Abstract
Purpose
This study was performed to evaluate prognostic factors for survival from first relapse (SFFR) in stage I-III breast cancer patients.
Materials and Methods
From June 1994 to June 2008, 3,835 patients were treated with surgery plus postoperative radiotherapy and adjuvant chemotherapy for stage I-III breast cancer at Samsung Medical Center. Among them, a total of 224 patients died by June 2009, and 175 deaths were of breast cancer. Retrospective review was performed on medical records of 165 patients who met the inclusion criteria of this study. Univariate and multivariate analysis were done on survivals according to variables, such as age, stage, hormone status of tumor, disease-free interval (DFI), sites of first failure, number of organs involved by recurrent disease (NOR), application of salvage treatments, and existence of brain or liver metastasis (visceral metastasis).
Results
Patients' median overall survival time was 38 months (range, 8 to 123 months). Median SFFR was 17 months (range, 5 to 87 months). Ninety percent of deaths occurred within 40 months after first recurrence. The patients with SFFR ≤1 year had tendency of triple-negativity, shorter DFI (≤2 years), larger NOR (>3), visceral metastasis for first relapse than the patients with SFFR >1 year. In multivariate analysis, longer DFI (>2 vs. ≤2 years), absence of visceral metastasis, and application of salvage treatments were statistically significant prognosticators for longer SFFR.
Conclusion
The DFI, application of salvage treatments, and visceral metastasis were significant prognostic factors for SFFR in breast cancer patients.
Keywords: Survival, Recurrence, Prognosis, Breast neoplasms
Introduction
Breast cancer is the most common type of malignancy and the leading cause of cancer death in female patients with malignancy. In 2008, total 458,400 patients died of breast cancer worldwide, accounting for 14% of the total cancer deaths [1]. Estimating life expectancy in breast cancer patients is important for physicians to decide on appropriate treatments and to inform patients about the prognosis of their diseases. There have been a few methods for predicting patients' survivals based on tumor size, nodal status, hormonal receptor status, and other prognostic factors that were found at the time of their breast cancer diagnosis [2-4]. Those methods can be used in optimizing initial treatment for newly diagnosed non-metastatic breast cancer patients. Studies also have reported on prognostic scores to forecast survivals for patients in the terminal stage of advanced cancers. Patient's performance status, existence of specific symptoms, and abnormal laboratory tests are considered as prognostic factors for life expectancies in incurable cancers [5-9]. There have also been some reports on predicting factors for survival from first recurrence (SFFR) of breast cancer. Those studies have shown that interval between initial treatment and relapse, extent of metastasis, presence of visceral metastasis, and hormone status of tumor are prognostic factors for survival after first relapse [10,11].
The current study analyzed important prognostic factors for SFFR in the patients who died of breast cancer. Through the analysis, we aimed at estimating patient's life expectancy after first disease recurrence according to prognostic factors.
Materials and Methods
1. Patients
Between June 1994 and June 2008, 3,835 patients were treated with surgery and postoperative radiotherapy plus adjuvant chemotherapy for stage I-III breast cancer at Samsung Medical Center. Until June 2009, a total of 224 patients died, including 175 who died of breast cancer. Among them, 165 met inclusion criteria of this study that stipulated as follows, 1) Patients who died of breast cancer during follow-ups after surgery plus postoperative radiotherapy and/or chemotherapy for stage I-III disease, 2) patients who had no previous history of other malignancy, 3) patients who had no history of chronic medical illness or accidents which could have resulted in death, and 4) patients who had clinical follow-ups within 6 months before death. The characteristics of the analyzed patients are summarized in Table 1.
Table 1.
Patients' characteristics
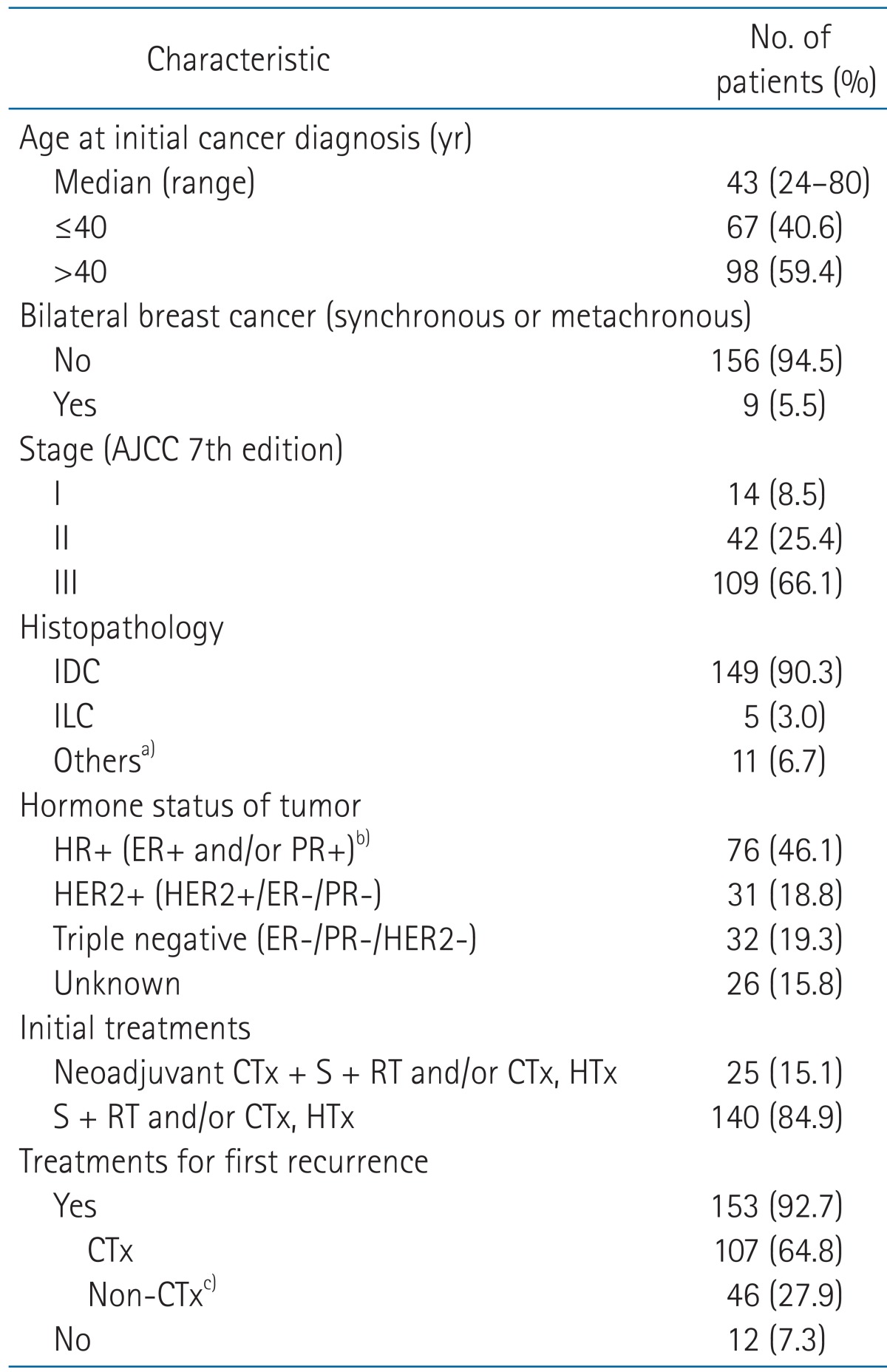
AJCC, American Joint Committee on Cancer; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; HR, hormone receptor; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; CTx, chemotherapy; S, surgery; RT, radiotherapy; HTx, hormone therapy.
a)Other types of histopathology were papillary (3), medullary (2), metaplastic (2), inflammatory (2), mixed mucinous and ductal (1), or angiosarcoma (1). b)Irrespective of HER2 status. c)Including hormone, radiotherapy, or surgery.
2. Treatment
All patients received standard combined treatment including surgery and adjuvant treatment at the initial disease presentation. After initial treatment, patients were followed up with history taking, physical examination, complete blood count, blood chemistry, chest X-ray, abdominal ultrasonography, and bone scan every 4 to 6 months for 5 years and repeated every 12 months thereafter. Computed tomography, magnetic resonance imaging, or 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) were conducted if patients complained of symptoms that suggested of tumor recurrence.
For patients who developed locoregional or distant failure after initial cancer treatments, chemotherapy, surgery, or radiotherapy were administered according to patient's performance status, sites of failure, and physicians' decision. Patients who had developed locoregional recurrence were treated with chemotherapy (11 patients), radiation therapy (4), surgery (3), hormonal therapy (3), no treatment (3), surgery followed by chemotherapy (1), or surgery followed by radiotherapy (1), respectively. For patients with distant metastasis, chemotherapy (59 patients), hormone therapy (14), radiation therapy followed by chemotherapy (27), no treatment (8), surgery followed by radiation and chemotherapy (4), chemotherapy and hormone therapy (3), or surgery followed by chemotherapy (1) were administered. The patients who had simultaneous locoregional and distant metastasis were treated with chemotherapy (10 patients), hormone therapy (2), radiotherapy followed by chemotherapy (10), or no treatment (1), respectively.
3. Definition of recurrence and statistical analysis
Overall survival time (OS) was defined as interval from initial treatment (surgery or neoadjuvant chemotherapy) to death. Disease-free interval (DFI) was specified as length of time from primary treatment to disease relapse. SFFR of breast cancer was calculated from the date of relapse to the date of death. Fisher exact test was used to compare the patients' characteristics between the groups. Survival curves were computed according to the Kaplan-Meier method, and the log-rank test was used to compare survival times between the groups with different variables. Locoregional recurrence was defined as recurrence in the operative bed, remnant breast, or regional lymph nodes (ipsilateral axillary, internal mammary, supraclavicular lymph nodes), and distant metastasis as failure in distant organs. Rules for counting a number of organs involved by recurrent disease (NOR) were as follows: 1) regional lymph nodes were considered as one organ and 2) separate lymph node regions were regarded as multiple organs. For example, NOR in patients who had cervical and mediastinal lymph nodes metastasis was considered as 2. 3) The bone was counted as one organ even in the presence of multiple bone metastasis. Single metastasis was defined as metastasis to one organ with a single measurable lesion. To determine independent prognostic factors for survival time, Cox regression analysis was used. Statistical significance was calculated at the 95% confidence interval (p < 0.05) and all analysis was performed using SAS ver. 9.1 (SAS Institute, Cary, NC, USA).
Results
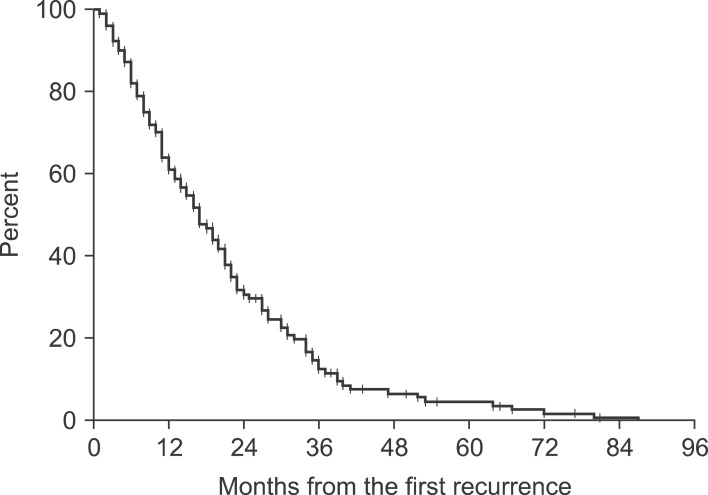
Median OS and DFI of the patients were 38 months (range, 8 to 123 months) and 21 months (range, 4 to 94 months), respectively. Median SFFR was 17 months (range, 5 to 87 months). Within 1 year after first recurrence, 38.7% of the patients died. Ninety percent of deaths occurred within 40 months after first recurrence (Fig. 1). In terms of first site of failure, 26 patients had locoregional recurrence and 116 patients developed distant metastasis. Locoregional and distant failure occurred simultaneously in 23 patients. Among the patients with distant failure, 18 patients had a single metastasis for first relapse, including 11 bone, 5 brain, and 2 other organ (contralateral supraclavicular and hilar lymph nodes) metastasis, respectively.
Fig. 1.
Survival from first recurrence in all patients.
We compared variables between the patients who had shorter than 1 year of SFFR (SFFR ≤ 1 year) and the patients who had longer than 1 year of SFFR (SFFR > 1 year). The percentage of patients with triple-negative tumors, shorter DFI (≤2 years), larger NOR (>3), and visceral metastasis was significantly higher in patients with SFFR ≤ 1 year than the patients with SFFR > 1 year (Table 2).
Table 2.
Comparisons the variables of patients who survived less than 1 year to the patients who survived more than 1 year after first recurrence of breast cancer
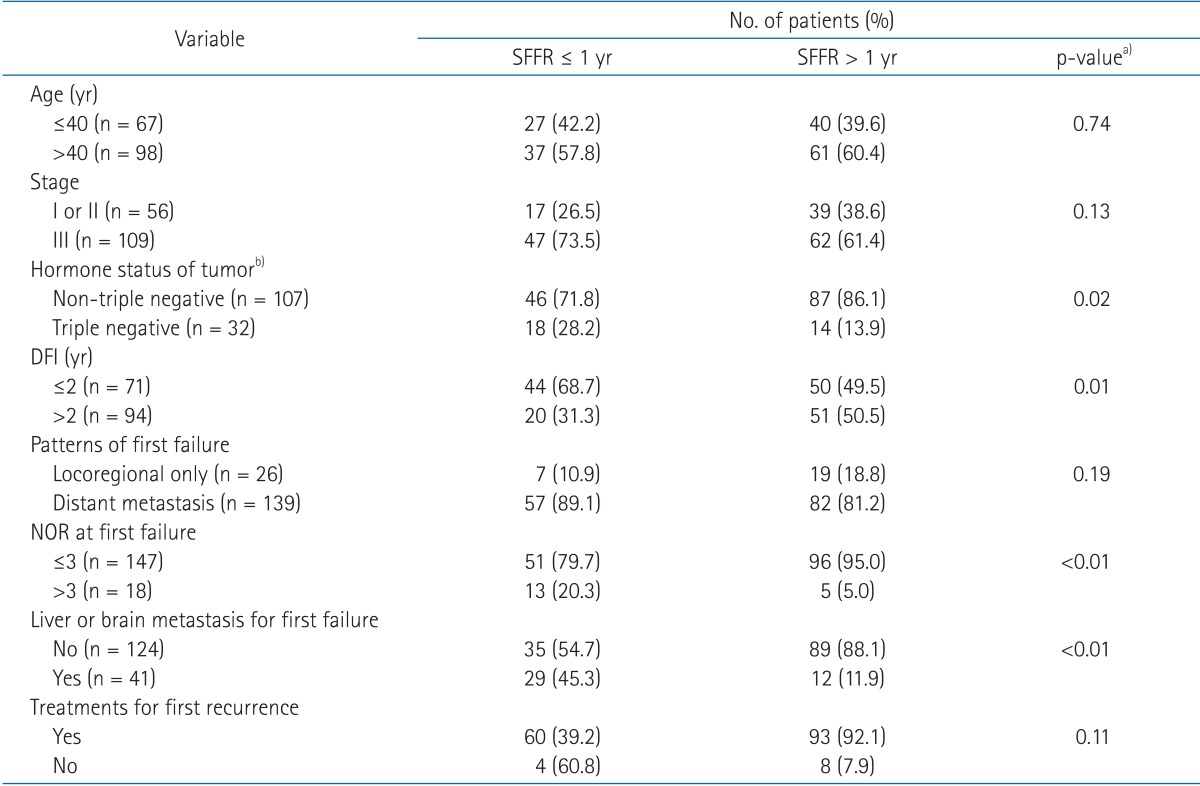
SFFR, survival from first relapse; DFI, disease free interval, NOR, number of organs involved by recurrent disease.
a)By Fisher exact test. b)In patients who had evaluable hormonal status of tumor.
To determine prognostic factors for SFFR, univariate analysis was performed. Triple-negative tumor, shorter DFI (≤2 years), larger NOR (>3), existence of liver or brain metastasis for first relapse, and absence of treatment for the first recurrence were related to shorter SFFR (Table 3). All the patients with triple-negative cancer died within 36 months after first relapse except for one patient. In multivariate analysis, DFI, existence of liver or brain metastasis, and non-treatment on first recurrence were statistically significant prognosticators for SFFR (Table 4).
Table 3.
Univariate analysis on prognostic factors for SFFR
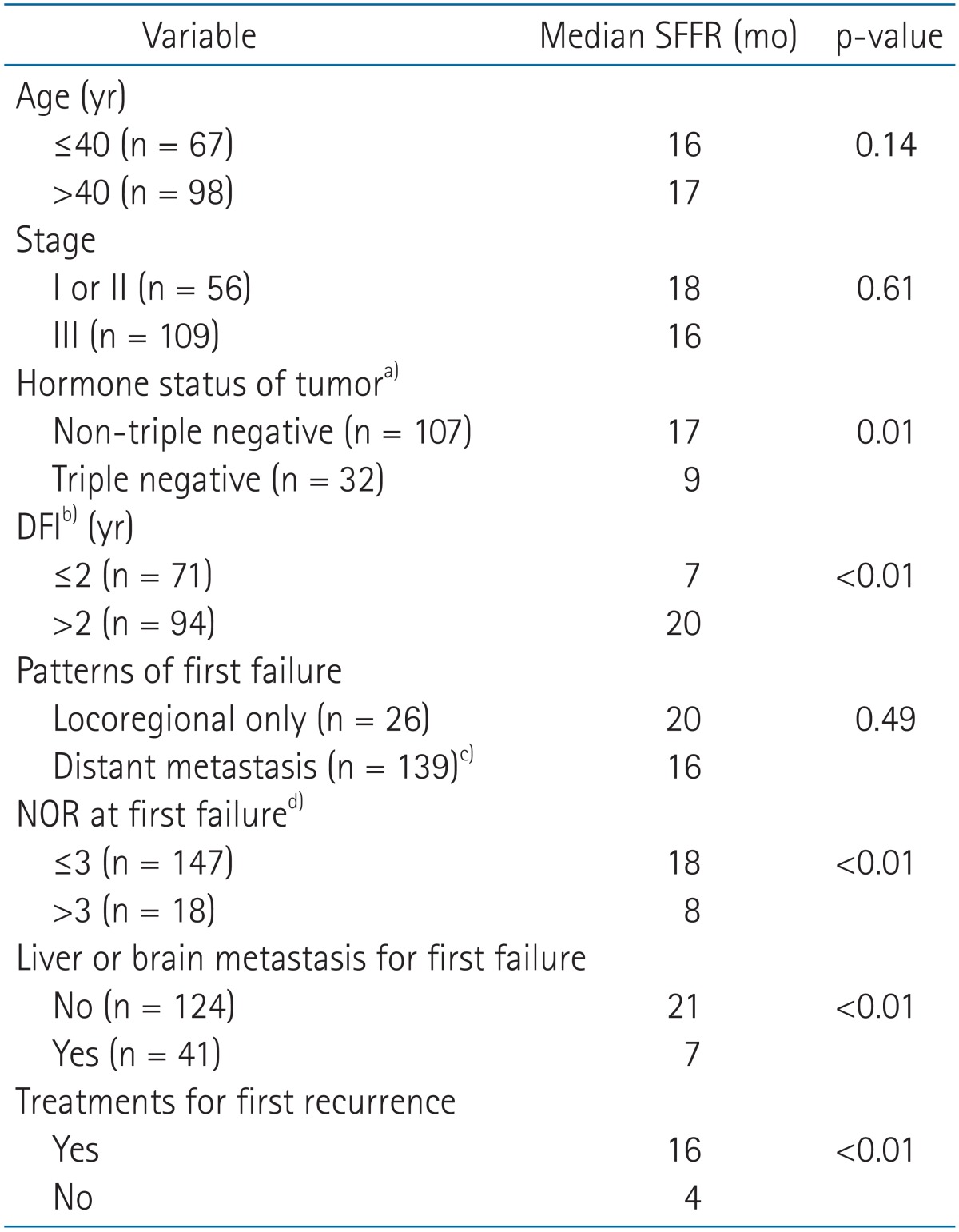
SFFR, survival from first relapse; DFI, disease free interval; NOR, number of organs involved by recurrent disease.
a)In patients who had evaluable hormonal status of tumor. b)Disease-free time between initial cancer treatment and recurrence. c)Simultaneous locoregional recurrence and distant metastasis were counted as distant metastasis. d)Rules for counting NOR were as follows: 1) regional lymph nodes were considered as one organ, 2) different lymph node regions were counted as multiple organs, for example, NOR in patients who had cervical and mediastinal lymph nodes metastasis was considered as 2, 3) The bone was counted as one organ even if multiple bones were involved by the disease.
Table 4.
Multivariate analysis on prognostic factors for SFFR
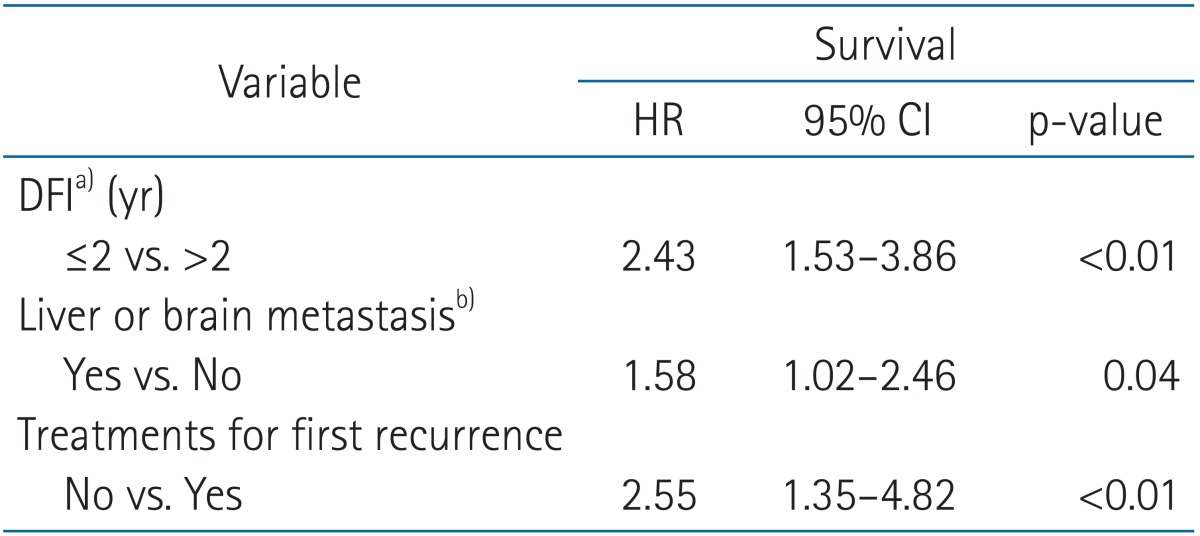
SFFR, survival from first relapse; HR, hazard ratio; CI, confidence interval; DFI, disease free interval.
a)Disease-free time between initial cancer treatment and recurrence. b)For the first failure.
Discussion and Conclusion
The current study demonstrated that DFI, metastasis to vital organs, and absence of treatment for the first recurrence in the first relapse were prognosticators for SFFR among patients who died of breast cancer. Our findings about the important prognostic factors for SFFR are similar to other studies [10-15]. In earlier report by Clark et al. [10], initial site of recurrence, DFI, estrogen receptor of tumor, and axillary lymph nodes status at the time of initial diagnosis were determinant for survival from the time of first recurrence. Meanwhile, Vogel et al. [11] showed that DFI, dominant metastasis to visceral organ, and estrogen receptor status were prognostic factors for survival after first relapse. Besides the aforementioned factors, recent reports also have found human epidermal growth factor receptor 2 (HER2) status of tumor, and administration of certain treatment, including trastuzumab or aromatase inhibitors, as prognostic factors for SFFR [14,16]. The current and other reports have found tumor characteristics, such as hormone or involved lymph nodes status, DFI, and metastatic site at first relapse are important factors for survival after first relapse of breast cancer.
Median SFFR of the patients was 17 months (range, 5 to 87 months), and ninety percent of deaths occurred within 40 months after first relapse in this study. This survival result is shorter than the results of other studies. The median survival time is currently reported as 20 to 28 months in metastatic breast cancer patients [11,16-18]. Unlike the previous results, the present analysis was done to the patients who died of breast cancer. Consequently, median SFFR was shorter than the survival time reported in the other studies that analyzed records of both living and deceased breast cancer patients.
There was a poor prognostic group of patients who died within 1 year after first recurrence in this study. These patients had higher tendencies of triple-negative tumors, shorter DFI, larger NOR, and visceral metastasis as first failure compared to the patients who survived more than 1 year after first relapse of breast cancer. Almost half of these poor prognostic patients had liver or brain metastasis as first relapse, and nearly 70% of these patients had shorter than 2 years of DFI. About a third of these patients had triple-negative tumors. Considering that triple-negative tumors accounts for 15% of all breast cancer cases [19], the proportion of triple-negative tumor in the poor prognostic group of patients in this study (28.2%) is thought to be high. The relatively high percentage of triple-negative breast cancer in the patients who died within 1 year after first recurrence in this study could have been caused by poor prognosis of triple-negative tumors. Moreover, median SFFR in patients with triple-negative tumors in this study was significantly shorter than the patients with non-triple negative tumors (7 months vs. 17 months, p < 0.01) in univariate analysis. Such poor prognosis of patients with triple-negative tumors was reported in other studies. In these studies, the patients with triple-negative breast cancer are associated with shorter overall survival, disease-free survival, and post-recurrence survival time in comparison to the patients with non-triple-negative breast cancer [20,21].
In the current analysis, the absence of salvage treatment at the first recurrence was a significant prognostic factor for SFFR. The median time of SFFR in the patients who received no treatment for the recurrence was 4 months, while it was 16 months for the patients treated with salvage therapy. It is possible that systemic treatment, such as chemotherapy or hormone therapy with or without locoregional treatment could have prolonged the patient's survival.
For the purpose of evaluating prognostic factors for SFFR in stage I-III breast cancer, this study analyzed factors associated with survival after first treatment failure in patients who died of breast cancer. Because all the patients were followed up until death, it was possible to estimate patient's life expectancy according to each individual's clinical factors. In the current study, we found that the patients with short DFI, visceral organ metastasis at first recurrence, and the patients who did not receive any salvage treatment had short life-expectancy after recurrence of disease. The factors found to be important in this study can be useful when counseling patients regarding their life expectancy.
In conclusion, the DFI, visceral metastasis, and application of salvage treatment for the recurrence were significant prognostic factors for SFFR in breast cancer patients. Shorter DFI than 2 years, brain or liver metastasis at first recurrence, and receiving no treatment at the time of first recurrence were found to be related to shorter SFFR in this study.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 3.Michaelson JS, Chen LL, Bush D, Fong A, Smith B, Younger J. Improved web-based calculators for predicting breast carcinoma outcomes. Breast Cancer Res Treat. 2011;128:827–835. doi: 10.1007/s10549-011-1366-9. [DOI] [PubMed] [Google Scholar]
- 4.Lundin J, Lundin M, Isola J, Joensuu H. A web-based system for individualised survival estimation in breast cancer. BMJ. 2003;326:29. doi: 10.1136/bmj.326.7379.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirovano M, Maltoni M, Nanni O, et al. A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage. 1999;17:231–239. doi: 10.1016/s0885-3924(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 6.Morita T, Tsunoda J, Inoue S, Chihara S. The Palliative Prognostic Index: a scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer. 1999;7:128–133. doi: 10.1007/s005200050242. [DOI] [PubMed] [Google Scholar]
- 7.Yun YH, Heo DS, Heo BY, Yoo TW, Bae JM, Ahn SH. Development of terminal cancer prognostic score as an index in terminally ill cancer patients. Oncol Rep. 2001;8:795–800. doi: 10.3892/or.8.4.795. [DOI] [PubMed] [Google Scholar]
- 8.Chuang RB, Hu WY, Chiu TY, Chen CY. Prediction of survival in terminal cancer patients in Taiwan: constructing a prognostic scale. J Pain Symptom Manage. 2004;28:115–122. doi: 10.1016/j.jpainsymman.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Bruera E, Miller MJ, Kuehn N, MacEachern T, Hanson J. Estimate of survival of patients admitted to a palliative care unit: a prospective study. J Pain Symptom Manage. 1992;7:82–86. doi: 10.1016/0885-3924(92)90118-2. [DOI] [PubMed] [Google Scholar]
- 10.Clark GM, Sledge GW, Jr, Osborne CK, McGuire WL. Survival from first recurrence: relative importance of prognostic factors in 1,015 breast cancer patients. J Clin Oncol. 1987;5:55–61. doi: 10.1200/JCO.1987.5.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Vogel CL, Azevedo S, Hilsenbeck S, East DR, Ayub J. Survival after first recurrence of breast cancer: the Miami experience. Cancer. 1992;70:129–135. doi: 10.1002/1097-0142(19920701)70:1<129::aid-cncr2820700122>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Robertson JF, Dixon AR, Nicholson RI, Ellis IO, Elston CW, Blamey RW. Confirmation of a prognostic index for patients with metastatic breast cancer treated by endocrine therapy. Breast Cancer Res Treat. 1992;22:221–227. doi: 10.1007/BF01840835. [DOI] [PubMed] [Google Scholar]
- 13.Harris JR, Hellman S. Observations on survival curve analysis with particular reference to breast cancer treatment. Cancer. 1986;57:925–928. doi: 10.1002/1097-0142(19860301)57:5<925::aid-cncr2820570508>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Shigematsu H, Kawaguchi H, Nakamura Y, et al. Significant survival improvement of patients with recurrent breast cancer in the periods 2001-2008 vs. 1992-2000. BMC Cancer. 2011;11:118. doi: 10.1186/1471-2407-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang J, Clark GM, Allred DC, Mohsin S, Chamness G, Elledge RM. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer. 2003;97:545–553. doi: 10.1002/cncr.11083. [DOI] [PubMed] [Google Scholar]
- 16.Puente J, Lopez-Tarruella S, Ruiz A, et al. Practical prognostic index for patients with metastatic recurrent breast cancer: retrospective analysis of 2,322 patients from the GEICAM Spanish El Alamo Register. Breast Cancer Res Treat. 2010;122:591–600. doi: 10.1007/s10549-009-0687-4. [DOI] [PubMed] [Google Scholar]
- 17.Mauri D, Polyzos NP, Salanti G, Pavlidis N, Ioannidis JP. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J Natl Cancer Inst. 2008;100:1780–1791. doi: 10.1093/jnci/djn414. [DOI] [PubMed] [Google Scholar]
- 18.Chia SK, Speers CH, D'yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–979. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 19.Oakman C, Viale G, Di Leo A. Management of triple negative breast cancer. Breast. 2010;19:312–321. doi: 10.1016/j.breast.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 21.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]