Abstract
Background and Purpose
Tissue type plasminogen activator (tPA) in combination with recombinant annexin A2 (rA2) is known to reduce acute brain damage after focal ischemia. Here, we ask whether tPA plus rA2 combination therapy can lead to sustained long term neurological improvements as well.
Methods
We compared the effects of intravenous high-dose tPA alone (10mg/kg) versus a combination of low-dose tPA (5mg/kg) plus 10 mg/kg rA2 in a model of focal embolic cerebral ischemia in rats. All rats were treated at 3 hours after embolization. Brain tissue and neurological outcomes were assessed at 1 month. Surrogate biomarkers for endogenous neurovascular remodeling in peri-infarct area were analyzed by immunohistochemistry.
Results
Compared to high-dose tPA alone, low-dose tPA plus rA2 significantly decreased infarction and improved neurological function at 1 month post-stroke. In peri-infarct areas, tPA-plus-rA2 combination therapy also significantly augmented microvessel density, VEGF and synaptophysin expression.
Conclusions
Compared to conventional high-dose tPA alone, combination low-dose tPA plus rA2 therapy may provide a safe and effective way to improve long term neurological outcomes after stroke.
Keywords: Annexin A2, tissue plasminogen activator, focal embolic stroke, rats, combination therapy, neurological outcomes
Introduction
Improving tPA thrombolytic therapy is a high priority in stroke research. The ability of tPA to efficiently convert plasminogen into clot-dissolving plasmin relies on an endogenous fibrinolytic assembly via a triple complex formation of tPA, annexin A2 and plasminogen1. Annexin A2 is a cell-surface protein, which, in complex with its binding partner p11, forms a heterotetrameric (A22p112) receptor for both plasminogen, the inactive precursor of plasmin, and its activator, tPA. By assembling tPA, annexin A2, and plasminogen, this complex increases the catalytic efficiency of tPA, enabling it to convert plasminogen to plasmin more efficiently than the same amount of tPA alone 2. We have hypothesized low-dose tPA plus recombinant annexin A2 protein (rA2) will improve reperfusion and neurological outcomes2. Our previous experiments have shown that combining rA2 with low-dose tPA successfully achieved reperfusion and reduced acute infarct size when treated at 2 hours, and also significantly decreased hemorrhagic transformation when treatment was delayed to 4 hours after focal embolic stroke in rats2-4. The purpose of the present study was to extend these promising findings by asking whether the benefits of tPA plus rA2 combination therapy can be sustained for long-term neurological outcomes.
Materials and Methods
Focal embolic cerebral ischemia in rats
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Wistar rats (280-330g) were subjected to focal embolic strokes as we previously described5.
Assessments of neurological function deficits
Modified neurological severity score (NSS) and foot fault test for motor coordination function, adhesive tap-removal test for sensorimotor neurological deficits were assessed on days 1, 3, 7, 14, 21, and 28 after stroke by following standard methods6-8.
Measurements of brain infarction and mortality
At 28 days after stroke, brains H&E infarction volume was examined as we previously described and expressed as % of hemisphere5. Within 28 days after stroke, dead animals were counted for mortality rates.
Immunohistochemistry and quantification
At 28 days after stroke, immunohistochemistry was performed on the coronal sections at −0.8 and −2.8mm from bregma (the maximal brain infarct area) by following standard methods8. Primary antibodies against vWF (Abbiotec, San Diego), VEGF (Santa Cruz biotechnology, Santa Cruz, CA), and synaptophysin (Chemicon, Temecula, CA) were used. Vessel density (vWF-positive vessels on 3 fields per section in peri-infarct cortex) was quantitated as percentage of vWF positive vessels area; VEGF expression (positive cells area on 3 fields per section in peri-infarct cortex) was quantitated as percentage of immunopositive area; and synaptophysin expression (positive signals on 8 fields per section in peri-infarct striatum) was quantitated as optical density.
Experimental Design
For this translation study, all STAIR and RIGOR guidelines were followed in terms of randomization, blinding and statistical powering 9, 10. Two experimental groups, standard rat dose of tPA 10 mg/kg 11 (Genentech Inc, San Francisco, CA), and combination of tPA 5 mg/kg plus rA2 (produced as previously described3) 10 mg/kg given intravenously at 3 hours after embolization. Non-treatment saline control was not included in this study due to unacceptable high mortality (over 50%) observed in our pilot study. Inclusion criteria was set as: (1) stable 50% or less rCBF of pre-ischemic baseline for up to 1 hour after embolization; (2) NSS score at 3 hours after stroke is ≥8. 30 rats per group were enrolled in this study.
Statistical analysis
Infarction volume and immunohistochemistry were analyzed by student t-test. Mortality rate was analyzed by two-sided Fisher’s exact test. Neurobehavioral assessments were analyzed by repeated measures ANOVA followed by post-hoc t-test. The most conservative multiple-test correction was applied using the Bonferroni method. Differences with P<0.05 were considered statistically significant.
Results
Neurological Function Deficits
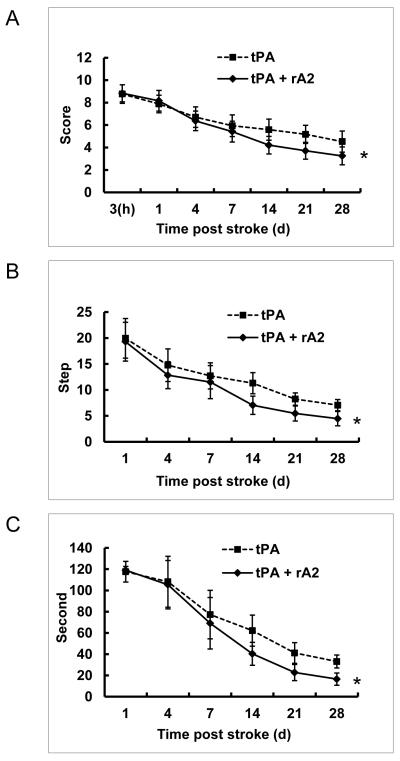
There was a statistically significant better performance in combination group compared to tPA alone controls on all 3 neurobehavioral assessments, and a significant interaction effect between treatments (combination versus tPA alone) and time for neurological functional recovery (up to 28 days after focal stroke in rats) in all 3 neurobehavioral assessments (Figure-1).
Figure-1.
Neurological Function Deficits. Neurological function deficits were assessed on days 1, 3, 7, 14, 21, and 28 after stroke. A. Modified neurological severity score. B. Foot fault test. C. Adhesive tap-removal test. *P<0.01, n=17 for tPA alone group, and n=24 for the combination group.
Brain infarction, mortality and vessel density
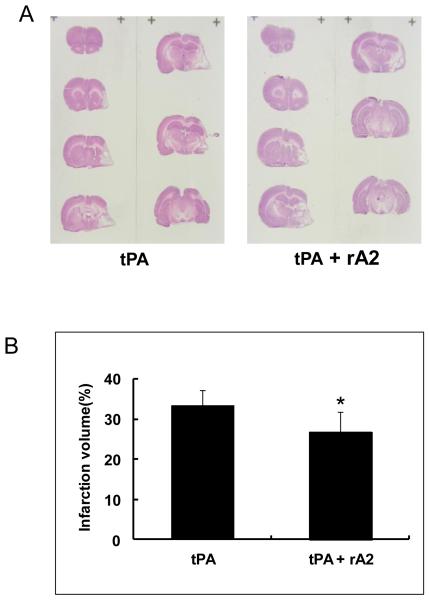
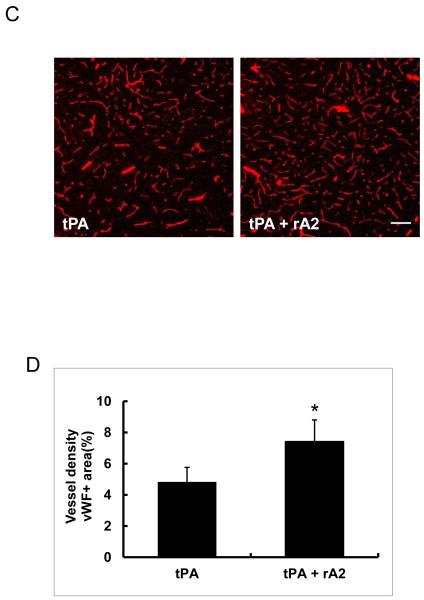
At 28 days after stroke, infarction volume in the tPA-plus-rA2 combination group was significantly smaller than the tPA alone group with reduction rate of 19.6% (Figure-2A, 2B), but There was no significant difference in mortality between tPA alone group (13/30, 43%) and the combination group (6/30, 20%) (P=0.095). Immunohistochemistry showed the combination treated animals had significantly higher cerebrovascular density in the peri-infarct cortex (Figure-2C, 2D),
Figure-2.
Brain Infarction volume and vessel density at 28 days after stroke. A. Representative H&E stained brain sections. B. Quantitation of brain infarction volume. *P<0.05, n=17 for tPA alone group, and n=24 for the combination group. C. Representative immunohistochemistry images of cerebral vessels labeled by endothelial cell maker vWF. Scale bar=100 μm. D. Quantitation of cerebrovascular density. *P<0.05, n=6 per group.
VEGF and synaptophysin expression in peri-infarct areas
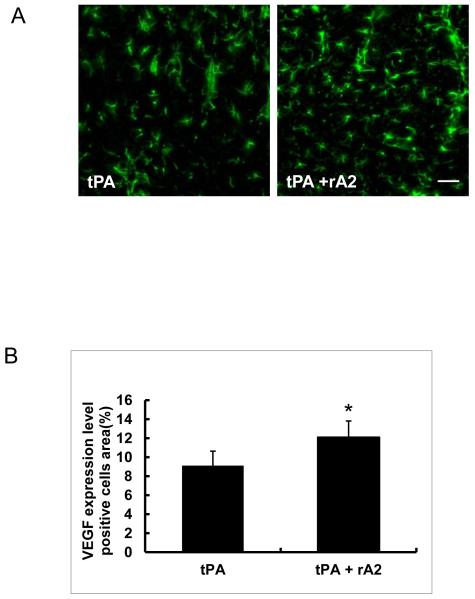
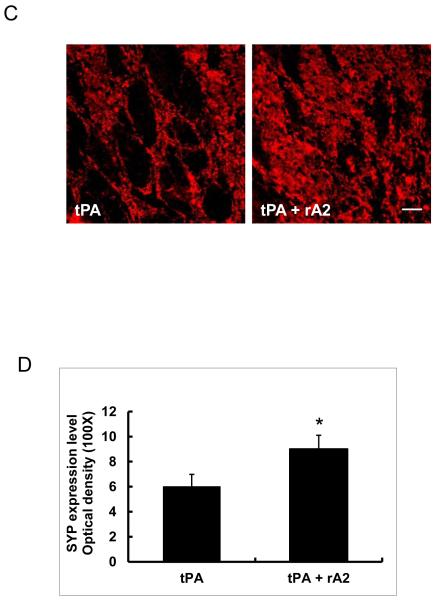
At 28 days after stroke, quantitation of immunohistochemistry showed rats treated with the tPA-plus-rA2 combination had significantly higher expression of the pro-angiogenic factor VEGF in peri-infarct cortex (Figure-3A, 3B), and significantly higher synaptic vesicle protein synaptophysin expression in peri-infarct striatum compared with tPA alone treated controls (Figure-3C, 3D).
Figure-3.
VEGF and synaptophysin expression in peri-infarct areas at 28 days after stroke. A. Representative immunohistochemistry images of VEGF expression in peri-infarct cortex area. Scale bar=50 μm. B. Quantitation of VEGF expression. C. Representative immunohistochemistry images of synaptophysin expression in peri-infarct striatum area. Scale bar=25 μm. D. Quantitation of synaptophysin expression. *P<0.05, n=6 per group.
Discussion
Existing data suggest that combining rA2 with tPA may allow one to use lower doses of tPA for effective thrombolysis2. However, acute neuroprotection may not always correspond to improvements in long-term outcomes. Hence, it was important to demonstrate that the beneficial effects of tPA-rA2 combination therapy would be sustained. Our present study showed that combination of low-dose tPA at 5 mg/kg plus rA2 10 mg/kg significantly reduced brain infarction and improved long-term neurological outcomes compared to standard high-dose tPA dose at 10 mg/kg alone.
During stroke recovery, neurovascular remodeling is induced in peri-infarct areas. The underlying mechanisms of these endogenous processes may involve matrix enzymes from the matrix metalloproteinase and plasminogen activator families12. So it was also important to determine whether manipulating tPA actions with rA2 could influence these recovery events. In this study, we measured microvessel density, VEGF and synaptophysin as representative surrogate markers of neurovascular remodeling in peri-infarct tissue. Along with infarct reduction and functional improvement, the combination of tPA plus rA2 did not dampen, and in fact, slightly augmented these indirect biomarkers of recovery. We speculate that in addition to the reduction in tPA-associated blood–brain barrier disruption, rA2-tPA complex might limit tPA brain penetration-associated neuronal excitotoxicity13, and rA2 might bind and neutralize angiostatin (one of tPA-plasminogen converting products)-associated endothelial toxicity 14. Ultimately, improved blood flow and fewer neurovascular side effects of the combination should translate into improved long-term outcome2. We acknowledge that stroke recovery involves complex cascades of neurovascular and gliovascular responses12, and how full spectrum of molecular events correlate with neurological outcomes remain to be elucidated. Nevertheless, our initial findings here may be consistent with a sustained long-term benefit of tPA plus rA2 therapy.
Taken together, our results demonstrated that combination treatment with tPA plus rA2 may sustain early brain tissue protective effects and preserve long term neurovascular remodeling and functional recovery. Further translational development of this tPA-plus-rA2 combination stroke therapy may be warranted.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported in part by NIH grant R01-NS065998 (to X.W.).
Footnotes
Disclosures: None.
reference
- 1.Kim J, Hajjar KA. Annexin ii: A plasminogen-plasminogen activator co-receptor. Front Biosci. 2002;7:d341–348. doi: 10.2741/kim. [DOI] [PubMed] [Google Scholar]
- 2.Fan X, Yu Z, Liu J, Liu N, Hajjar KA, Furie KL, et al. Annexin a2: A tissue plasminogen activator amplifier for thrombolytic stroke therapy. Stroke. 2010;41:S54–58. doi: 10.1161/STROKEAHA.110.596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu H, Fan X, Yu Z, Liu J, Murata Y, Lu J, et al. Annexin a2 combined with low-dose tpa improves thrombolytic therapy in a rat model of focal embolic stroke. J Cereb Blood Flow Metab. 2010;30:1137–1146. doi: 10.1038/jcbfm.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walvick RP, Bratane BT, Henninger N, Sicard KM, Bouley J, Yu Z, et al. Visualization of clot lysis in a rat embolic stroke model: Application to comparative lytic efficacy. Stroke. 2011;42:1110–1115. doi: 10.1161/STROKEAHA.110.602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan X, Qiu J, Yu Z, Dai H, Singhal AB, Lo EH, et al. A rat model of studying tissue-type plasminogen activator thrombolysis in ischemic stroke with diabetes. Stroke. 2011;43:567–70. doi: 10.1161/STROKEAHA.111.635250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 7.Stroemer RP, Kent TA, Hulsebosch CE. Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with d-amphetamine therapy after neocortical infarction in rats. Stroke. 1998;29:2381–2393. doi: 10.1161/01.str.29.11.2381. discussion 2393-2385. [DOI] [PubMed] [Google Scholar]
- 8.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 9.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. Rigor guidelines: Escalating stair and steps for effective translational research. Transl Stroke Res. 2013;4:279–285. doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–565. [PubMed] [Google Scholar]
- 12.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: More than a thrombolytic. Trends Neurosci. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Tuszynski GP, Sharma MR, Rothman VL, Sharma MC. Angiostatin binds to tyrosine kinase substrate annexin ii through the lysine-binding domain in endothelial cells. Microvascular research. 2002;64:448–462. doi: 10.1006/mvre.2002.2444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.