ABSTRACT
BACKGROUND
Lack of regular physical activity is highly prevalent in U.S. adults and significantly increases mortality risk.
OBJECTIVE
To examine the clinical impact of a newly implemented program (“Exercise as a Vital Sign” [EVS]) designed to systematically ascertain patient-reported exercise levels at the beginning of each outpatient visit.
DESIGN AND PARTICIPANTS
The EVS program was implemented in four of 11 medical centers between April 2010 and October 2011 within a single health delivery system (Kaiser Permanente Northern California). We used a quasi-experimental analysis approach to compare visit-level and patient-level outcomes among practices with and without the EVS program. Our longitudinal observational cohort included over 1.5 million visits by 696,267 adults to 1,196 primary care providers.
MAIN MEASURES
Exercise documentation in physician progress notes; lifestyle-related referrals (e.g. exercise programs, nutrition and weight loss consultation); patient report of physician exercise counseling; weight change among overweight/obese patients; and HbA1c changes among patients with diabetes.
KEY RESULTS
EVS implementation was associated with greater exercise-related progress note documentation (26.2 % vs 23.7 % of visits, aOR 1.12 [95 % CI: 1.11–1.13], p < 0.001) and referrals (2.1 % vs 1.7 %; aOR 1.14 [1.11–1.18], p < 0.001) compared to visits without EVS. Surveyed patients (n = 6,880) were more likely to report physician exercise counseling (88 % vs. 76 %, p < 0.001). Overweight patients (BMI 25–29 kg/m2, n = 230,326) had greater relative weight loss (0.20 [0.12 – 0.28] lbs, p < 0.001) and patients with diabetes and baseline HbA1c > 7.0 % (n = 30,487) had greater relative HbA1c decline (0.1 % [0.07 %–0.13 %], p < 0.001) in EVS practices compared to non-EVS practices.
CONCLUSIONS
Systematically collecting exercise information during outpatient visits is associated with small but significant changes in exercise-related clinical processes and outcomes, and represents a valuable first step towards addressing the problem of inadequate physical activity.
KEY WORDS: primary care, health systems, health services research, health IT, exercise, diabetes, weight loss
The United States is experiencing an epidemic of physical inactivity. Recent national survey data indicate that over one-third of U.S. adults engage in no physical activity,1 and less than 10 % achieve the recommended dose of 150 minutes of moderate activity or 75 minutes of vigorous activity per week when measured by accelerometry.2 This lack of regular moderate to vigorous physical activity (MVPA) has important consequences for our nation’s health: epidemiologic studies have consistently shown a strong association of inactivity with morbidity and mortality risk.3,4 Indeed, physical inactivity was the fourth leading cause of death in 2005, responsible for an estimated 200,000 deaths;5 and the combination of inactivity and overweight/obesity contributed to 27 % of U.S. health care costs.6
Multiple interventional and observational studies have demonstrated the clinical benefit of increasing physical activity, especially among high-risk populations such as patients with cardiovascular disease and type 2 diabetes.7–10 Because many middle-aged and older adults regularly engage with the health system through primary care visits, primary care providers are well positioned within care systems to address lack of regular MVPA through screening, initial counseling, and referral to behavioral specialists and programs to increase healthy lifestyles.11 However, primary care interactions are time-limited, and patients report that fewer than one-third of visits include any exercise or lifestyle counseling.12–14 System-level innovations are needed to more effectively translate the evidence of physical activity’s health benefits into clinical practice.
Beginning in April, 2010, Kaiser Permanente Northern California (KPNC), an integrated health care delivery system serving over 3.4 million members, implemented a new program (“Exercise as a Vital Sign”) to improve recognition and treatment of insufficient physical activity. For all outpatient visits, medical assistants asked patients about frequency and duration of MVPA when initially bringing them into the exam room, and these data were entered into the electronic medical record for the primary care provider (PCP) to review during the visit along with blood pressure and other traditional vital signs. We tested the hypothesis that a program designed to increase ascertainment and recording of patients’ self-reported physical activity as part of the initial visit intake would improve exercise-related clinical care processes (increased progress note documentation, exercise counseling, and lifestyle-related referrals) and provide clinical benefit (weight loss among overweight/obese patients, improved glycemic control among patients with diabetes).
RESEARCH DESIGN AND METHODS
Setting and Participants
Kaiser Permanente Northern California is a nonprofit health care delivery system providing comprehensive medical care to nearly one-third of the population in Northern California. The distribution of patient demographic and socioeconomic factors is diverse and similar to that of the area population, except at the extremes of the income distribution.15
The Exercise as a Vital Sign (EVS) program included a change to medical assistant workflow and an added feature to the electronic medical record that allowed rapid, structured exercise data collection by medical assistants prior to the physician entering the room. Medical assistants were trained to ask patients two questions:
“How many days a week do you engage in moderate to strenuous exercise (like a brisk walk)?”
“On average, how many minutes per day do you exercise at this level?”
Moderate activity was defined as activities inducing a light sweat, such as dancing, swimming, walking fast, biking, and mowing the lawn. Responses were entered into the “Vital Signs” section of the electronic medical record (a version of EPIC®), and the total weekly minutes of MVPA were presented as a single value adjacent to other vital signs.
The EVS program was sequentially implemented at four of 15 KPNC San Francisco Bay Area regional medical centers between April 2010 and March 2011 before being rolled out system-wide beginning November 2011. These four regional medical centers were comprised of 78 primary care practices (11 to 25 practices per medical center), with an average of 80 different PCPs per center (range 39–154). Our study observation period of April 2010–October 2011 included 50 total months of EVS implementation at the primary care practices of four medical centers (Fig. 1). We excluded the initial ramp-up program implementation period (1–2 months) at each pilot medical center when training was being conducted at each of the practices, and fewer than 50 % of overall visits had data collection. These 18,196 excluded visits (1.2 % of total visits) were grouped with the control arm. Because this study focuses on the clinical impact of the EVS program relative to practices without the program, we did not analyze the MVPA data collected in the EVS practices.
Figure 1.
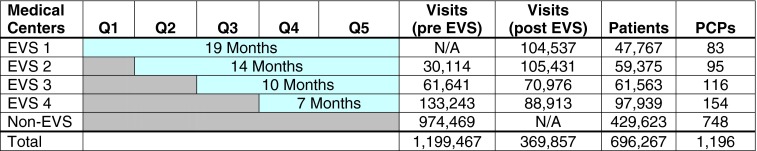
Sequential implementation of the “Exercise as a Vital Sign” program at four medical centers (EVS 1 – 4) from 4/2010 to 10/2011; 11 other medical centers (Non-EVS) in the same geographic region did not implement the EVS program during the study analysis period. N/A = not applicable. Note: The month during initial program implementation at each pilot medical center had <50 % exercise data collection per visit; these months were grouped with the “pre-EVS” months.
Patient Eligibility
We restricted our analyses to adults ages 18–89 with continuous plan membership who visited their assigned primary care physician within one of the 15 KPNC San Francisco Bay area medical centers during the study observation period (April 2010 to October 2011, n = 824,311). We excluded 128,044 patients who switched medical center affiliation during the study period, had recent hospitalizations or pregnancies, or had comorbidities that might limit exercise capacity (e.g. cancers other than skin cancer, end-stage renal disease, liver failure, lower extremity amputation, dementia), leaving 696,267 eligible patients for analysis.
Outcomes
Our primary hypothesis was that the systematic collection of exercise information at the beginning of primary care visits by medical assistants would lead to corresponding changes in exercise-related clinical care. Our conceptual model was that EVS data collection would directly increase physician and patient awareness of inadequate physical activity. This increased awareness would in turn trigger corresponding clinical actions, such as lifestyle counseling and referrals to support healthy lifestyle changes including weight loss, particularly when patients are both sedentary and overweight or diabetic. These healthy lifestyle changes would be expected to result in increased exercise, weight loss, and (among patients with inadequately controlled diabetes) improved glycemic control. Thus, the EVS program would act as a “red flag” to help trigger a broad range of health-related preventive actions.
Physician documentation of exercise in the progress notes was based on a key word search algorithm (see Appendix). Manual clinician review of 200 progress notes identified by our search algorithm either with exercise documentation (n = 100) or without exercise documentation (n = 100) yielded 90 % specificity and 98 % sensitivity for identifying progress notes with exercise-related documentation. Referrals for lifestyle-related counseling were captured using our system’s electronic referral system and/or registered attendance in lifestyle-related classes. We grouped together all referrals related to weight management, exercise, nutrition, or other healthy behavior counseling and educational services.
KPNC randomly selects approximately 10 % of patients with recent visits for mailed surveys (response rate 48 %). During the study period, surveys that were sent to patients insured by Medicare included two additional questions related specifically to exercise counseling: “In the last 12 months, has a doctor or other health provider talked with you about your level of exercise or physical activity? Has a doctor or other health provider advised you to start, increase, or maintain your level of exercise or physical activity?” We used these randomly collected survey data among Medicare patients (n = 6880) to assess patient-reported exercise counseling in EVS and non-EVS practices. Weight, body mass index, and HbA1c results were extracted from the electronic medical record databases.
Study Design and Statistical Methods
For visit-based outcomes (progress note documentation, referrals, and patient survey results), we compared patient visits to the four medical centers after EVS was implemented with visits to the medical centers where EVS was not yet implemented (which included the four EVS medical centers prior to EVS implementation and the 11 medical centers without EVS implementation during the study period, see Fig. 1). We analyzed progress note documentation and referrals using logistic regression models (SAS PROC GLIMMIX). We controlled for practice-level differences by including both baseline year exercise progress note documentation rates and trends for each medical center, and we accounted for patient-level differences by including baseline patient demographic and comorbidity variables in our models (Table 1). We also adjusted for repeated visits per patient to account for clustering of data within patients. Because of the high outcome prevalence in patient survey results, we used modified Poisson regression models (SAS PROC GENMOD) with robust error variance to estimate relative likelihood of exercise counseling after adjusting for patient demographic and clinical variables.16 Use of modified Poisson models allowed us to estimate relative risks rather than odds ratios, a more appropriate effect measure when outcomes are not rare.
Table 1.
Patient Characteristics
| ALL | EVS Medical Centers | Non-EVS Medical Centers | |
|---|---|---|---|
| Patients, No. (%) | 696,267 | 266,644 (38.3 %) | 429,623 (61.7 %) |
| Women, No. (%) | 363,948 (52.3 %) | 139,864 (52.5 %) | 224,084 (52.2 %) |
| Age, years (sd) | 51.4 (16.4) | 51.0 (16.4) | 51.6 (16.3) |
| Race, No. (%) | |||
| Asian | 118,768 (17.1 %) | 43,615 (16.4 %) | 75,153 (17.5 %) |
| Black | 45,832 (6.6 %) | 18,028 (6.8 %) | 27,804 (6.5 %) |
| Hispanic | 92,732 (13.3 %) | 37,917 (14.2 %) | 54,815 (12.8 %) |
| White | 324,712 (46.6 %) | 123,292 (46.2 %) | 201,420 (46.9 %) |
| Other | 114,223 (16.4 %) | 43,792 (16.4 %) | 70,431 (16.4 %) |
| HH income, No. (%) | |||
| < $40 K | 73,540 (10.9 %) | 32,584 (12.6 %) | 40,956 (9.8 %) |
| $40 K–$74.9 K | 370,123 (54.8 %) | 127,300 (49.4 %) | 242,823 (58.2 %) |
| ≥ $75 K | 231,453 (34.3 %) | 97,851 (38.0 %) | 133,602 (32.0 %) |
| BMI, mean (sd) | 28.0 (6.1) | 27.9 (6.1) | 28.1 (6.1) |
| BMI categories, No. (%) | |||
| Normal (15–24.9) | 215,220 (33.7 %) | 84,414 (34.6 %) | 130,806 (33.1 %) |
| Overweight (25–29.9) | 231,862 (36.3 %) | 87,847 (36.0 %) | 144,015 (36.4 %) |
| Obese ( ≥ 30) | 192,447 (30.1 %) | 71,831 (29.4 %) | 120,616 (30.5 %) |
| Comorbid Conditions, No. (%) | |||
| Hypertension | 235,829 (33.9 %) | 88,075 (33.0 %) | 147,754 (34.4 %) |
| Dyslipidemia | 197,073 (28.3 %) | 73,502 (27.6 %) | 123,571 (28.8 %) |
| Diabetes | 72,229 (10.4 %) | 27,154 (10.2 %) | 45,075 (10.5 %) |
| Osteoarthritis | 62,221 (8.9 %) | 24,218 (9.1 %) | 38,003 (8.9 %) |
| COPD / Asthma | 51,825 (7.4 %) | 18,359 (6.9 %) | 33,466 (7.8 %) |
| CVD | 41,674 (6.0 %) | 15,714 (5.9 %) | 25,960 (6.0 %) |
| Total primary care visits, mean (sd) | 2.3 (1.5) | 2.2 (1.5) | 2.3 (1.5) |
| Patients with diabetes | 72,229 | 27,154 | 45,075 |
| HbA1c (%), Mean (sd) | 7.2 (1.4) | 7.2 (1.4) | 7.2 (1.4) |
| Baseline (pre-study) | |||
| Exercise documentation/visit | 21.0 % | 22.5 % | 20.0 % |
Numbers are number and percent [No. (%)], or mean (standard deviation, sd)
EVS Exercise as a Vital Sign program; HH Income Household income by US Census 2000 block group; BMI body mass index (kg/m2); COPD chronic obstructive pulmonary disease; CVD cardiovascular disease
Primary care visits are for the 12 months preceding the study period
All differences between EVS and non-EVS site patients are significant (p < 0.001). Proportions exclude missing data (< 3 % for all variables except BMI, missing = 8.2 %)
For the patient-level outcomes of weight loss and HbA1c change, we used a difference-in-differences approach to compare changes from baseline (most recent measurement preceding the first EVS implementation 4/2010) to last measure in the observation period between study arms. This analysis was restricted to patients with at least two measurements (91.0 % of cohort for weight; 97.0 % of patients with diabetes for HbA1c), and we compared all eligible patients receiving primary care in the four EVS medical centers to all eligible patients receiving primary care in the 11 non-EVS medical centers over the 16-month study period. Mean interval between first and last BMI measurements was 427.1 (132.6) days for patients in the four EVS medical centers and 429.4 (131.5) days for patients in the 11 non-EVS medical centers (p < 0.001). Mean interval between first and last HbA1c measurements was 463 (95.6) and 451.7 (102.3) days (p < 0.001) for patients with diabetes attending EVS and non-EVS medical centers, respectively.
All analyses were intention-to-treat, meaning that visits to primary care practices that had implemented the EVS program were counted as EVS visits, regardless of whether MVPA was collected for that patient at that visit. Results were also stratified by BMI category (normal, overweight, obese). In an exploratory analysis, we also examined our primary visit-based outcomes in an “on-treatment” analysis comparing EVS visits where MVPA data were actually collected to control visits. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA). These analyses were conducted as part of the Natural Experiments for Translation in Diabetes (NEXT-D) Study, a network of academic, community, industry, and policy partners collaborating to advance the methods and practice of natural experimental research, and to identify and prioritize the best policies to prevent and control diabetes. This study was approved by the institutional review board of the Kaiser Foundation Research Institute.
RESULTS
Participants
After excluding patients based on age, non-continuous membership, and comorbidity, there were 1,569,324 visits by 696,267 eligible patients to 1,196 primary care providers during the study period. There were 594,855 visits by 266,644 eligible patients to primary care practices of four medical centers with EVS implemented (224,998 visits before and 369,857 visits after EVS implementation); and 974,469 visits by 429,623 eligible patients to primary care practices of 11 medical centers without EVS implemented (Fig. 1). In the four medical centers where EVS was implemented, exercise data were recorded in the medical record by medical assistants at 73.9 % of eligible patient visits, and 83.4 % of eligible patients had exercise data collected during at least one visit. There were few clinically important differences between the eligible patients with and without EVS collection (data not shown).
Mean age for the overall cohort was 51.4 ± 16.4 years; 52.3 % were women, and 46.6 % were non-Hispanic White. Mean baseline BMI was 28.0 ± 6.1 kg/m2, with 30.1 % of the cohort obese and 36.3 % overweight. Prevalence of type 2 diabetes was 10.4 %. Although often statistically significant, differences between patients in EVS vs. non-EVS practices were small (Table 1). Among patients with recorded EVS data, mean self-reported MVPA was 156 ± 182 minutes per week (median 120 minutes/week, interquartile range: 0 to 240), and 29.9 % reported no MVPA (0 minutes/week).
Physician Exercise Documentation and Lifestyle-Related Referrals
Physicians were more likely to document exercise in their progress notes during visits where EVS was implemented (26.2 % vs 23.7 % of notes, including 29.4 % vs 26.0 % among obese patients; Table 2). In a multivariate model adjusting for demographic differences and repeated measures, the adjusted odds of exercise documentation in PCP progress notes increased by 12 % (aOR 1.12, 95 % CI: 1.11-1.13) among practices that had implemented EVS compared to practices without EVS.
Table 2.
Proportion of Visits with Progress Note Exercise Documentation and Referrals for Lifestyle-Related Counseling, Comparing Practices with and Without EVS Implementation (n = 1,569,324 Visits)
| EVS implemented | EVS Not implemented | Adjusted OR [95 % CI] | p value | |
|---|---|---|---|---|
| Visits, All eligible patients | 369,857 | 1,199,467 | ||
| Progress Note, % | 26.2 % | 23.7 % | 1.12 [1.11, 1.13] | < 0.001 |
| Referral, % | 2.1 % | 1.7 % | 1.14 [1.11, 1.18] | |
| Visits, Patients with BMI ≥ 30 | 111,435 | 358,938 | ||
| Progress Note, % | 29.4 % | 26.0 % | 1.14 [1.12, 1.16] | < 0.001 |
| Referral, % | 4.0 % | 3.2 % | 1.12 [1.08, 1.17] |
Models are adjusted for repeated measures, and baseline differences in patient demographic variables, comorbidities, and pre-study medical center exercise documentation rates
EVS Exercise as a Vital Sign program; SD standard deviation; CI confidence interval
Implementation of EVS was associated with a large relative (although small absolute) increase in referrals. Rates of referral per visit were greater if EVS had been implemented (2.1 % vs. 1.7 % of visits), a difference that was greater among obese patients (4.0 % vs. 3.2 % of visits, Table 2). In a multivariate model adjusted for demographic differences and repeated measures, the per-visit odds of referral increased by 14 % (aOR 1.14, 95 % CI: 1.11–1.18) among practices that had implemented EVS.
Limiting our analysis to EVS visits with MVPA collected (“On-treatment”) resulted in even greater odds of exercise-related progress note documentation (aOR 1.16 [1.14, 1.17], p < 0.001; compared to 1.12 [1.11, 1.13], p < 0.001 in the intention to treat analysis) and greater odds of healthy lifestyle-related referrals (aOR 1.20 [1.16-1.24], p < 0.001; compared to aOR 1.14 [1.11-1.18], p < 0.001) when compared to control visits.
Patient-Reported Exercise Discussions
A random subset of Medicare-insured patients in our analysis completed post-visit patient surveys (n = 6,880). Patients in this survey subset were 73.1 (7.1) years old; 59 % were women, and 68 % were white. 68 % were overweight/obese. More respondents with EVS visits reported that their physician discussed exercise than respondents with non-EVS visits (88 % vs. 76 %, p <0.001). After adjusting for age and baseline BMI, survey respondents were 14 % more likely to report that their PCP discussed exercise (aRR 1.14, 95 % CI: 1.11–1.17) after a visit to an EVS practice (n = 2,312) compared to a visit to a non-EVS practices (n = 4,568, p < 0.001).
Changes in Weight and HbA1c
Overall, overweight and obese patients attending primary care practices at the four EVS medical centers lost more weight over the study period compared to patients in the 11 non-EVS medical centers (adjusted difference-in-differences 0.16 lbs [95 % CI: 0.10–0.21], p < 0.001). This difference between study arms was driven primarily by weight loss in the overweight group. In a multivariate linear model adjusting for baseline differences, attendance at an EVS primary care practice was associated with 0.20 lb greater weight loss among overweight patients (95 % CI: 0.12–0.28 lbs, p <0.001; Table 3). Applying this small calculated benefit to the overall population of overweight patients in our system (n = 230,326), we estimate that full implementation of EVS would be associated with 46,065 lbs (23 tons) of additional weight loss.
Table 3.
Changes in Weight Over Time Among Patients Attending EVS vs. non-EVS Medical Centers (n = 633,864 Patients with at Least Two Weight Measurements)
| EVS Medical Centers (n = 4) | Non-EVS Medical Centers (n = 11) | p value | ||
|---|---|---|---|---|
| Weight change from baseline (SD) | Weight change from baseline (SD) | Difference-in-Differences (95 % CI) | ||
| All eligible patients, n = 634,202 | −0.18 (10.3) | −0.08 (10.5) | 0.16 [0.10–0.21] | < 0.001 |
| Overweight patients, n = 230,326 | −0.21 (9.2) | −0.04 (9.3) | 0.20 [0.12–0.28] | < 0.001 |
| Obese patients, n = 189,973 | −1.64 (13.7) | −1.52 (13.9) | 0.12 [−0.01–0.25] | 0.08 |
Weights are in pounds
Difference-in-differences analyses between study arms are adjusted for baseline differences in patient demographic variables and comorbidities
Interval between first and last weight = 427 days (EVS medical centers), 429 days (Non-EVS medical centers)
EVS Exercise as a Vital Sign program; BMI body mass index (kg/m2; SD standard deviation; CI confidence interval
Overweight = BMI ≥ 25 and < 30; Obese = BMI ≥ 30
Among eligible patients with diabetes (10.4 % of cohort), attendance in an EVS primary care practice was associated with 0.06 % more favorable decline in A1c (95 % CI: 0.05 % to 0.08 %’ Table 4), a benefit that was greater among patients with A1c > 7 % at baseline (0.1 % relatively greater decline, 95 % CI: 0.07 % to 0.13 %) and among patients with A1c > 8 % (0.15 %, 95 % CI: 0.09 % to 0.21 %) compared to patients in non-EVS practices.
Table 4.
Changes in HbA1c Over Time Among Patients with Diabetes Attending EVS Versus Non-EVS Medical Centers (n = 70,083 Patients with at Least Two HbA1c Measurements)
| EVS Medical Centers (n = 4) | Non-EVS Medical Centers (n = 11) | p value | ||
|---|---|---|---|---|
| HbA1c change from baseline (SD) | HbA1c change from baseline (SD) | Difference-in-Differences (95 % CI) | ||
| All patients with diabetes, n = 70,083 | 0.23 % (1.21) | 0.29 % (1.22) | 0.06 % [0.05–0.08] | < 0.001 |
| Patients with HbA1c > 7, n = 30,487 | −0.13 % (1.54) | −0.02 % (1.53) | 0.1 % [0.07–0.13] | < 0.001 |
| Patients with HbA1c > 8, n = 13,440 | −0.63 % (1.85) | −0.47 % (1.84) | 0.15 % [0.09–021] | < 0.001 |
Difference-in-differences analyses between study arms are adjusted for baseline differences in patient demographic variables and comorbidities;
Interval between first and last HbA1c = 463 days (EVS medical centers), 452 days (Non-EVS medical centers)
EVS Exercise as a Vital Sign program; HbA1c Hemoglobin A1c; SD standard deviation; CI confidence interval
DISCUSSION
In the past several decades, Americans have become increasingly sedentary and obese.17 This epidemic of unhealthy lifestyle behaviors has had a tremendous impact on the overall U.S. health and health care costs.5,6,9 We found that a health system-level intervention to improve the recognition and assessment of insufficient physical activity was associated with improved exercise related care processes (e.g. increased physician exercise documentation, exercise counseling, and lifestyle-related referrals) and with small but significant clinical improvements (e.g. weight loss among overweight/obese adults and improved HbA1c among patients with diabetes and elevated HbA1c) compared to usual care. Although individually averaged weight loss was small, these changes correspond to substantial total weight loss when applied to the overall medical center population. Our results indicate that a relatively simple intervention to collect patient-reported exercise levels at a key step in the care process can have significant effects on population-level health outcomes.
The “Exercise as a Vital Sign” intervention combined a modification to an existing electronic health record with changes to the clinical workflow and staff responsibilities. The program was designed to efficiently elicit each patient’s weekly MVPA and to present these data in a timely way to the primary care physician during the clinical encounter. Prior work to validate these data has demonstrated good discriminant and face validity.18 This program incorporated key elements of the Chronic Care Model,19 including practice reorganization, new clinical roles, and the innovative use of health information technology (IT). Results from this study demonstrate the potential clinical benefits that can be derived from the meaningful use of electronic health records.20 However, the absolute clinical improvement, while consistent across different patient subgroups, was relatively small, suggesting that this approach needs to be linked to more intensive and effective tools to help patients increase their physical activity. Indeed, as has been seen in other EHR-based studies, information-only interventions seldom have a clinically significant impact if not linked to effective means for patients and/or providers to act on the information provided.21,22 The U.S. Preventive Services Task Force recently concluded that substantially increasing physical activity requires medium-intensity and high-intensity counseling interventions.11 Taken together, these findings suggest that the next step in improving physical activity among primary care populations may be to link the initial identification and counseling about insufficient physical activity during the primary care visit to a more robust referral system for behavioral health interventions.
Prior work has shown that physician physical activity counseling has more impact in patients experiencing health problems compared to their healthy counterparts.23 Weight change associated with the EVS intervention in our study was greatest among patients who are overweight, suggesting that these patients may be most amenable to primary care-initiated health behavior changes.24 However, while prior research has found that physician counseling can be effective,25 the severe time constraints typical of primary care visits may require alternative means of implementing behavioral interventions.26,27
Our results must be considered in the context of the study design. First, we conducted a nonrandomized parallel comparison. While investigator-driven randomization is the gold-standard design for evenly distributing potential confounders between study arms, the allocation to early vs. later EVS implementation in our system was a decision made at medical center leadership level rather than at the provider level, and thus this pseudo-randomized design is not likely confounded by intervention allocation. Indeed, in reviewing the circumstances of the initial roll-out, we found that while there was some evidence of greater willingness to test this new program at the medical center leadership level, this was not driven by “grassroots” enthusiasm at the clinician level from the many different practices within each medical center. Second, without audiotaping visit encounters, it is difficult to accurately assess the content of patient–provider discussions. Future research into how the EVS health IT component prompted changes in physician behavior may be helpful. We used a sensitive and specific algorithm to detect physician progress note documentation, but such documentation likely under-reports actual visit-based counseling, and our measurement of this variable at the physician level did not allow for true difference-in-difference analysis at the patient level. Indeed, in the subset of patients who were mailed member surveys by KPNC, the prevalence of patient-reported physician exercise counseling was markedly higher than the prevalence documented in progress notes. With either measure, however, the relative differences between study arms remain significant. Third, rates of referral for lifestyle-associated counseling were relatively low, and may have undercounted programs that patients could have participated in without formal referral or registered attendance. However, because such programs are available to all patients, this potential under-measurement would not have created biased results. Fourth, we compared changes in weight and Hba1c between the four medical centers that implemented EVS and the 11 medical centers that had not yet implemented EVS. This comparison strategy may have underestimated the impact of EVS because this program was phased in sequentially at the four implementation sites over a 1-year period, rather than being fully implemented for the entire observation period (Fig. 1). Finally, our study was conducted within a large, integrated care system, and thus our findings may not be immediately generalizable to other systems. Indeed, overall prevalence of patient-reported physician counseling was markedly higher in our health system than nationally.12–14 However, this organizational model is well-suited for testing advanced approaches to population-level health interventions, and with over 1.5 million visits by nearly 700,000 patients to over 1,000 PCPs, our results are not likely to be biased by self-selection or outlier biases that can occur in smaller, traditional randomized trials. Further research with longer follow-up will be required to ascertain how well these changes are maintained over time.
Insufficient physical activity is a highly prevalent problem with significant health consequences. We found that the systematic collection of patient-reported exercise levels during initial primary care visit intake was associated with increased physician progress note documentation, lifestyle-related referrals, and patient-reported physician exercise counseling. Care process changes were associated with small but significantly improved weight loss and glycemic control for large numbers of patients in our system. These results support a care model in which the identification and initial discussion of exercise can be increased by the systematic collection of patient-reported exercise data as part of usual clinical workflow. Greater clinical impact on exercise-related health outcomes may require combining this approach with more intensive interventions outside of the primary care visit.
Acknowledgements
This study was funded by the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (no. U58 DP002721) and by the Division of Research, Kaiser Permanente Northern California. RWG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
APPENDIX
The Keyword Search
Physician progress notes that included “EXERCI” (after excluding EXERCISE or EXERCISE-INDUCED or KEGEL EXERCISE or EXERCISE STRESS TEST) or “PHYSICAL ACTIV” or “PHYSICALLY ACTIV” or “LIFESTYLE MOD” or “LIFE STYLE MOD” or “LIFESTYLE CHANG” or “LIFE STYLE CHANG” or “HEALTHY LIFE”
REFERENCES
- 1.State-specific prevalence of no leisure-time physical activity among adults with and without doctor-diagnosed arthritis—United States, 2009 2011. [PubMed]
- 2.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the physical activity guidelines for Americans. Am J Prev Med. 2011;40(4):454–461. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Willis BL, Gao A, Leonard D, Defina LF, Berry JD. Midlife fitness and the development of chronic conditions in later life. Arch Intern Med. 2012;27:1–8. doi: 10.1001/archinternmed.2012.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vigen R, Ayers C, Willis B, DeFina L, Berry JD. Association of cardiorespiratory fitness with total, cardiovascular, and noncardiovascular mortality across 3 decades of follow-up in men and women. Circ Cardiovasc Qual Outcome. 2012;5(3):358–364. doi: 10.1161/CIRCOUTCOMES.111.963181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson LH, Martinson BC, Crain AL, et al. Health care charges associated with physical inactivity, overweight, and obesity. Prev Chron Dis. 2005;2(4):A09. [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn EB, Ramsey LT, Brownson RC, et al. The effectiveness of interventions to increase physical activity. A systematic review. Am J Prev Med. 2002;22(4 Suppl):73–107. doi: 10.1016/S0749-3797(02)00434-8. [DOI] [PubMed] [Google Scholar]
- 8.Herring MP, Puetz TW, O'Connor PJ, Dishman RK. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(2):101–111. doi: 10.1001/archinternmed.2011.696. [DOI] [PubMed] [Google Scholar]
- 9.Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 10.Leitzmann MF, Park Y, Blair A, et al. Physical activity recommendations and decreased risk of mortality. Arch Intern Med. 2007;167(22):2453–2460. doi: 10.1001/archinte.167.22.2453. [DOI] [PubMed] [Google Scholar]
- 11.Lin JS, O'Connor E, Whitlock EP, Beil TL. Behavioral counseling to promote physical activity and a healthful diet to prevent cardiovascular disease in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153(11):736–750. doi: 10.7326/0003-4819-153-11-201012070-00007. [DOI] [PubMed] [Google Scholar]
- 12.Wee CC, McCarthy EP, Davis RB, Phillips RS. Physician counseling about exercise. JAMA. 1999;282(16):1583–1588. doi: 10.1001/jama.282.16.1583. [DOI] [PubMed] [Google Scholar]
- 13.Barnes PM, Schoenborn CA. Trends in adults receiving a recommendation for exercise or other physical activity from a physician or other health professional. NCHS Data Brief. 2012;86:1–8. [PubMed] [Google Scholar]
- 14.Glasgow RE, Eakin EG, Fisher EB, Bacak SJ, Brownson RC. Physician advice and support for physical activity: results from a national survey. Am J Prev Med. 2001;21(3):189–196. doi: 10.1016/S0749-3797(01)00350-6. [DOI] [PubMed] [Google Scholar]
- 15.Karter AJ, Moffet HH, Liu J, et al. Achieving good glycemic control: initiation of new antihyperglycemic therapies in patients with type 2 diabetes from the Kaiser Permanente Northern California diabetes registry. Am J Manag Care. 2005;11(4):262–270. [PMC free article] [PubMed] [Google Scholar]
- 16.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 17.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 18.Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise "vital sign" in electronic medical records. Med Sci Sports Exerc. 2012;44(11):2071–2076. doi: 10.1249/MSS.0b013e3182630ec1. [DOI] [PubMed] [Google Scholar]
- 19.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544. doi: 10.2307/3350391. [DOI] [PubMed] [Google Scholar]
- 20.Jha AK. Meaningful use of electronic health records: the road ahead. JAMA. 2010;304(15):1709–1710. doi: 10.1001/jama.2010.1497. [DOI] [PubMed] [Google Scholar]
- 21.Grant RW, Middleton B. Improving primary care for patients with complex chronic diseases: can health information technology play a role? CMAJ. 2009;181(1–2):17–18. doi: 10.1503/cmaj.091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang JW, Kushner RF, Cameron KA, Hicks B, Cooper AJ, Baker DW. Electronic tools to assist with identification and counseling for overweight patients: a randomized controlled trial. J Gen Intern Med. 2012;27(8):933–939. doi: 10.1007/s11606-012-2022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss DR, Wolfson C, Yaffe MJ, Shrier I, Puts MT. Physician counseling of older adults about physical activity: the importance of context. Am J Health Promot. 2012;27(2):71–74. doi: 10.4278/ajhp.100804-QUAL-263. [DOI] [PubMed] [Google Scholar]
- 24.Phillips EM, Kennedy MA. The exercise prescription: a tool to improve physical activity. PM & R: J Inj Funct Rehab. 2012;4(11):818–825. doi: 10.1016/j.pmrj.2012.09.582. [DOI] [PubMed] [Google Scholar]
- 25.Calfas KJ, Long BJ, Sallis JF, Wooten WJ, Pratt M, Patrick K. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med. 1996;25(3):225–233. doi: 10.1006/pmed.1996.0050. [DOI] [PubMed] [Google Scholar]
- 26.Ostbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bock C, Diehl K, Schneider S, Diehm C, Litaker D. Behavioral counseling for cardiovascular disease prevention in primary care settings: a systematic review of practice and associated factors. Med Care Res Rev. 2012;69(5):495–518. doi: 10.1177/1077558712441084. [DOI] [PubMed] [Google Scholar]


