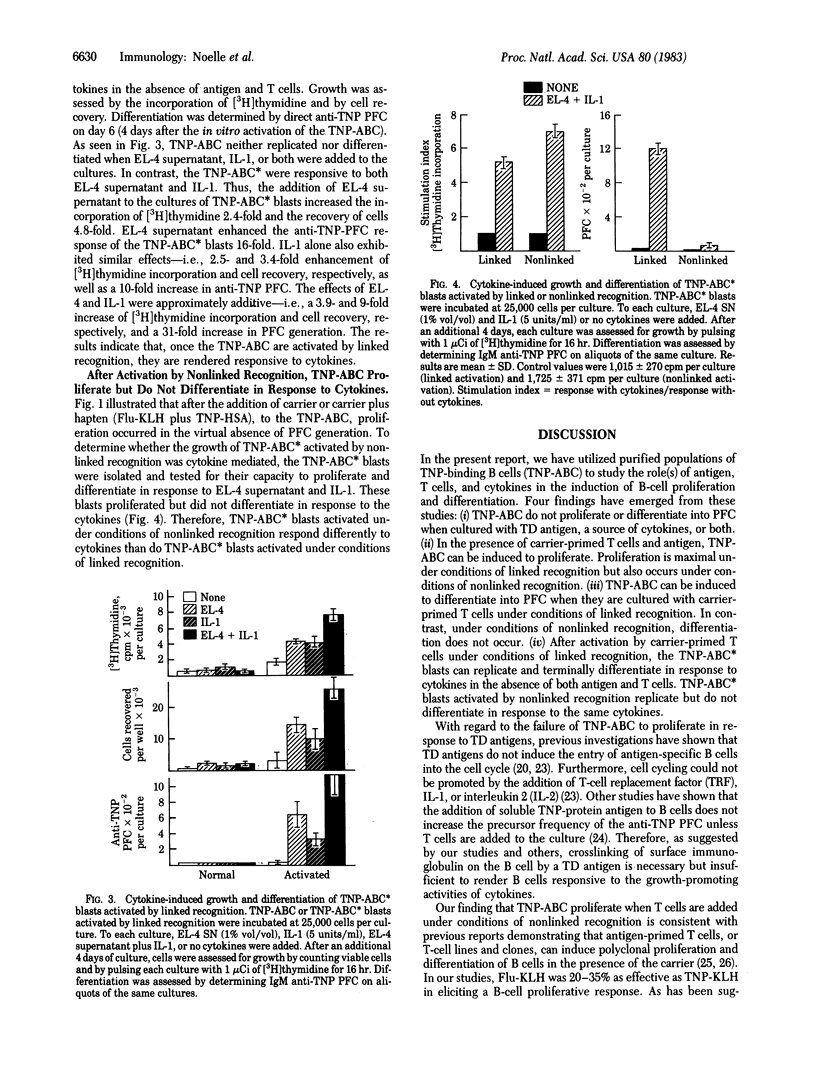
Abstract
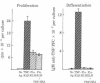
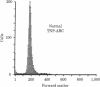
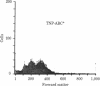
T cells and cytokines were used to activate highly enriched populations of 2,4,6-trinitrophenyl (TNP)-binding B cells (TNP-ABC). TNP-ABC did not proliferate or differentiate when they were cultured with thymus-dependent (TD) antigen, even in the presence of supernatants known to contain B-cell growth and differentiation factors. However, purified TNP-ABC did proliferate and differentiate when they were cultured with TD antigen in the presence of carrier-primed T cells and antigen (TNP-keyhole limpet hemocyanin)--i.e., linked recognition. TNP-ABC blasts generated under conditions of linked recognition proliferated and differentiated in response to cytokines in the absence of T cells and antigen. In contrast, under conditions of nonlinked recognition (hapten and carrier on different molecules) TNP-ABC blasts also proliferated but did not differentiate in response to the same cytokines. These results indicate that antigen-specific "resting" B cells must be activated by T cells and antigen prior to becoming responsive to cytokines. Furthermore, activation under conditions of linked and nonlinked recognition generates two different types of blasts with regard to their subsequent response to cytokines.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Schreier M. H., Melchers F. T-cell-dependent B-cell stimulation is H-2 restricted and antigen dependent only at the resting B-cell level. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1612–1616. doi: 10.1073/pnas.77.3.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y., Hodes R. J. T cell regulation of B cell activation. T cells independently regulate the responses mediated by distinct B cell subpopulations. J Exp Med. 1982 May 1;155(5):1267–1276. doi: 10.1084/jem.155.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y., Shigeta M., Fathman C. G., Singer A., Hodes R. J. Role of the major histocompatibility complex in T cell activation of B cell subpopulations. A single monoclonal T helper cell population activates different B cell subpopulations by distinct pathways. J Exp Med. 1982 Aug 1;156(2):350–360. doi: 10.1084/jem.156.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y., Singer A., Hodes R. J. Role of the major histocompatibility complex in T cell activation of B cell subpopulations. Major histocompatibility complex-restricted and -unrestricted B cell responses are mediated by distinct B cell subpopulations. J Exp Med. 1981 Oct 1;154(4):1100–1115. doi: 10.1084/jem.154.4.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. W., Rittenberg M. B. In vitro-initiated secondary anti-hapten response. II. Increasing cell avidity for antigen. J Exp Med. 1970 Nov;132(5):926–940. doi: 10.1084/jem.132.5.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. C., Monroe J. G., Neale M. J. Definition of conditions that enable antigen-specific activation of the majority of isolated trinitrophenol-binding B cells. J Exp Med. 1982 Dec 1;156(6):1635–1649. doi: 10.1084/jem.156.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., Janeway C. A., Jr Cooperative interaction of B lymphocytes with antigen-specific helper T lymphocytes is MHC restricted. Nature. 1981 Aug 6;292(5823):547–549. doi: 10.1038/292547a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., von Boehmer H., Sidman C. L. Dissociation of two signals required for activation of resting B cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1989–1993. doi: 10.1073/pnas.79.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller D. M., Swierkosz J. E., Marrack P., Kappler J. W. Two types of functionally distinct, synergizing helper T cells. J Immunol. 1980 Mar;124(3):1350–1359. [PubMed] [Google Scholar]
- Kettman J., Dutton R. W. Radioresistance of the enhancing effect of cells from carrier-immunized mice in an in vitro primary immune response. Proc Natl Acad Sci U S A. 1971 Apr;68(4):699–703. doi: 10.1073/pnas.68.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishmoto T., Ishizaka K. Immunologic and physicochemical properties of enhancing soluble factors for IgG and IgE antibody responses. J Immunol. 1975 Apr;114(4):1177–1184. [PubMed] [Google Scholar]
- Mayer L., Fu S. M., Kunkel H. G. Human T cell hybridomas secreting factors for IgA-specific help, polyclonal B cell activation, and B cell proliferation. J Exp Med. 1982 Dec 1;156(6):1860–1865. doi: 10.1084/jem.156.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Andersson J., Lernhardt W., Schreier M. H. H-2-unrestricted polyclonal maturation without replication of small B cells induced by antigen-activated T cell help factors. Eur J Immunol. 1980 Sep;10(9):679–685. doi: 10.1002/eji.1830100905. [DOI] [PubMed] [Google Scholar]
- Pure E., Isakson P. C., Kappler J. W., Marrack P., Krammer P. H., Vitetta E. S. T cell-derived B cell growth and differentiation factors. Dichotomy between the responsiveness of B cells from adult and neonatal mice. J Exp Med. 1983 Feb 1;157(2):600–612. doi: 10.1084/jem.157.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puré E., Isakson P. C., Takatsu K., Hamaoka T., Swain S. L., Dutton R. W., Dennert G., Uhr J. W., Vitetta E. S. Induction of B cell differentiation by T cell factors. I. Stimulation of IgM secretion by products of a T cell hybridoma and a T cell line. J Immunol. 1981 Nov;127(5):1953–1958. [PubMed] [Google Scholar]
- Schimpl A., Wecker E. A third signal in B cell activation given by TRF. Transplant Rev. 1975;23:176–188. doi: 10.1111/j.1600-065x.1975.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Severinson E., Bergstedt-Lindqvist S., van der Loo W., Fernandez C. Characterization of the IgG response induced by polyclonal B cell activators. Immunol Rev. 1982;67:73–85. doi: 10.1111/j.1600-065x.1982.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Snow E. C., Noelle R. J., Uhr J. W., Vitetta E. S. Activation of antigen-enriched B cells. II. Role of linked recognition in B cell proliferation to thymus-dependent antigens. J Immunol. 1983 Feb;130(2):614–618. [PubMed] [Google Scholar]
- Snow E. C., Vitetta E. S., Uhr J. W. Activation of antigen-enriched b cells. I. Purification and response to thymus-independent antigens. J Immunol. 1983 Feb;130(2):607–613. [PubMed] [Google Scholar]
- Swain S. L., Dennert G., Warner J. F., Dutton R. W. Culture supernatants of a stimulated T-cell line have helper activity that acts synergistically with interleukin 2 in the response of B cells to antigen. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2517–2521. doi: 10.1073/pnas.78.4.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu K., Tanaka K., Tominaga A., Kumahara Y., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). III. Establishment of T cell hybrid clone continuously producing TRF and functional analysis of released TRF. J Immunol. 1980 Dec;125(6):2646–2653. [PubMed] [Google Scholar]
- Tse H. Y., Mond J. J., Paul W. E. T lymphocyte-dependent B lymphocyte proliferative response to antigen. I Genetic restriction of the stimulation of B lymphocyte proliferation. J Exp Med. 1981 Apr 1;153(4):871–882. doi: 10.1084/jem.153.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]