ABSTRACT
BACKGROUND
In response to epidemic levels of prescription opioid overdose, abuse, and diversion, routine urine drug tests (UDTs) are recommended for patients receiving chronic opioid therapy (COT) for chronic pain. However, UDT ordering for COT patients is inconsistent in primary care, and little is known about how to increase UDT ordering or the impact of increased testing on rates of aberrant results.
OBJECTIVE
To compare rates and results of UDTs for COT patients before versus after implementation of an opioid risk reduction initiative in a large healthcare system.
DESIGN
Pre-post observational study.
PATIENTS
Group Health patients on COT October 2008–September 2009 (N = 4,821), October 2009–September 2010 (N = 5,081), and October 2010–September 2011 (N = 5,498).
INTERVENTION
Multi-faceted opioid risk reduction initiative.
MAIN MEASURES
Annual rates of UDTs and UDT results.
KEY RESULTS
Half of COT patients received at least one UDT in the year after the initiative was implemented, compared to only 7 % 2 years prior. The adjusted odds of COT patients having at least one UDT in the first year of the opioid initiative were almost 16 times (adjusted OR = 15.79; 95 % CI: 13.96–17.87) those 2 years prior. The annual rate of UDT detection of marijuana and illicit drugs did not change (12.6 % after initiative implementation), and largely reflected marijuana use (detected in 11.1 % of all UDTs in the year after initiative implementation). In the year after initiative implementation, 10.7 % of UDTs were negative for opioids.
CONCLUSIONS
The initiative appeared to dramatically increase urine drug testing of COT patients in the healthcare system without impacting rates of aberrant results. The large majority of aberrant results reflected marijuana use or absence of opioids in the urine. The utility of increased urine drug testing for COT patient safety and prevention of diversion remains uncertain.
KEY WORDS: urine drug test, urine drug screen, chronic opioid therapy, chronic pain, opioid risk reduction
INTRODUCTION
Dramatic increases in the prescribing of opioid medications for chronic non-cancer pain (CNCP) in the U.S. have resulted in epidemic levels of prescription opioid overdose, abuse, addiction, and diversion.1–5 In 2010, there were 16,651 fatal overdoses involving prescription opioids.6 Prescription opioid misuse and abuse resulted in almost 660,000 emergency department visits in 2010, over twice as many as in 2004.7 Substance abuse treatment admissions for opiates other than heroin as the primary drug of abuse increased more than six-fold from 1999 to 2009.8
Routine urine drug tests (UDTs) for patients on chronic opioid therapy (COT) are widely recommended as a strategy to increase patient safety and decrease diversion.1,9,10 UDTs may help identify diversion (through absence of a prescribed opioid) or substance misuse and abuse (through detection of illicit drug or prescription opioid or sedative-hypnotic use unknown to the opioid prescriber). A recent editorial concluded, “to improve the safe and effective use of opioids, …healthcare systems and individual practices will need to be redesigned to support routine urine drug testing in conjunction with other opioid monitoring strategies” (p. 495).11 However, evidence indicates low UDT use in primary care, where most chronic pain is managed.1 In one study of primary care COT patients, only 8 % received a UDT;3 in another, only 26 % of patients on very high opioid doses and 20 % of patients on lower doses received UDTs in the prior year.12 It is well-established that changing physician practice behaviors, including adherence to clinical guidelines, is difficult.13,14
We sought to determine whether UDT ordering can be substantially increased in primary care. No prior studies have assessed efforts to increase UDTs among COT patients, or evaluated the impact of increased testing on rates of aberrant results. Increased use of UDTs could be inefficient if it results in more testing of COT patients without aberrant findings.
In response to concerns about prescription opioid safety, Group Health (GH) implemented a multi-faceted risk reduction initiative targeting COT patient management.15 This initiative, which included UDT guidelines, aimed to reduce risks of opioid overdose, misuse, abuse, and diversion. We evaluated the initiative’s impact on rates of UDTs and of aberrant UDT results.
METHODS
Setting and Sample
GH is a large nonprofit healthcare system in Washington State with an integrated group practice in 25 ambulatory medical centers. The GH initiative,15 implemented in September 2010, targeted COT in the group practice and included a comprehensive COT patient care guideline and practice tools (see Text Box). The guideline provides UDT recommendations according to patient opioid dose and other risk factors (Table 1), and recommends use of a specific “pain management” UDT for routine use with COT patients (see Text Box). Primary care clinicians are given lists of their COT patients.
Table 1.
Study COT Risk Category Definitions and Group Health UDT Recommendations
| Risk category | MED/methadone category | UDT recommendation |
|---|---|---|
| High | ≥ 120 mg MED/day or on methadone | UDT at least twice a year |
| Medium | 20–< 120 mg MED/day | UDT at least once a year |
| Low | < 20 mg MED/day | Consider UDT once a year |
COT chronic opioid therapy; MED morphine-equivalent dose; UDT urine drug test
Text Box. Components of Group Health Opioid Risk Reduction Initiative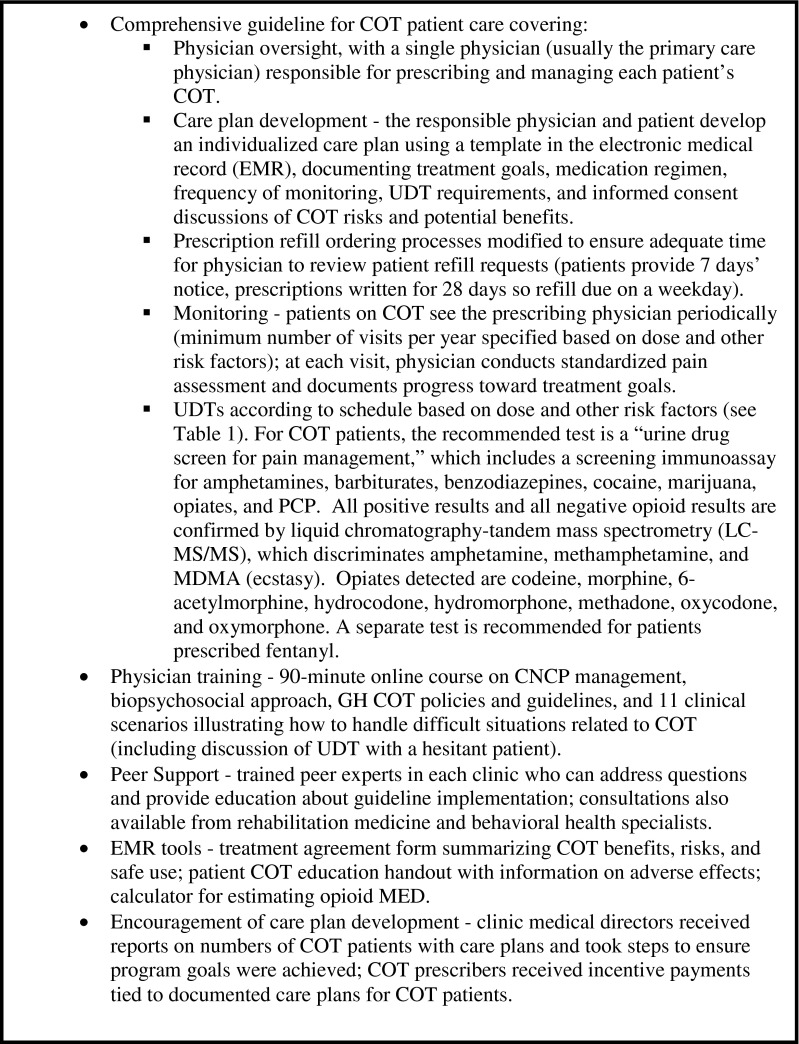
To evaluate the initiative’s impact on UDT rates, we examined GH electronic data for 3 years: (1) the initiative implementation year (October 2010–September 2011), (2) the initiative planning year (October 2009–September 2010), and (3) the year prior to the initiative planning year (baseline year; October 2008–September 2009). Our study sample for each year consisted of the GH patient population aged 18 years and older targeted by the initiative; i.e., Western Washington integrated group practice clinic patients receiving COT for CNCP. To define COT, we identified prescriptions for non-liquid oral and transdermal opioids (except buprenorphine) filled in the three calendar months before each month in each year. We calculated total days’ supply for these prescriptions in each 3-month period for each patient. The GH initiative defined COT as receipt of ≥ 70 days’ supply of opioids in the previous 3 months. For this study, we expanded this criterion to a 1-year period: For each study year, the sample consisted of patients who received ≥ 70 days’ supply of opioids in the previous 3 months for ≥ 6 of the 12 months. To ensure pharmacy data availability, we included only patients enrolled at GH continuously from 3 months before the study year to the end of the study year. To limit the sample to CNCP patients, we excluded patients who, during this period, had hospice care, an opioid prescription from an oncologist or radiation oncologist, or visits for a cancer diagnosis (except non-melanoma skin cancer).
The initiative categorized COT patients as appropriate for high, medium, or low intensity monitoring (including UDTs) according to their risk profiles, based on opioid dose, methadone use, and other factors (e.g., history of alcohol or drug abuse). To categorize patients for this study, we used only the GH dose and methadone use criteria (Table 1), because information on the other risk factors was unavailable through automated data. The study was approved by the GH Institutional Review Board.
Measures
UDT Measures
We identified all UDTs in each study year that tested for opiates; amphetamines; methamphetamine; 3,4-methylenedioxymethamphetamine (MDMA; ecstasy); barbiturates; benzodiazepines; cocaine; phencyclidine (PCP); and tetrahydrocannabinol (THC; marijuana). We examined results from confirmatory tests after positive screening immunoassays. We examined opiate results only for the recommended “pain management” UDT in the initiative implementation year, because only this UDT was followed consistently by confirmatory testing for opiates, regardless of the screening result (with the exception of methadone, which is confirmed only if present in the screening assay).
Opioid Dose
For patients who received a UDT in each year, we calculated mean daily opioid dose in the 90 days prior to their first UDT in that year. For patients without a UDT in the year, we calculated dose for a 90-day period prior to a randomly selected date in that year. We calculated the total morphine-equivalent dose (MED) for each opioid prescription filled during the 90-day period as the quantity of pills dispensed, multiplied by the dose (in mg), multiplied by the opioid-to-morphine equivalent conversion factor.16 The mean daily opioid dose was the total MED for the 90 days divided by 90.
Covariates
From GH electronic databases, we obtained patient age and gender.
Statistical Analysis
We used repeated measures logistic regression to examine differences across the three study years in UDT rates among COT patients. Patients were included only in the years they met COT criteria. The outcome was one or more UDT in the year (no, yes). The independent variable was study year (baseline, initiative planning, initiative implementation). Covariates were age, gender, number of months in the year meeting COT criteria, and mean daily opioid dose. The model was estimated using generalized estimating equations (GEE).17,18 We used an independence working correlation matrix and estimated standard errors using the robust sandwich estimator to account for dependence between some observations (i.e., patients who were in more than one study year).19 For each year, we calculated the percent of UDT confirmatory tests with aberrant results. For the implementation year, we calculated the percent of “pain management” UDT confirmatory tests negative for opioids.
RESULTS
Sample Characteristics
Over the three study years, the number of patients who met the study criteria for COT increased (Table 2). In each year, 61–62 % of these patients met COT criteria in all 12 months and 82 % met the criteria in at least 9 months. There was overlap in patients across study years: 2,711 patients were included in all 3 years, 1,964 in 2 years, and 3,339 in only 1 year. The mean daily opioid dose decreased over the 3 years by 17 %. The proportions of COT patients in medium and high opioid dose categories also decreased across study years (from 15.7 % on high and 48.0 % on medium doses in the baseline year to 11.9 % on high and 45.2 % on medium doses in the implementation year), while the proportion in the low dose category increased (from 36.3 % in the baseline year to 42.9 % in the implementation year).
Table 2.
Sample N, Gender, and Age in Each Study Year
| Sample characteristic | Baseline year (10/2008–9/2009) | Initiative planning year (10/2009–9/2010) | Initiative implementation year (10/2010–9/2011) |
|---|---|---|---|
| Patients meeting study COT definition, n (% of Group Health* enrollees) | 4,821 (2.2 %) | 5,081 (2.3 %) | 5,498 (2.5 %) |
| Female, n (%) | 3,062 (63.5) | 3,210 (63.2) | 3,430 (62.4) |
| Age,† mean years (SD) | 57.8 (14.9) | 58.1 (14.7) | 58.5 (14.3) |
| Daily opioid MED, mg‡ | |||
| Mean (SD) | 59.3 (90.3) | 53.9 (83.1) | 49.4 (78.8) |
| Median (IQR) | 29.7 (14.3–66.5) | 27.6 (13.8–60.7) | 24.4 (12.0–55.5) |
COT chronic opioid therapy; SD standard deviation; MED morphine-equivalent dose; IQR interquartile range
* Western Washington integrated group practice clinics. In the baseline/initiative planning/initiative implementation year, 15,745/17,282/19,359 patients had at least one 3-month period of COT (at least 70 days’ supply of opioids in 90 days); among these, 6,490/6,999/7,821 patients were excluded because they had less than 6 months of COT in the year, 6/6/4 were excluded because they were under age 18 years, 1,574/1,942/2,480 were excluded because they were not enrolled continuously in Group Health from 3 months before the study year to the end of the study year, 2,505/2,892/3,187 were excluded because they were not treated at a Western Washington integrated group practice clinic, and 349/362/369 were excluded because of a cancer exclusion criterion
† At beginning of study year
‡ Average daily dose in 90 days prior to UDT or comparable period for patients who did not receive UDT in the year
Changes in UDT Rates
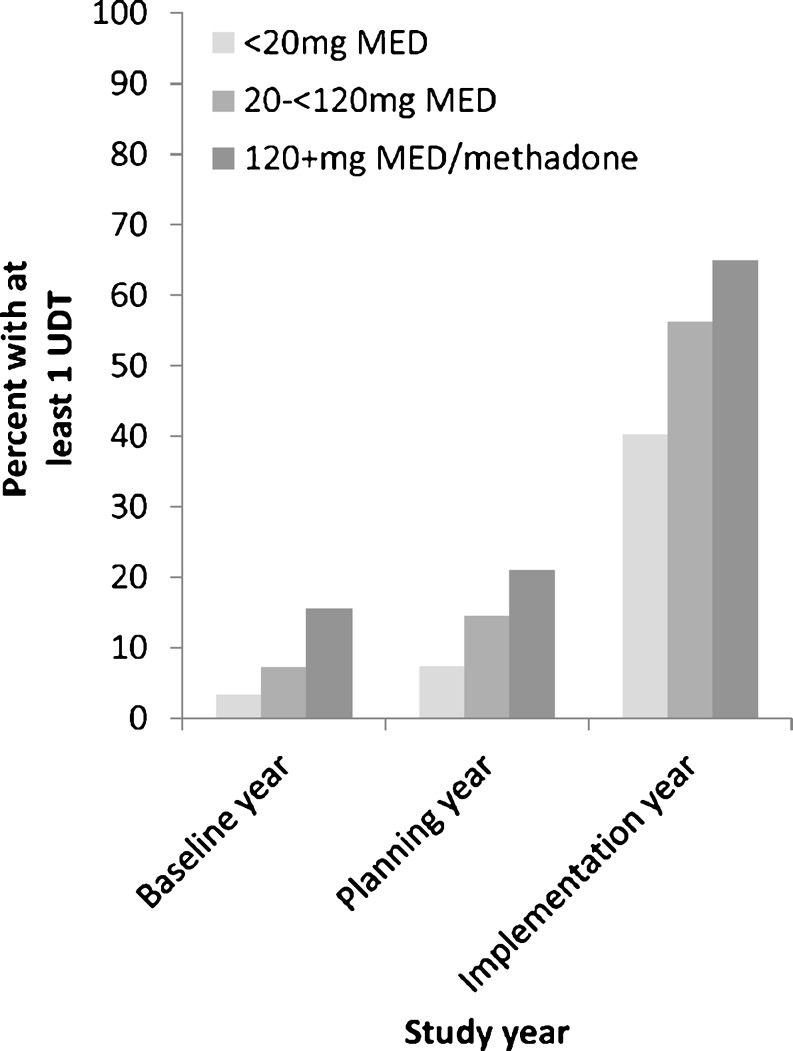
The proportion of COT patients who received at least one UDT increased from 7 % (n = 343/4,821) in the baseline year and 13 % (n = 641/5,081) in the planning year to 50 % (n = 2,773/5,498) in the implementation year. Adjusting for age, gender, opioid dose, and months in the year meeting criteria for COT, the odds of COT patients having at least one UDT were approximately twice in the planning year (adjusted OR = 1.99; 95 % CI: 1.75–2.26) and 16 times in the implementation year (adjusted OR = 15.79; 95 % CI: 13.96–17.87), compared to the baseline year. There was a large increase in UDTs in the implementation year for COT patients in all three opioid dose groups (Fig. 1). In the high dose group, for whom at least two UDTs per year were recommended, the proportion of patients who received more than one UDT also increased substantially over the study years: 3.8 % in the baseline year, 6.8 % in the planning year, and 20.9 % in the implementation year.
Figure 1.
Percent of chronic opioid therapy patients who received at least one UDT in each year by opioid dose group.
Aberrant UDT Results
Across all 3 years, 12.2–14.3 % of confirmatory tests were positive for THC, cocaine, amphetamine, or methamphetamine (Table 3). Most of these were due to THC (detected in 9.4–11.8 % of all UDTs); none were positive for MDMA (ecstasy), 6-acetylmorphine (6-AM; heroin), or PCP. In the implementation year, the rate of tests positive for any of these substances was similar to that in the baseline year, but the increased number of tests resulted in many more positive tests (434 versus 56), largely reflecting THC (88 % of positive tests). Only five more tests were positive for cocaine in the implementation year than in the baseline year, despite 2,985 more UDTs. The rate of specimens too dilute to analyze decreased from 7.4 % in the baseline year to 4.4 % in the implementation year.
Table 3.
UDT Results Among Chronic Opioid Therapy Patients in Each Study Year
| UDT result | Baseline year N = 460 UDTs n (%) |
Planning year N = 895 UDTs n (%) |
Implementation year N = 3,445 UDTs n (%) |
|---|---|---|---|
| Positive for 6-AM, amphetamine, cocaine, MDMA, methamphetamine, PCP, or THC | 56 (12.2) | 128 (14.3) | 434 (12.6) |
| Positive for THC | 43 (9.4) | 106 (11.8) | 381 (11.1) |
| Positive for cocaine | 8 (1.7) | 6 (0.7) | 13 (0.4) |
| Positive for amphetamine | 9 (2.0) | 17 (1.9) | 55 (1.6) |
| Positive for methamphetamine | 1 (0.2) | 6 (0.7) | 7 (0.2) |
| Specimen too dilute to analyze* | 34 (7.4) | 53 (5.9) | 151 (4.4) |
6-AM 6-acetylmorphine (heroin); MDMA 3,4-methylenedioxymethamphetamine (ecstasy); PCP phencyclidine; THC tetrahydrocannabinol (marijuana); UDT urine drug test
*Dilute urines can result from a variety of causes, including a purposeful attempt to make the results not analyzable
Among the 2,781 “pain management” UDTs in the implementation year (a subset of the 3,445 total UDTs), 24 (0.9 %) were too dilute to analyze and 16 were for patients whose only opioid prescription in the previous 30 days was for fentanyl (not detected by this UDT). Among the remaining 2,741 tests, 292 (10.7 %) were negative for opioids.
DISCUSSION
There has been a call to develop and evaluate prescription opioid monitoring programs, including routine urine drug testing, in primary care settings, with the goal of providing safer, more effective care of patients receiving COT for CNCP.11 GH’s implementation of a multi-faceted COT risk reduction initiative that included UDT recommendations achieved a substantial increase in UDTs: half of COT patients received at least one UDT in the year after the initiative was implemented compared to only 7 % 2 years prior, a 16-fold increase in odds. It is not possible to know which initiative elements most affected UDT ordering. The incentivized COT patient care plans included UDT components. Physician education may have increased recognition of the importance of UDTs, comfort ordering UDTs, and confidence responding to aberrant results. UDT ordering is easier with a single test with which physicians and clinic staff are familiar. Finally, the GH guideline creates a standard that reduces concern that patients may feel singled out.
Nonetheless, the rate remained well below that targeted. The initiative first focused on high-dose patients, then expanded to all COT patients. Some patients, especially those on lower doses, may not have completed a UDT until after the study period, due to the time required for physicians to meet with all COT patients to develop care plans and order UDTs. Other plausible explanations for UDT non-completion include the guideline’s recommendation only to “consider UDT” for patients on low doses and physician beliefs that their patients are unlikely to abuse or divert drugs, concerns about negative impact on physician–patient relationships, difficulty interpreting UDT results, and discomfort responding to unexpected results.1,11
Greater UDT ordering could result in lowered rates of aberrant findings if proportionally more patients not abusing drugs are tested. However, at GH, increased testing did not lower the rate of tests positive for marijuana and drugs of abuse—12 % in both the baseline and implementation years. We do not know how physicians changed their UDT practices, including what proportion of their COT patients they tested and how they selected patients for testing, after the initiative. Aberrant result rates would not change with increased testing if physicians were inaccurate in estimating patients’ risks for abuse or if proportions of UDTs ordered routinely versus selectively remained similar.
The rate of urine specimens too dilute to analyze was somewhat lower in the implementation year (4.4 % versus 7.4 % in the baseline year). Dilute urine samples are considered aberrant, because they may reflect purposeful attempts to avoid detection of illicit substances. However, they can also result from other causes.20
Rates of detection of possible illicit drug use were very low. In the initiative implementation year, less than 1 % of tests detected cocaine or methamphetamine, and only 1.6 % detected amphetamine (which may or may not have reflected illicit use). Despite almost 3,000 more UDTs than 2 years prior, only five more detected cocaine. None detected PCP, heroin, or ecstasy. Detection of true PCP use is rare because the drug is no longer widely available in the U.S.21 Heroin can be detected only if used shortly before the urine sample is provided.20,22 The GH rates of tests positive for cocaine (0.4–1.7 %) were substantially lower than the 18 % rate reported at an academic internal medicine clinic, where UDTs may have been ordered selectively for patients suspected of substance abuse.23 We think it likely that the low rates of illicit drug detection at GH reflect the GH population, which is largely middle class and representative of the general Western Washington population. GH opioid prescribing policies could also deter drug abusers from enrolling.
THC was responsible for the great majority of aberrant results. Although marijuana use is not known to increase risk of opioid overdose, it may be associated with opioid abuse or diversion,24 and little is known regarding its impact on COT patient safety or outcomes. We do not know how many patients with tests positive for THC were using marijuana for pain or with prior knowledge of their physicians. At the time of our study, marijuana use for medical purposes, including pain relief, was legal under Washington State law. There is currently no consensus regarding actions to take when a COT patient’s UDT indicates marijuana use, and appropriate actions may vary according to state laws. GH guidelines recommend a range of responses for providers to consider (e.g., discontinuing COT, evaluating for substance abuse, referring for substance abuse treatment).
In the initiative implementation year, 10.6 % of “pain management” UDT confirmatory tests were negative for opioids. Although such a result is considered unexpected and has been found to be associated with illicit drug use,25 the extent to which this reflected opioid diversion is unknown. Absence of opioids may reflect no use in the previous several days (with or without diversion); diluted or adulterated urine; or altered opioid metabolism (e.g., due to poor absorption, genetic variability, or use of certain other medications).26–29 GH recommends that the prescribing physician discuss negative opioid results with the patient, inquire about reasons, and respond accordingly.
Study limitations include the observational study design in a single institution with a historical cohort comparison. Differences in UDT rates across study years may have reflected factors in addition to the opioid initiative, such as greater UDT ordering due to increased awareness of COT risks from media and journal articles; the Washington State Interagency Guideline on Opioid Dosing for Chronic Non-cancer Pain30 (originally published in March 2007; the July 2010 update provides detailed UDT guidance); and clinical guideline recommendations.10 However, UDT rates increased dramatically immediately after implementation of the initiative.
We did not assess whether UDT results were consistent with patients’ prescribed medications (e.g., opioids, benzodiazepines, stimulants). Clinical interpretation of UDT results is complex, requiring knowledge of all drugs used by a patient, as well as of other factors that can affect test results.21,26 Patients can avoid drug detection by adulterating, substituting, or diluting urine samples.20,21
An important unanswered question is whether, given the low rates of illicit drug detection and lack of guidance regarding concomitant use of marijuana, routine UDTs for all COT patients at GH and similar settings are warranted, given the costs and demands on provider and patient time. Some patients withhold information or provide incorrect information regarding drug use31 and behavioral monitoring alone does not identify all problematic substance use,32 supporting the potential value of UDTs in providing objective information regarding patient use of prescribed and nonprescribed drugs. Awareness of illicit or nonprescribed drug use can help identify patients with substance use disorders, which can then be treated. UDTs negative for opioids may help identify and stop diversion, potentially reducing illegal activity and opioid overdoses and deaths among people who acquire the drugs illegally. However, data are lacking concerning actual impact of UDTs on COT patient or public health safety.
It is unknown whether increased testing at GH enhanced the safety of COT for patients or prevented diversion of opioids. More patients who may be using marijuana, cocaine, amphetamines, and methamphetamine are being identified, but, other than marijuana, these numbers are very small relative to the number of UDTs. The 11 % rate of no opioid detected requires further investigation. UDTs may be more useful or cost-effective for subgroups of patients, such as patients on high doses or with known risk factors for opioid misuse, including age younger than 45 years and history of alcohol or other substance abuse or mental health disorders.3
In conclusion, in a setting with organizational resources and capabilities, urine drug testing for COT patients was rapidly and dramatically increased. This resulted in only a small increase in the number of patients identified who might have been using illicit drugs. There was a large increase in the number of tests positive for marijuana, but the implications of marijuana use for patient safety and outcomes, and optimal responses to COT patients using marijuana, remain unknown. These knowledge gaps point to important areas for further research, which also include the consequences of routine UDTs for decreasing opioid diversion and abuse and improving patient and public health safety, as well as optimal UDT procedures and frequency for specific patient subgroups and clinical settings.
Acknowledgements
Contributors
The authors thank Lisa C. Murphy, MD, Group Health, for her helpful comments regarding the study results.
Funders
The study was funded by NIH NIA grant 1R01 AG034181. The findings and conclusions do not necessarily represent views of Group Health.
Prior Presentations
Portions of this work were presented as a poster at the annual meeting of the American Pain Society, May 17, 2012; and at the International Association for the Study of Pain World Congress on Pain, August 29, 2012.
Conflict of Interest
Dr. Von Korff has received grant funding to Group Health Research Institute from Janssen Pharmaceuticals, which also supported Dr. Turner, Dr. LeResche, and Ms. Saunders. Dr. Von Korff has also received grant funding to Group Health Research Institute from Pfizer. Dr. Shortreed has served as a biostatistician on a grant to Group Health Research Institute from Pfizer. Ms. Saunders owns stock in Merck. The other authors declare that they do not have any conflicts of interest.
REFERENCES
- 1.Starrels JL, Becker WC, Alford DP, Kapoor A, Williams A, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152:712–20. doi: 10.7326/0003-4819-152-11-201006010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Warner M, Chen LH, Makuc DM, Anderson RN, Minino AM. Drug poisoning deaths in the United States, 1980–2008. NCHS data brief, no. 81. Hyattsville, MD, 2011. [PubMed]
- 3.Starrels J, Becker W, Weiner M, Li X, Heo M, Turner B. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011;26:958–64. doi: 10.1007/s11606-011-1648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–21. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305(13):1346–7. doi: 10.1001/jama.2011.369. [DOI] [PubMed] [Google Scholar]
- 6.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–9. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 7.Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality. The DAWN report: highlights of the 2010 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Rockville, MD, July 2, 2012. [PubMed]
- 8.Substance Abuse and Mental Health Services Administration. Treatment Episode Data Set (TEDS) 1999–2009: National Admissions to Substance Abuse Treatment Services [Accessed September 5, 2013]. Available at: http://www.samhsa.gov/data/DASIS/teds09/TEDS2k9NTbl1.1a.htm.
- 9.Rolfs RT, Johnson E, Williams NJ, Sundwall DN. Utah clinical guidelines on prescribing opioids for treatment of pain. J Pain Palliat Care Pharmacother. 2010;24(3):219–35. doi: 10.3109/15360288.2010.503265. [DOI] [PubMed] [Google Scholar]
- 10.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bair MJ, Krebs EE. Why is urine drug testing not used more often in practice? Pain Pract. 2010;10(6):493–6. doi: 10.1111/j.1533-2500.2010.00425.x. [DOI] [PubMed] [Google Scholar]
- 12.Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010;151:625–32. doi: 10.1016/j.pain.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCracken LM, Boichat C, Eccleston C. Training for general practitioners in opioid prescribing for chronic pain based on practice guidelines: a randomized pilot and feasibility trial. J Pain. 2012;13(1):32–40. doi: 10.1016/j.jpain.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Hanbury A, Wallace L, Clark M. Use of a time series design to test effectiveness of a theory-based intervention targeting adherence of health professionals to a clinical guideline. Br J Health Psychol. 2009;14(3):505–18. doi: 10.1348/135910708X369558. [DOI] [PubMed] [Google Scholar]
- 15.Trescott CE, Beck RM, Seelig MD, Von Korff M. Group Health’s initiative to avert opioid misuse and overdose among patients with chronic noncancer pain. Health Aff. 2011;30:1420–4. doi: 10.1377/hlthaff.2011.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Korff M, Saunders K, Ray GT, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–7. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman & Hall; 1989. [Google Scholar]
- 18.Liang JK-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 19.Zeger SL, Liang JK-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. doi: 10.2307/2531248. [DOI] [PubMed] [Google Scholar]
- 20.Cone EJ, Caplan YH. Urine toxicology testing in chronic pain management. Postgrad Med. 2009;121:91–102. doi: 10.3810/pgm.2009.07.2035. [DOI] [PubMed] [Google Scholar]
- 21.Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66–76. doi: 10.4065/83.1.66. [DOI] [PubMed] [Google Scholar]
- 22.Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436–48. doi: 10.1080/10550887.2010.509277. [DOI] [PubMed] [Google Scholar]
- 23.Hariharan J, Lamb GC, Neuner JM. Long-term opioid contract use for chronic pain management in primary care practice. A five year experience. J Gen Intern Med. 2007;22:485–90. doi: 10.1007/s11606-006-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reisfield GM, Wasan AD, Jamison RN. The prevalence and significance of cannabis use in patients prescribed chronic opioid therapy: a review of the extant literature. Pain Med. 2009;10(8):1434–41. doi: 10.1111/j.1526-4637.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- 25.Pesce A, West C, Gonzales E, et al. Illicit drug use correlates with negative urine drug test results for prescribed hydrocodone, oxycodone, and morphine. Pain Physician. 2012;15:E687–92. [PubMed] [Google Scholar]
- 26.Alford DP. Chronic back pain with possible prescription opioid misuse. JAMA. 2013;309(9):919–25. doi: 10.1001/jama.2013.522. [DOI] [PubMed] [Google Scholar]
- 27.Reisfield GM, Salazar E, Bertholf RL. Rational use and interpretation of urine drug testing in chronic opioid therapy. Ann Clin Lab Sci. 2007;37(4):301–14. [PubMed] [Google Scholar]
- 28.McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19(1):4–16. doi: 10.1111/j.1521-0391.2009.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123–43. [PubMed] [Google Scholar]
- 30.Washington State Agency Medical Directors’ Group. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy, 2010 update. Olympia, WA. 2010. www.agencymeddirectors.wa.gov/opioiddosing.asp. Accessed September 5 2013.
- 31.Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15:184–91. doi: 10.1097/00002508-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097–102. doi: 10.1213/01.ANE.0000080159.83342.B5. [DOI] [PubMed] [Google Scholar]