enhanced CLIC4 expression. TGF-β treatment of fibroblasts enhances CLIC4 (Figure 1b, left panel), and this is concurrent with increase in α-SMA expression. Notably, these proteins are co-expressed in the same cells (Figure 1b, right panel) as shown by immunofluorescence. The increase in CLIC4 expression by tumor conditioned media occurs at the transcriptional level and coincides with induction of α-SMA transcripts (gene name Acta2) (Figure 1c) consistent with the progressive increase in TGF-β levels in these conditioned media. Treatment of fibroblasts with ALK5 blocker SB431542 prior to conditioned medium treatment abolishes both tumor induced CLIC4 and α-SMA expression. These results indicate that expression of CLIC4 and α-SMA in stromal fibroblasts is regulated by TGF-β released by tumor cells.
Figure 1.
Tumor cells induce CLIC4 and α-SMA expression in fibroblasts via TGF-β signaling. (a) Left Primary mouse dermal fibroblasts were treated with serum free conditioned media from either of the human breast cell lines MI, MII, MIII or MIV or the murine squamous cell lines S1 or PAM 212 cells. Expression of CLIC4 was analyzed by immunoblotting. Right TGF-β concentrations in conditioned media from human and mouse cell lines were determined by ELISA and normalized to total protein content. Data sets were compared for statistical significance with MI or S1(non-tumorigenic lines). (b) Primary dermal fibroblasts were treated for different time periods with TGF-β (10ng/ml) and immunoblotted (left) for CLIC4 and α-SMA. (Right) Co-immunofluorescence for CLIC4 (green) and α-SMA (red) in primary untreated fibroblasts or fibroblasts treated with TGF-β for 48h. (c) Primary dermal fibroblasts were treated with conditioned media as in A with or without pretreatment with the ALK5 inhibitor SB431542 (5µM). Expression of CLIC4 and α-SMA (gene name Acta2) was analyzed by real time PCR normalized to respective GAPDH levels and plotted as relative to MI or S1. Data sets were compared as indicated by lines for statistical significance.
CLIC4 is required for TGF-β dependent conversion of fibroblasts to myofibroblasts
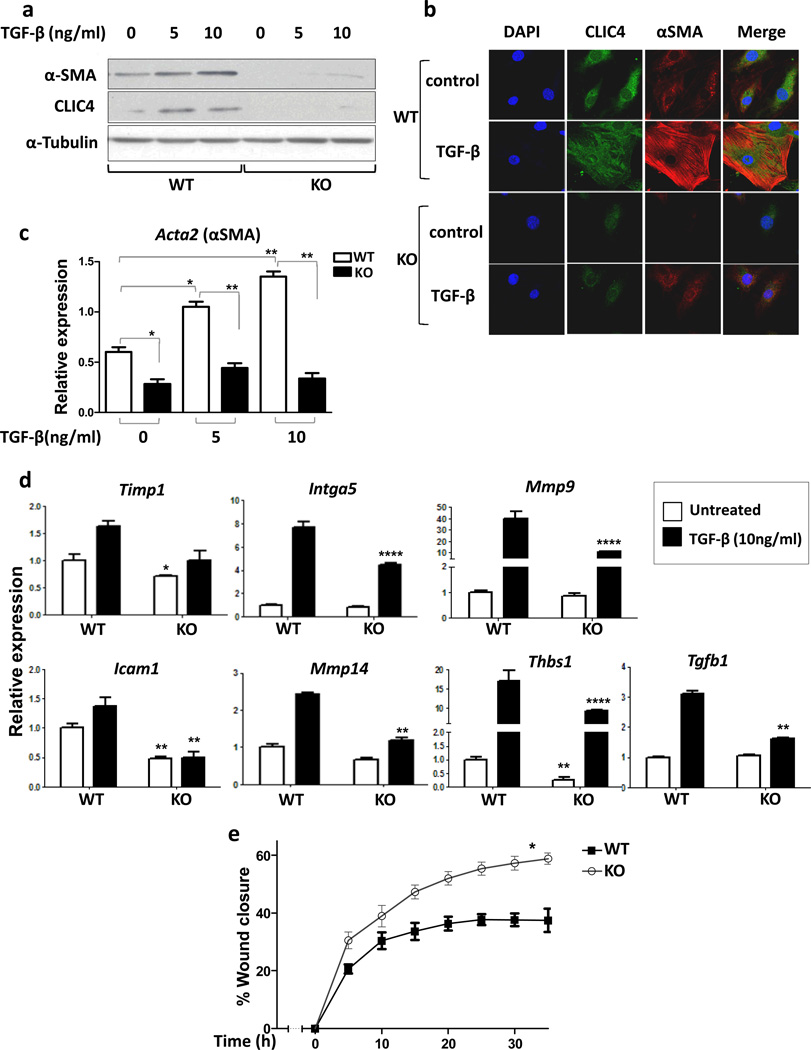
To determine if CLIC4 is required for fibroblast to myofibroblast conversion, we used the Cre-lox system to delete exon 2 of CLIC4 in floxed mice (10). Fibroblasts isolated from these mice were ablated of CLIC4 in vitro by adenoviral expression of Cre recombinase. In response to TGF-β (Figure 2a and 2b) CLIC4 null fibroblasts did not convert to myofibroblasts as defined by induced expression of α-SMA and cell spreading. The induction of α-SMA transcript by TGF-β requires the presence of CLIC4 in fibroblasts (Figure 2c). Furthermore, the expression of exogenous CLIC4 by adenoviral transduction enhances α-SMA expression even in the absence of TGF-β treatment and further enhances the induction by TGF-β (Supplementary Figure 1).
Figure 2.
CLIC4 is required for TGF-β dependent conversion of fibroblasts to myofibroblasts. (a) Adenoviral Cre recombinase transduced CLIC4 wild-type (WT) and floxed fibroblasts (KO) were treated with TGF-β for 48h at varying concentrations and immunoblotted for CLIC4 and α-SMA. α-Tubulin was used as loading control. (b) Co-immunofluorescence for CLIC4 and α-SMA in CLIC4 WT and KO fibroblasts treated with or without TGF-β (10ng/ml). (c) CLIC4 WT and KO fibroblasts treated with TGF-β for 24h at varying concentrations and analyzed for α-SMA by real time PCR. Data sets were compared as indicated by lines for statistical significance. (d) Real time PCR analysis of various ECM genes in CLIC4 WT and KO fibroblasts with or without treatment with 10ng/ml TGF-β for 24h. For statistical analysis, untreated KO were compared to untreated WT. TGF-β treated KO were compared to TGF-β treated WT. (e) Scratch assay on WT and KO fibroblasts in media containing 0.2% serum with 10ng/ml TGF-β. Migration was recorded and quantified using Incucyte technology. The two curves were compared for statistical significance by Mann Whitney t-test.
Myofibroblasts play a central role in the synthesis, degradation and remodeling of the extracellular matrix, a process that is TGF-β regulated (19). CLIC4 ablated fibroblasts have reduced basal and TGF-β induced expression of ECM genes Timp1, Itga5, Mmp9, Icam1, Mmp14, Thbs1 and TGF-β1 compared to their wild-type counterparts (Figure 2d). Myofibroblasts are also characterized by a reduced motility and rate of migration (20; 21). CLIC4 ablated fibroblasts have greater mobility compared to wild-type fibroblasts in the presence of TGF-β (Figure 2e). This implies that TGF-β reduces fibroblast motility owing to their myofibroblast conversion (21), but has a smaller influence on fibroblasts devoid of CLIC4. Thus, in the absence of CLIC4, TGF-β induced fibroblast to myofibroblast conversion is greatly impaired by multiple parameters.
TGF-β regulates myofibroblast conversion via p38MAPK signaling
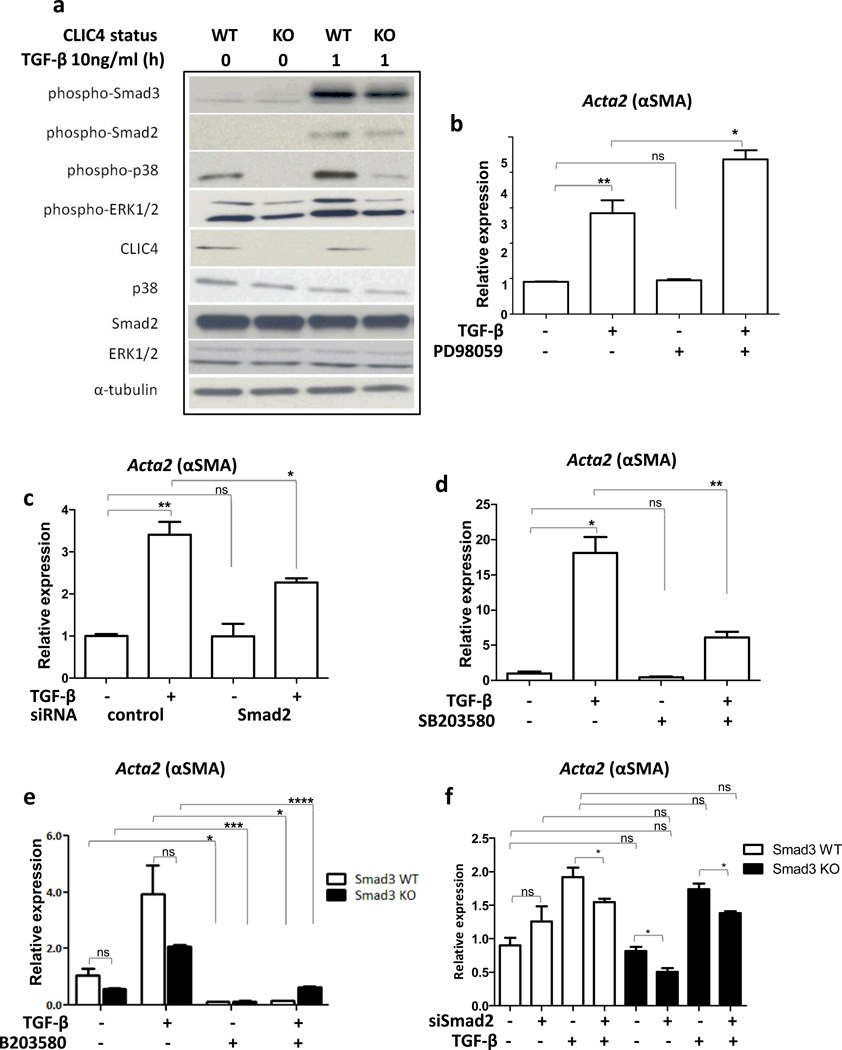
TGF-β signals through Smad dependent and independent pathways to affect cellular responses. Upon TGF-β treatment, CLIC4 ablated fibroblasts had markedly reduced activation of p38MAPK and a small decrease in activated Smads 2/3 and p-ERK1/2 (Figure 3a). Chemical blockade of ERK1/2 signaling using PD98059 (Figure 3b) did not reduce the level of TGF-β induced α-SMA transcript, and siRNA knockdown of Smad 2 (Figure 3c) had only a partial effect. In contrast, p38MAPK blockade using SB203580 substantially inhibited the capacity of TGF-β to upregulate α-SMA expression (Figure 3d). To assess a possible contribution of Smad3 in α-SMA induction, we used primary fibroblasts from Smad3 KO or WT mice. As shown in Figure 3e, α-SMA expression was lower (although statistically not significant) in Smad3 KO fibroblasts at the basal level and upon TGF-β treatment, indicating a potential partial role for Smad3 in the regulation of α-SMA in fibroblasts. However, the basal and induced levels of α-SMA were markedly reduced by SB203580 pretreatment (Figure 3e) indicating a major function for the p38 pathway in TGF-β mediated α-SMA expression. Combined loss-of-function of Smad2 and Smad3 by siRNA mediated knockdown of Smad2 in Smad3 KO fibroblasts did not reduce α-SMA expression significantly more than knockdown of Smad2 alone (Figure 3f). In keratinocytes, TGF-β causes CLIC4 nuclear translocation in conjunction with Schnurri-2, and nuclear CLIC4 prolongs Smad signaling by inhibiting phospho-Smad 2/3 interaction with the phosphatase PPM1a (13). Similarly, Schnurri-2 is equally abundant in fibroblasts, (Supplementary Figure 2a), and CLIC4 inhibits the interaction of phospho-Smads with PPM1a (Supplementary Figure 2b). Thus, this mechanism defined in keratinocytes is also intact in TGF-β treated fibroblasts, but the targeting of CLIC4 on TGF-β mediated p38 activation appears to be the dominant pathway responsible for the regulation of fibroblast to myofibroblast conversion by TGF-β. We further defined the p38 isoform responsible for TGF-β dependent α-SMA expression in primary dermal fibroblasts. Upon siRNA mediated knockdown of p38α, p38β and p38δ independently, PCR analysis showed that α-SMA expression is most compromised when p38α expression is reduced and unaffected by ablation of p38δ (Supplementary Figure 3).
Figure 3.
CLIC4 dependent p38 and Smad activation contribute to increased α-SMA expression in primary dermal fibroblasts. (a) Lysates of CLIC4 WT and KO fibroblasts untreated or treated with TGF-β (10ng/ml) for 1h were immunoblotted for proteins involved in TGF-β signaling. α-Tubulin was used as loading control. (b–d) Primary dermal fibroblasts were pretreated with (b) 60µM MEK1 inhibitor PD98059 for 30 min, (c) Smad2 siRNA for 48h or (d) 10µM p38 inhibitor SB 203580 for 30 min before TGF-β treatment (10ng/ml) for 24h. α-SMA expression was analyzed by real time PCR, normalized to respective GAPDH levels and expressed as relative to untreated control.(e,f) Primary dermal fibroblasts from Smad3 wild-type or knockout mice were (e) pretreated with 10µM p38 inhibitor SB203580 for 30min, or (f) transfected with nonsilencing control (NS) or Smad2 siRNA for 48h before TGF-β treatment for 24h. α-SMA expression was analyzed by real time PCR, normalized to respective GAPDH levels and expressed as relative to untreated control. b-f, Data sets were compared as indicated by lines for statistical significance. ns=not significant.
CLIC4 reduces p38 dephosphorylation
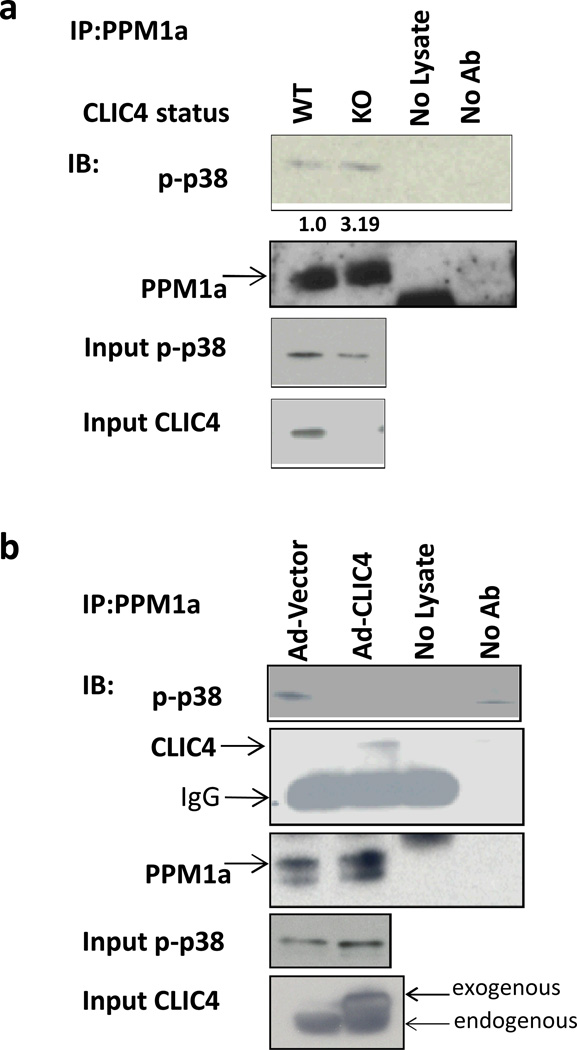
We next examined the interaction of CLIC4 with p38. Studies have shown that in addition to Smad2/3 (22), PPM1a dephosphorylates other phospho-proteins including p38, MKK6, JNK and SEK1 (23), CDK2 and CDK6 (24), and IKKβ (25) leading to inactivation. Figure 4 indicates that dephosphorylation of p38 in fibroblasts is modified by CLIC4. In the absence of fibroblast CLIC4, more phospho-p38 was associated with PPM1a in co-immunoprecipitation assays than was associated in wild-type fibroblasts (Figure 4a) and the content of p-p38 is reduced (Figure 4a, input p-p38). Conversely, in fibroblasts overexpressing CLIC4, as seen in cancer stroma, the amount of phospho-p38 that co-IPs with PPM1a (Figure 4b) is reduced, indicating that CLIC4 interferes with the interaction of phospho-p38 with its primary phosphatase, PPM1a, resulting in higher levels of activated p-38 (Figure 4b, input p-p38). CLIC4 and PPM1a co-immunoprecipitate in pull down experiments in lysates from fibroblasts overexpressing CLIC4 (Figure 4b), raising the possibility that CLIC4 interaction with PPM1a might cause reduced binding of PPM1a to multiple substrates.
Figure 4.
CLIC4 prolongs p38 phosphorylation by inhibiting interaction of p-p38 and its phosphatase PPM1a. (a) Lysates from CLIC4 WT and KO fibroblasts were immunoprecipitated with anti-PPM1a antibody and immunoblotted for phospho-p38 and PPM1a. Non-immunoprecipitated (input) lysates were immunoblotted for phospho-p38 and CLIC4. Immunoprecipitated p-p38 bands were quantified using Image J software, normalized to their respective input p-p38 bands and expressed relative to WT. (b) Primary dermal fibroblasts were transduced with vector or CLIC4 expressing adenovirus. Lysates were immunoprecipitated with anti-PPM1a antibody and immunoblotted for phospho-p38, CLIC4 and PPM1a. Non-immunoprecipitated (input) lysates were immunoblotted for phospho-p38 and CLIC4. A,B-Samples undergoing the immunoprecipitation process without any protein lysate (no lysate) or without antibody (no Ab) were used as controls.
Stromal CLIC4 enhances migration, invasion and epithelial to mesenchymal transition of tumor cells
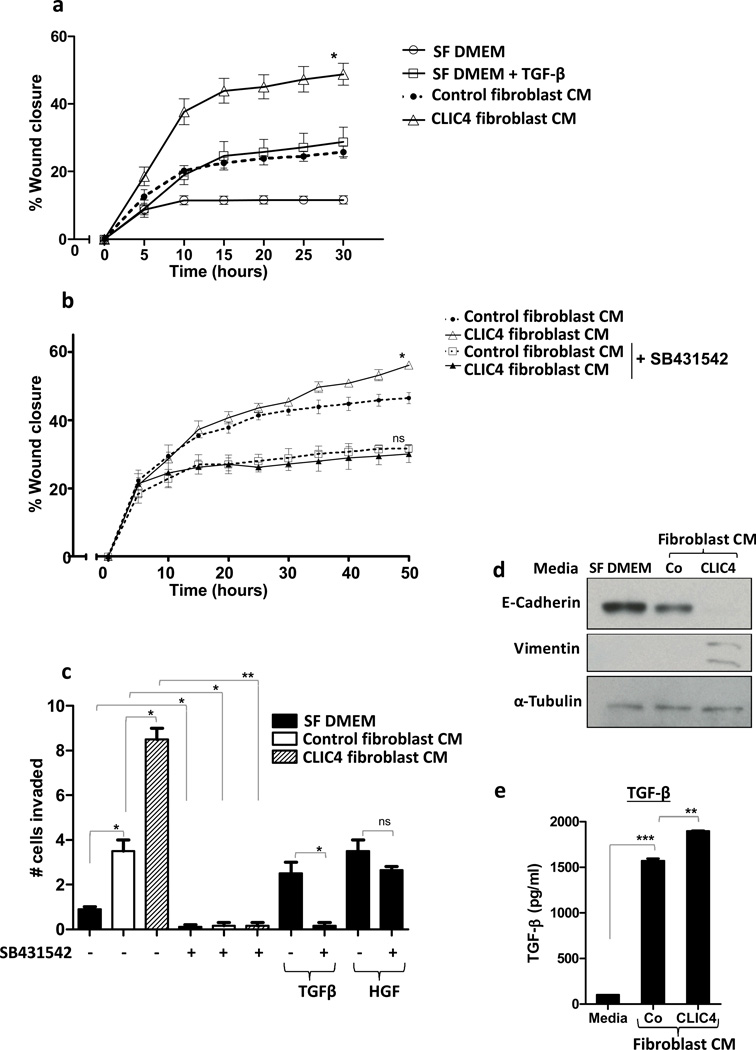
Since CLIC4 is highly expressed in cancer stroma in vivo (12), we examined the potential consequences of high stromal CLIC4 on tumor cells in vitro. Migration of PAM212 cells was enhanced when treated with conditioned medium from adeno-CLIC4 overexpressing fibroblasts when compared to medium conditioned by control fibroblasts infected with the adeno-vector only (Figure 5a). Notably the effect of conditioned medium from CLIC4 overexpressing fibroblasts was lost upon pretreatment of PAM212 cells with the ALK5 blocker SB431542 (Figure 5b). This suggests that stromal CLIC4 via TGF-β signaling imparts an enhanced migratory capacity to PAM212 cells. When conditioned media from CLIC4 overexpressing fibroblasts was placed in the lower chamber in a matrigel invasion assay (Figure 5c), a greater number of PAM212 cells migrated across the matrigel when compared to control conditioned medium. Pretreatment of PAM212 cells with SB431542 and presence of SB431542 in the conditioned media ablated the effect of conditioned media on invasion. When TGF-β and HGF were used as chemoattractants in the lower chamber, SB431542 blocked TGF-β dependent invasion but not HGF dependent invasion of PAM212 cells, indicating the specificity of SB431542 for blocking TGF-β activity. Enhanced migration and invasion could be a consequence of these tumor cells undergoing epithelial to mesenchymal transition (EMT) (26). Figure 5d shows that conditioned media from CLIC4 overexpressing fibroblasts suppressed expression of E-Cadherin and upregulated Vimentin in PAM212 cells, two molecular events that characterize EMT. ELISA of conditioned media confirmed that fibroblast conditioned medium contained considerable amounts of TGF-β, which was greater in medium from fibroblasts expressing exogenously elevated levels of CLIC4 (Figure 5e). To confirm that squamous tumor cells were sensitive to stromal CLIC4 in vivo, we constructed a xenograft model at the orthotopic site on the dorsal skin with human SCC13 keratinocytes (27) mixed with stromal fibroblasts modified to induce exogenous CLIC4 upon treatment of the host with Doxycycline (Supplementary Figure 4a). While tumor size increased in parallel prior to CLIC4 induction, subsequent growth was accelerated substantially once CLIC4 was induced in the stroma. Correspondingly, 100% (5/5) of orthografts of oncogenic ras transformed wild-type primary keratinocytes formed well-differentiated squamous cell carcinomas in wild-type hosts while only 1/5 of the same donor cells were tumorigenic in CLIC4 KO hosts (Supplementary Figure 4b).
Figure 5.
Stromal CLIC4 enhances tumor cell migration and invasion via TGF-β signaling. (a) Scratch assay on PAM212 cells treated with either serum free media (SF DMEM) +/− TGF-β (10ng/ml) or serum free conditioned media from fibroblasts transduced with adeno-vector (Control fibroblast CM)) or adeno-CLIC4 (CLIC4 fibroblast CM). Control CM curve was compared to CLIC4 CM curve for statistical analysis using Mann Whitney t-test. (b) Scratch assay on PAM212 cells treated with serum free conditioned media from fibroblasts transduced with adeno-vector control or adeno-CLIC4 +/− ALK5 blocker SB431542 (5µM). For statistical analysis, untreated control CM curve was compared to untreated CLIC4 CM curve while SB431542 treated control CM curve was compared to treated CLIC4 CM curve using Mann Whitney t-test. A,B-Time lapse monitoring of migration of PAM212 cells was conducted using Incucyte technology. (c) Matrigel invasion assay on PAM212 cells plated in matrigel coated upper chambers of a 24-transwell plate. Serum free media (SF DMEM)or serum free conditioned media from fibroblasts transduced with adeno-vector (Control fibroblast CM) or adeno-CLIC4 (CLIC4 fibroblast CM) was placed in the lower chamber. ALK5 blocker SB431542 (5µM) was added to the upper and lower chambers and TGF-β or HGF added to the lower chambers as indicated. Invasion through matrigel was analyzed after 24h. Data sets were compared as indicated by lines for statistical significance. (d) PAM212 cells were treated with SF DMEM or serum free conditioned media from fibroblasts transduced with adeno-vector (Co) or adeno-CLIC4 (CLIC4) for 6 days. Whole cell lysates were immunoblotted for E-Cadherin, Vimentin and α-Tubulin. (e) ELISA analysis of TGF-β levels in SF DMEM (Media) or serum free conditioned media from vector transduced (Co) or CLIC4 overexpressing (CLIC4) fibroblasts. For statistical analysis, Co data set was compared to SF DMEM and CLIC4 to Co.
Discussion
The importance of the tumor stroma has long been appreciated and it is now widely accepted that tumor associated fibroblasts and myofibroblasts are pivotal in supporting tumor growth, progression and invasion (28; 29). In the present study, we show that endogenous fibroblast CLIC4 is important for TGF-β driven conversion of fibroblasts to myofibroblasts. The importance of CLIC4 in the cancer stroma is likely to be physiologically significant since tumor cells themselves upregulate CLIC4 expression in the vicinal stroma (present study) and fibroblasts overexpressing CLIC4 enhance tumor growth in vivo (12). Tumor cells cause a variety of changes in their surroundings to aid their own prosperity. Our data indicate that the paracrine activity of tumor cells responsible for CLIC4 induction is through TGF-β signaling. TGF-β is a key cancer cell derived cytokine that affects the stromal compartment to enhance matrix remodeling, secrete other growth and angiogenic factors, produce anti-apoptotic molecules and stimulate cancer progression (30–32). This is accomplished by stimulating conversion of stromal fibroblasts into myofibroblasts (33). We show that cell lines with increasing tumorigenicity secrete progressively higher levels of TGF-β and induce CLIC4 and α-SMA expression coordinately suggesting that CLIC4 is a key mediator in the TGF-β regulated myofibroblast conversion program and hence contributes to cancer progression.
In the absence of CLIC4, myofibroblast conversion is markedly reduced, at least as measured by α-SMA expression. α-SMA positive myofibroblasts are major components of the tumor microenvironment and modulate tumor growth in a paracrine way (34). α-SMA expression imparts greater contractility to the fibroblasts (35; 36). Stromal expression of α-SMA is a marker of aggressive basal cell carcinoma (34) and predicts disease recurrence in colorectal cancer (37). Our data suggest that CLIC4 is integral to the TGF-β dependent induction of α-SMA via p38MAPK activation. A number of signaling pathways mediated by TGF-β have been implicated in myofibroblast conversion of fibroblasts. The induction of α-SMA in lung myofibroblasts is controversial and has been reported to be predominantly either Smad 2, Smad 3 or Erk dependent (38–41) in different analyses. p38 inhibitors prevent myofibroblast conversion of human tendon fibroblasts (42). Likewise, it is the activation of p38MAPK that we find the most compelling evidence for regulating TGF-β mediated α-SMA expression in murine primary dermal fibroblasts. While Smad2 had a partial effect, Smad3 knockdown or Erk blockade did not reduce α-SMA expression in these fibroblasts. A previous study suggested that the contribution of TGF-β and CLIC4 to myofibroblast conversion of the fetal lung fibroblast cell line MRC5 was mediated by reactive oxygen (43) but we could not reproduce that result in primary skin fibroblasts (data not shown). We attribute the impact of CLIC4 on activation of p38MAPK to reduced interaction of phospho-p38 with its phosphatase PPM1a, thus enhancing the p38 signaling in response to TGF-β. The association of CLIC4 with PPM1a could negatively influence binding of PPM1a to all its substrates and inhibit their subsequent dephosphorylation. We have previously shown that CLIC4 prolongs Smad2/3 signaling by inhibiting their dephosphorylation via reduced access to PPM1a. The present results with p38 strengthen the likelihood of CLIC4 operating via a common mechanism such as binding to PPM1a to prevent its interaction with PPM1a substrates. Theoretically, CLIC4 could either bind the catalytic domain of PPM1a (44) (Supplementary Figure 5, top panel) and hinder binding to PPM1a substrates or bind in a non-catalytic (beta pleated) region to change the molecular conformation and disrupt the substrate binding site. By molecular modeling and sequence analysis, it is predicted that CLIC4 may be phosphorylated (45) (Supplementary Figure 5bottom panel) and in such a circumstance, phospho-CLIC4 might bind PPM1a as a substrate. Unphosphorylated CLIC4 may bind the substrate binding (catalytic) pocket of PPM1a as well. It is possible that the smaller size of CLIC4 makes for higher affinity binding to PPM1a than its other substrates. Although, binding of CLIC4 to the non-catalytic domain of PPM1a is possible, if this binding were to be such that it caused a substantial conformational change and destroyed the substrate binding pocket of PPM1a, it would leave PPM1a at a high energy unstable state and hence this possibility is less likely. Therefore by structural modeling prediction, the first possibility seems more probable. These predictions, until experimentally verified, remain mere possibilities and would be a subject of future studies.
We show that stromal CLIC4 helps tumor cells migrate, invade and undergo EMT. Underlying this property of CLIC4 is its action to enhance secretion of TGF-β by fibroblasts and its essential role in myofibroblast generation, induction of α-SMA and expression of ECM genes. Myofibroblasts are proinvasive, located mainly at the invasive front in cancer of the colon, breast, liver, lung and pancreas (46). TGF-β produced by activated tumor stroma is a critical mediator of epithelial-mesenchymal transition (EMT) of nearby carcinoma cells which would enhance their invasiveness (47). Enhanced MMP secretion would increase basement membrane degeneration. Thus enhanced stromal CLIC4 expression presents a multitude of factors to aid cancer growth and invasion.
With increasing evidence for the importance of tumor-stromal interactions in affecting the behavior of cancer cells, the viability of stromal therapy is being considered (48). Targeting stromal signals that influence tumor growth and invasion would limit the stromal reaction and cripple the tumor. In this regard, CLIC4 or p38 are attractive targets. Downregulation of CLIC4 in the stroma through antisense /siRNA means would lead to reduced fibroblast transdifferentiation and ECM remodeling and hamper tumor growth. The reduction in tumor growth in absence of stromal CLIC4 in our orthograft assay points to the merit of this approach. Several TGF-β inhibitors are being developed for anticancer therapy (49; 50). However, targeting the TGF-β pathway could be fraught with severe side effects in light of the friend and foe dual role of TGF-β (50–52). Considering the very high level of CLIC4 expressed in the myofibroblast stroma of many epithelial cancers (11; 12) and the marked reduction of myofibroblast markers in the absence of CLIC4, perhaps targeting CLIC4 or p38 would be a more specific approach to inhibit the tumor enhancing aspect of TGF-β signaling and therefore would give better therapeutic outcome.
Materials and methods
Cell culture, expression vectors, pathway inhibitors and transfection
Primary dermal fibroblasts from newborn CLIC4 wild-type mice on a C57Bl/6 background or mice from the same breeding colony in which the second exon of CLIC4 is flanked by loxP sites to generate CLIC4 ablated cells upon recombination with Cre recombinase (floxed mice) (10) were prepared and cultured according to established methods (53) in DMEM supplemented with 10% treated fetal bovine serum. Primary fibroblasts from Smad3 knockout mice were kindly provided by Dr. Lalage Wakefield (National Cancer Institute). Well established human breast epithelial cell lines of increasing tumorigenicity in xenograft assays, MI, MII, MIII and MIV (17; 18) were also provided by Dr. Lalage Wakefield and cultured in DMEM. The non-tumorigenic mouse keratinocyte cell line S1 and the mouse squamous cell carcinoma cell line PAM212 were cultured in Eagle’s Minimum Essential medium supplemented with 0.05 mM CaCl2 and 8% chelex treated fetal bovine serum. Human SCC 13 squamous cancer cells (27) were provided by James Rheinwald (Harvard Medical School). To delete CLIC4 from the floxed fibroblasts, cultured wild-type and floxed cells were exposed to an adenovirus encoding the Cre recombinase (adeno-Cre) (kind gift of Dr. Frank Gonzalez, NCI) for 48 hours on day 4 of culture and used for experiments accordingly. Deletion of CLIC4 was confirmed by immunoblotting and these cells are referred to as CLIC4 KO.
Generation of conditioned media
Conditioned media from mouse keratinocyte cell lines S1 and PAM212 or human breast epithelial cell lines (Ml – MIV) were generated by culturing these cell lines in serum free media for 72h. Culture supernatants were then centrifuged at 10,000 rpm and filtered. For generation of conditioned media from CLIC4 overexpressing or control fibroblasts, fibroblasts were transduced with either empty vector or CLIC4 expressing adenovirus for 48h, and serum free conditioned medium was subsequently centrifuged and filtered as above. TGF-β concentration was measured in conditioned media using the R&D Systems Quantikine TGF-β1 immunoassay according to manufacturer’s instructions. It was normalized to total cell protein.
Antibodies, immunoprecipitation and immunoblotting
Monospecific polyclonal antibody generated against the N-terminal peptide of CLIC4 has been described elsewhere (54). The polyclonal serum was purified through a protein A column (Pharmacia) following manufacturer’s specifications and dialyzed in borate buffer. Phospho-p38 (p-p38), total p38, p-ERK1/2, total ERK1/2, phospho-Smad2, total Smad2 and Vimentin antibodies were from Cell Signaling Technologies. PPM1a antibody was from Abcam, α-SMA from Sigma, E-cadherin from Santa Cruz and α-tubulin from Invitrogen. Protein expression was analyzed by immunoblotting. Cells were washed and scraped into lysis buffer (Cell Signaling Technologies). 25 µg of protein was subjected to SDS-PAGE and immunoblotting and visualized using enhanced chemiluminescence (Pierce Biotechnology, Inc). For immunoprecipitation, cells were washed with cold phosphate-buffered saline (PBS) and lysed in M-PER (Pierce Biotechnology, Inc) lysis buffer containing 5 mM EGTA, 20 µM leupeptin, 10 µg aprotinin, 1 mM phenylmethylsulfonyl fluoride, 200 µM NaVO3 and 10 mM NaF. Lysates were immunoprecipitated with anti-PPM1a antibody at 4°C using the Exacta Cruz™ protocol. Beads were washed in immunoprecipitation buffer, centrifuged, resuspended and boiled prior to electrophoresis.
Real time PCR
Total RNA from fibroblasts was isolated using Trizol (Invitrogen), and reverse transcribed using SuperScript III First Strand kit (Invitrogen). SYBR Green (Biorad) based real-time PCR analysis was carried out using predesigned RT2 qPCR primer assay primers (Qiagen), BioRad iQ5 iCycler and Gene Expression Macro. Results are expressed as relative units after normalization to GAPDH expression levels.
Scratch assay
Scratch Assay was performed using the IncuCyte technology. PAM212 cells plated in 24 well IncuCyte image lock plates were scratched using IncuCyte Wound Maker Tool and washed twice with medium. Subsequently, conditioned media, TGF-β or pathway blockers were added to the wells and the scratched area (wound) imaged in a time lapse manner for 24h using IncucyteFLR. Results were plotted using the IncucyteFLRsoftware in terms of percentage wound closure as a function of time.
Matrigel migration assay
BD Biocoat matrigel invasion chambers in a 24 well plate were rehydrated in serum free DMEM for 2h in a humidified incubator at 37C, 5%CO2. 750ul of conditioned media with or without pathway blockers was added to lower chambers followed immediately by addition of 50,000 PAM212 cells per well in the top chambers. Plates were incubated at 37C for 24h. The top side of the inserts was scrubbed, and the inserts stained in Diff Quick stain, washed and dried. The membranes were cut, mounted on a slide and stained cells were counted under a microscope.
Confocal microscopy
Cells grown in glass chamber slides were fixed in 4% paraformaldehyde/PBS (phosphate buffered saline) for 20 min, washed with PBS and permeabilized with 0.5% Triton for 10 min. Samples were then blocked in 1% bovine serum albumin (BSA)/PBS + 0.1% horse serum for 10 min. This was followed by incubation with anti-CLIC4 antibody (1:100) for 72h at 4 °C and anti-α-SMA antibody (1:1000) for the last 1h. Slides were washed with PBS and incubated in the dark with fluorescence-labeled anti-mouse together with anti-rabbit secondary antibody for 45min. The plates were washed again, Vectashield mounting medium with DAPI (Vector Laboratories) was added for nuclear staining and cells were analyzed by confocal microscopy (Zeiss-NLO microscope).
Statistics
All experiments were repeated a minimum of two times and data were subjected to unpaired t-test. Line graphs were evaluated using Mann Whitney t-tests. P-values are indicated in Figures. **** p<0.0001, *** p<0.0005, ** p<0.005, *p<0.05
Supplementary Material
Acknowledgements
The authors thank Dr. Lalage Wakefield, National Cancer Institute, Bethesda for providing Smad3 KO mice and MI, MII, MIII, MIV cell lines, Dr. Ed Leof, Department of Biochemistry and Molecular Biology, Mayo Clinic, Minnesota for providing anti-phospho Smad3 antibody and Dr. Akira Oshima, National Cancer Institute for help with immunofluorescence. This work was supported by the intramural program of the Center for Cancer Research, National Cancer Institute.
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- 1.Suh KS, Yuspa SH. Intracellular chloride channels: critical mediators of cell viability and potential targets for cancer therapy. Curr Pharm Des. 2005;11(21):2753–2764. doi: 10.2174/1381612054546806. [DOI] [PubMed] [Google Scholar]
- 2.Littler DR, Harrop SJ, Goodchild SC, Phang JM, Mynott AV, Jiang L, et al. The enigma of the CLIC proteins: Ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010 May 17;584(10):2093–2101. doi: 10.1016/j.febslet.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Littler DR, Harrop SJ, Fairlie WD, Brown LJ, Pankhurst GJ, Pankhurst S, et al. The intracellular chloride ion channel protein CLIC1 undergoes a redox-controlled structural transition. J Biol Chem. 2004 Mar 5;279(10):9298–9305. doi: 10.1074/jbc.M308444200. [DOI] [PubMed] [Google Scholar]
- 4.Littler DR, Assaad NN, Harrop SJ, Brown LJ, Pankhurst GJ, Luciani P, et al. Crystal structure of the soluble form of the redox-regulated chloride ion channel protein CLIC4. FEBS J. 2005 Oct;272(19):4996–5007. doi: 10.1111/j.1742-4658.2005.04909.x. [DOI] [PubMed] [Google Scholar]
- 5.Shorning BY, Wilson DB, Meehan RR, Ashley RH. Molecular cloning and developmental expression of two Chloride Intracellular Channel (CLIC) genes in Xenopus laevis. Dev Genes Evol. 2003 Oct;213(10):514–518. doi: 10.1007/s00427-003-0356-2. [DOI] [PubMed] [Google Scholar]
- 6.Duncan RR, Westwood PK, Boyd A, Ashley RH. Rat brain p64H1, expression of a new member of the p64 chloride channel protein family in endoplasmic reticulum. J Biol Chem. 1997 Sep 19;272(38):23880–23886. doi: 10.1074/jbc.272.38.23880. [DOI] [PubMed] [Google Scholar]
- 7.Bohman S, Matsumoto T, Suh K, Dimberg A, Jakobsson L, Yuspa S, et al. Proteomic analysis of vascular endothelial growth factor-induced endothelial cell differentiation reveals a role for chloride intracellular channel 4 (CLIC4) in tubular morphogenesis. J Biol Chem. 2005 Dec 23;280(51):42397–42404. doi: 10.1074/jbc.M506724200. [DOI] [PubMed] [Google Scholar]
- 8.Tung JJ, Hobert O, Berryman M, Kitajewski J. Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis. 2009 Feb 27; doi: 10.1007/s10456-009-9139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulmasov B, Bruno J, Gordon N, Hartnett ME, Edwards JC. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Pathol. 2009 Mar;174(3):1084–1096. doi: 10.2353/ajpath.2009.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padmakumar VC, Speer K, Pal-Ghosh S, Masiuk KE, Ryscavage A, Dengler SL, et al. Spontaneous skin erosions and reduced skin and corneal wound healing characterize CLIC4(NULL) mice. Am J Pathol. 2012 Jul;181(1):74–84. doi: 10.1016/j.ajpath.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronnov-Jessen L, Villadsen R, Edwards JC, Petersen OW. Differential expression of a chloride intracellular channel gene, CLIC4, in transforming growth factor-beta1-mediated conversion of fibroblasts to myofibroblasts. Am J Pathol. 2002 Aug;161(2):471–480. doi: 10.1016/s0002-9440(10)64203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh KS, Crutchley JM, Koochek A, Ryscavage A, Bhat K, Tanaka T, et al. Reciprocal modifications of CLIC4 in tumor epithelium and stroma mark malignant progression of multiple human cancers. Clin Cancer Res. 2007 Jan 1;13(1):121–131. doi: 10.1158/1078-0432.CCR-06-1562. [DOI] [PubMed] [Google Scholar]
- 13.Shukla A, Malik M, Cataisson C, Ho Y, Friesen T, Suh KS, et al. TGF-beta signalling is regulated by Schnurri-2-dependent nuclear translocation of CLIC4 and consequent stabilization of phospho-Smad2 and 3. Nat Cell Biol. 2009 Jun;11(6):777–784. doi: 10.1038/ncb1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla A, Yuspa SH. CLIC4 and Schnurri-2: A dynamic duo in TGF-beta signaling with broader implications in cellular homeostasis and disease. Nucleus. 2010 Mar;1(2):144–149. doi: 10.4161/nucl.1.2.10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol. 2001 Apr;11(2):97–104. doi: 10.1006/scbi.2000.0361. [DOI] [PubMed] [Google Scholar]
- 16.De WO, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008 Nov 15;123(10):2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 17.Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, et al. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res Treat. 2001 Jan;65(2):101–110. doi: 10.1023/a:1006461422273. [DOI] [PubMed] [Google Scholar]
- 18.Tang B, Vu M, Booker T, Santner SJ, Miller FR, Anver MR, et al. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression.1. J Clin Invest. 2003 Oct;112(7):1116–1124. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinz B. Formation and function of the myofibroblast during tissue repair.1. J Invest Dermatol. 2007 Mar;127(3):526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 20.Ronnov-Jessen L, Petersen OW. A function for filamentous alpha-smooth muscle actin: retardation of motility in fibroblasts.1. J Cell Biol. 1996 Jul;134(1):67–80. doi: 10.1083/jcb.134.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenmoehl J, Miller SN, Hofmann C, Vogl D, Falk W, Scholmerich J, et al. Transforming growth factor-beta 1 induces intestinal myofibroblast differentiation and modulates their migration. World J Gastroenterol. 2009 Mar 28;15(12):1431–1442. doi: 10.3748/wjg.15.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, et al. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell. 2006 Jun 2;125(5):915–928. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takekawa M, Maeda T, Saito H. Protein phosphatase 2Calpha inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J. 1998 Aug 17;17(16):4744–4752. doi: 10.1093/emboj/17.16.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng A, Kaldis P, Solomon MJ. Dephosphorylation of human cyclin-dependent kinases by protein phosphatase type 2C alpha and beta 2 isoforms. J Biol Chem. 2000 Nov 3;275(44):34744–34749. doi: 10.1074/jbc.M006210200. [DOI] [PubMed] [Google Scholar]
- 25.Sun W, Yu Y, Dotti G, Shen T, Tan X, Savoldo B, et al. PPM1A and PPM1B act as IKKbeta phosphatases to terminate TNFalpha-induced IKKbeta-NF-kappaB activation. Cell Signal. 2009 Jan;21(1):95–102. doi: 10.1016/j.cellsig.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):314–323. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 1981 May;41(5):1657–1663. [PubMed] [Google Scholar]
- 28.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999 Oct 1;59(19):5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005 May 6;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Tuxhorn JA, McAlhany SJ, Dang TD, Ayala GE, Rowley DR. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 2002 Jun 1;62(11):3298–3307. [PubMed] [Google Scholar]
- 31.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003 Jul;200(4):429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 32.Lu SL, Herrington H, Reh D, Weber S, Bornstein S, Wang D, et al. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006 May 15;20(10):1331–1342. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996 Jan;76(1):69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 34.Adegboyega PA, Rodriguez S, McLarty J. Stromal expression of actin is a marker of aggressiveness in basal cell carcinoma. Hum Pathol. 2010 Aug;41(8):1128–1137. doi: 10.1016/j.humpath.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001 Sep;12(9):2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001 Sep;159(3):1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007 Apr 1;13(7):2082–2090. doi: 10.1158/1078-0432.CCR-06-2191. [DOI] [PubMed] [Google Scholar]
- 38.Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol. 2003 Sep;29(3 Pt 1):397–404. doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- 39.Uemura M, Swenson ES, Gaca MD, Giordano FJ, Reiss M, Wells RG. Smad2 and Smad3 play different roles in rat hepatic stellate cell function and alpha-smooth muscle actin organization. Mol Biol Cell. 2005 Sep;16(9):4214–4224. doi: 10.1091/mbc.E05-02-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans RA, Tian YC, Steadman R, Phillips AO. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp Cell Res. 2003 Jan 15;282(2):90–100. doi: 10.1016/s0014-4827(02)00015-0. [DOI] [PubMed] [Google Scholar]
- 41.Caraci F, Gili E, Calafiore M, Failla M, La RC, Crimi N, et al. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res. 2008 Apr;57(4):274–282. doi: 10.1016/j.phrs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Meyer-Ter-Vehn T, Gebhardt S, Sebald W, Buttmann M, Grehn F, Schlunck G, et al. p38 inhibitors prevent TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2006 Apr;47(4):1500–1509. doi: 10.1167/iovs.05-0361. [DOI] [PubMed] [Google Scholar]
- 43.Yao Q, Qu X, Yang Q, Wei M, Kong B. CLIC4 mediates TGF-beta1-induced fibroblast-to-myofibroblast transdifferentiation in ovarian cancer. Oncol Rep. 2009 Sep;22(3):541–548. doi: 10.3892/or_00000469. [DOI] [PubMed] [Google Scholar]
- 44.Das AK, Helps NR, Cohen PT, Barford D. Crystal structure of the protein serine/threonine phosphatase 2C at 2.0 A resolution. EMBO J. 1996 Dec 16;15(24):6798–6809. [PMC free article] [PubMed] [Google Scholar]
- 45.Qian Z, Okuhara D, Abe MK, Rosner MR. Molecular cloning and characterization of a mitogen-activated protein kinase-associated intracellular chloride channel. J Biol Chem. 1999 Jan 15;274(3):1621–1627. doi: 10.1074/jbc.274.3.1621. [DOI] [PubMed] [Google Scholar]
- 46.Micke P, Ostman A. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer. 2004 Aug;45(Suppl 2):S163–S175. doi: 10.1016/j.lungcan.2004.07.977. [DOI] [PubMed] [Google Scholar]
- 47.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009 Jun;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M, Xu J, Deng H. Tangled fibroblasts in tumor-stroma interactions. Int J Cancer. 2011 Apr 5; doi: 10.1002/ijc.26116. [DOI] [PubMed] [Google Scholar]
- 49.Saunier EF, Akhurst RJ. TGF beta inhibition for cancer therapy. Curr Cancer Drug Targets. 2006 Nov;6(7):565–578. doi: 10.2174/156800906778742460. [DOI] [PubMed] [Google Scholar]
- 50.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004 Dec;3(12):1011–1022. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 51.Connolly EC, Saunier EF, Quigley D, Luu MT, De SA, Hann B, et al. Outgrowth of drug-resistant carcinomas expressing markers of tumor aggression after long-term TbetaRI/II kinase inhibition with LY2109761. Cancer Res. 2011 Mar 15;71(6):2339–2349. doi: 10.1158/0008-5472.CAN-10-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006 Jul;6(7):506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 53.Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3(5):799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandez-Salas E, Sagar M, Cheng C, Yuspa SH, Weinberg WC. p53 and tumor necrosis factor α regulate the expression of a mitochondrial chloride channel protein. J Biol Chem. 1999;274(51):36488–36497. doi: 10.1074/jbc.274.51.36488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.