Abstract
Purpose
This prospective, randomized phase III intergroup trial of the Gynecologic Oncology Group and National Cancer Institute of Canada Clinical Trials Group was designed to test the effectiveness and safety of adding the hypoxic cell sensitizer tirapazamine (TPZ) to standard cisplatin (CIS) chemoradiotherapy in locally advanced cervix cancer.
Patients and Methods
Patients with locally advanced cervix cancer were randomly assigned to CIS chemoradiotherapy versus CIS/TPZ chemoradiotherapy. Primary end point was progression-free survival (PFS). Secondary end points included overall survival (OS) and tolerability.
Results
PFS was evaluable in 387 of 402 patients randomly assigned over 36 months, with enrollment ending in September 2009. Because of the lack of TPZ supply, the study did not reach its original target accrual goal. At median follow-up of 28.3 months, PFS and OS were similar in both arms. Three-year PFS for the TPZ/CIS/RT and CIS/RT arms were 63.0% and 64.4%, respectively (log-rank P = .7869). Three-year OS for the TPZ/CIS/RT and CIS/RT arms were 70.5% and 70.6%, respectively (log-rank P = .8333). A scheduled interim safety analysis led to a reduction in the starting dose for the TPZ/CIS arm, with resulting tolerance in both treatment arms.
Conclusion
TPZ/CIS chemoradiotherapy was not superior to CIS chemoradiotherapy in either PFS or OS, although definitive commentary was limited by an inadequate number of events (progression or death). TPZ/CIS chemoradiotherapy was tolerable at a modified starting dose.
INTRODUCTION
Historically, invasive cervical cancer has been treated with either surgery or radiation therapy. In early-stage disease (ie, International Federation of Obstetrics and Gynecology [FIGO] stage ≤ IIA), surgery and radiotherapy are equally efficacious, with differing toxicity profiles. In locally advanced–stage disease (ie, FIGO stage IIB to IVA), the role of surgery is limited, and curative therapy remains chemoradiotherapy using cisplatin (CIS).1 In 2012, approximately 12,170 patient cases of invasive cervical cancer were diagnosed, and 4,220 women died as a result of this disease in the United States, with advanced-stage disease accounting for both the majority of patient cases diagnosed and the associated deaths. Despite improvements in progression-free survival (PFS) with chemoradiotherapy, there is still a significant risk of both local recurrence and distant relapse in patients with locally advanced cervix cancer.2
Current investigations and previously published studies by large multi-institutional groups have examined the role of chemotherapy as a radiation sensitizer in the treatment of locally advanced cervical cancer. On the basis of the positive results of these five trials, the National Cancer Institute (NCI) released a clinical announcement in 1999 supporting the use of chemoradiotherapy as the standard of care in the treatment of patients with locally advanced cervical cancer.3–7
Additional trials have not demonstrated regimens more efficacious than irradiation and CIS. The Gynecologic Oncology Group (GOG) compared the efficacy of fluorouracil (FU) versus CIS as radiation sensitizers.8 This trial closed when a planned interim futility analysis demonstrated adding protracted venous FU infusion to irradiation was unlikely to provide a PFS benefit when compared with standard CIS chemoradiotherapy. Another GOG trial evaluated the addition of erythropoietin to CIS chemoradiotherapy to maintain a minimal hemoglobin level > 12.0 g/dL versus chemoradiotherapy with standard hemoglobin support to maintain a hemoglobin level > 10.0 g/dL.9 Unfortunately, this attempt to optimize oxygen delivery was associated with toxicity, leading to early study closure with no evaluable survival outcome.
Tirapazamine (TPZ) is a bioreductively activated, hypoxia-selective antitumor agent of the benzotriazine series, which is 35× to 450× more cytotoxic (dependent on tumor cell line studied) to hypoxic cells than well-oxygenated cells.10 It is bioactivated to a cytotoxic metabolite by electron transfer.11,12 This one-electron reduction product, an oxidizing radical anion, causes extensive single- and double-strand breaks in DNA. It may also accentuate DNA damage induced by irradiation or DNA-damaging cytotoxic agents by inhibiting DNA repair.13 Use of TPZ in a chemoradiotherapy regimen for cervical cancer makes biologic sense, because tumor hypoxia occurs in solid tumors and seems to be a contributing factor to irradiation resistance and treatment failure.14 Of further importance is the finding from preclinical studies that TPZ is synergistic with CIS by markedly increasing CIS cytotoxicity.15 This may be the primary cause of antitumor efficacy, because in a number of models, the level of cytotoxicity of TPZ alone is quite low, whereas the combination of TPZ and CIS increases the level of cell kill by many orders of magnitude.16,17 In the 5 years preceding this trial, TPZ had been used experimentally with CIS with or without radiation therapy in other solid tumors.18,19 Given the evidence suggesting patients with cervical cancer are adversely affected by hypoxic conditions, it was reasonable to evaluate TPZ in an attempt to improve outcomes in this group of patients. Early clinical studies in head and neck and non–small-cell lung cancers support the preclinical findings of TPZ/CIS synergism.20 Toxicity was mainly limited to fatigue and muscle cramps in patients with lung cancer. Hematologic toxicity was seen in those with head and neck cancer, related to dose and schedule.20,21
Clinical development of TPZ in cervix cancer was most promising when administered in combination with CIS in patients with recurrent cervical cancer. In a phase II trial by Malof et al,22 36 patients were treated with TPZ/CIS, with an overall response rate (partial and complete responses) of 27.8%. In a separate phase II trial conducted by the Southwest Oncology Group, 53 patients were treated with TPZ/CIS, yielding an overall response rate of 32.1%.23
The only experience in patients with cervical cancer undergoing chemoradiotherapy before the initiation of this study was limited to 15 patients in a phase I/II trial. These patients presented with locally advanced cervical cancer and were treated with TPZ once per week, CIS once every other week, and radiation therapy once per day.24 The maximum-tolerated dose chosen from this study was TPZ 290 mg/m2 and CIS 75 mg/m2 on days 1, 15, and 29 and TPZ 220 mg/m2 on days 8, 10, 12, 22, 24, and 26 concurrent with radiation therapy. Of the 15 patients treated in this trial, pelvic control was achieved in 12 (80%) of 15, with a minimum follow-up of 3 years.
On the basis of the potential clinical advantages of the addition of TPZ to CIS and with a known maximum-tolerated dose for this regimen, GOG initiated the current study, a phase III randomized trial of weekly CIS and irradiation versus CIS and TPZ (IND #46525) and irradiation in patients with stage IB2 or IIA (tumor size > 4 cm) or IIB, IIIB, or IVA cervical carcinoma limited to the pelvis.
PATIENTS AND METHODS
GOG Protocol 219 was a prospective, randomized phase III trial comparing the addition of TPZ to standard CIS-based chemoradiotherapy in patients with locally advanced cervical cancer. Patients with primary, untreated, histologically confirmed invasive squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma of the uterine cervix—clinical stage IB2 or IIA (tumor size > 4 cm) or IIB, IIIB, or IVA—were eligible for participation. Staging was defined by FIGO guidelines and based on clinical criteria. Surgical staging was not permitted, although lymph node status at the time of surgery (if performed) could be used for eligibility criteria purposes. Although computed tomography scans were required for eligibility purposes, they were not part of the staging criteria. Eligibility was confirmed through central review by the GOG Pathology Committee. Eligibility criteria also included the following: no evidence of para-aortic lymph node or distant metastasis on pretreatment imaging; adequate bone marrow, renal, and hepatic function; GOG performance status ≤ 3; no concurrent malignancy or prior malignancy within 5 years, excluding nonmelanoma skin cancer; signed informed consent; and approval by the institutional review board at each participating institution.
Study Design
The primary end point of the study was to determine if combining TPZ with CIS during radiation therapy increases PFS when compared with weekly CIS and radiation therapy in this patient population. Secondary end points included the impact on overall survival (OS) and assessment of treatment-related toxicity. Patients were stratified based on stage at diagnosis, cooperative group from which they were enrolled, type of brachytherapy (low-dose [LDR] v high-dose radiation [HDR]), and whether para-aortic lymph nodes were assess surgically (yes v no).
Treatment
Patients were randomly assigned to receive either CIS 40 mg/m2 on days 1, 8, 15, 22, 29, and 36 or TPZ 290 mg/m2 and CIS 75 mg/m2 on days 1, 15, and 29 and TPZ 220 mg/m2 on days 8, 10, 12, 22, 24, and 26 of chemoradiotherapy. After a planned interim safety analysis, the GOG Data Safety Monitoring Committee recommended decreasing TPZ from 290 to 220 mg/m2 and CIS from 75 to 60 mg/m2 on days 1, 15, and 29 in the experimental regimen.
Patients were also prescribed 41.4 to 45 Gy external-beam radiation therapy delivered homogenously to the pelvis in 23 to 25 fractions of 1.8 Gy. Intensity-modulated radiation therapy was not permitted in this study. After completion of external-beam radiation therapy, patients received 35 to 43.6 Gy to point A by intracavitary implant with radium or its equivalent if treated by LDR. Total HDR brachytherapy dose to point A was 27 to 31.5 Gy over five doses.
Statistical Methods
Random assignment with equal probability of assignment to each treatment regimen was carried out by dynamic allocation, balancing treatment assignment within cooperative group (GOG, National Cancer Institute of Canada Clinical Trials Group, or other), FIGO stage (IB2, IIA, IIB, IIIB, or IVA), type of brachytherapy (LDR or HDR), and para-aortic lymph nodes sampled (yes or no). The accrual goal was set at 750 eligible patients, with follow-up until 256 events (146 in control arm).25 This sample size would provide a statistical power of at least 85% to detect a 30% decrease in the progression hazards rate when testing at a significance level of .05 (one-sided test).
The design included an interim safety analysis after the first 60 patients. If toxicity of grade ≥ 3 was seen in a significant number of these patients (predetermined rule had been defined a priori), it was proposed that dose modifications should be recommended. An analogous second safety analysis was planned to further evaluate resolution of any safety concern. In addition, interim efficacy and futility analysis was to occur at 50% of the information time (ie, after observing at least 128 events). Stopping rules assumed two sequential tests were made using O'Brien Fleming spending functions (to determine test boundaries) and that the hazards were proportional.26,27
PFS was calculated as time in months from study enrollment to disease progression or death for noncensored observations (events) or to date of last contact for censored observations. OS was calculated as time in months from date of study enrollment to death for noncensored observations (events) or to date of last contact for censored observations (ie, patients alive, regardless of disease status). Recurrence site was classified as local if within the pelvic field, as locoregional if in vagina, para-aortic lymph nodes, or abdomen, and as distant otherwise.
Estimated product limits were computed using the Kaplan-Meier method.28 Differences in PFS and OS by treatment were evaluated using the log-rank test according to the intent-to-treat principle of eligible patients.29,30 Treatment effect on PFS and OS while adjusting for known prognostic factors and enrollment period (pre– v post–dose modification amendment) was accomplished using Cox regression.31 Screening for chance imbalances between clinical/pathologic characteristics and treatment assignment was performed using Pearson's χ2 test at a significance level of 0.1.32 The Mann-Whitney U test was used when the characteristic was continuous (eg, age). Fisher's exact test was used to assess differences in incidence of maximum adverse event grade (Common Terminology Criteria for Adverse Events, version 3) by treatment regimen.
RESULTS
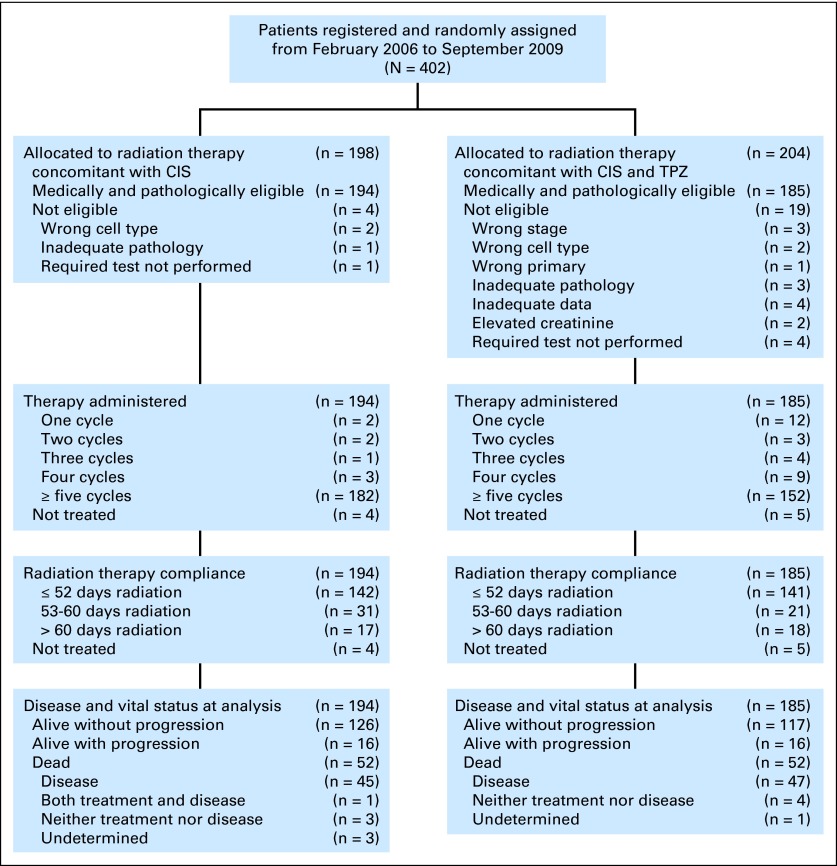
A total of 402 patients were enrolled from February 6, 2006, until study closure on September 9, 2009, because of lack of study drug. Of those, 379 were eligible and evaluable in the intent-to-treat analysis (Fig 1). The most common reasons for ineligibility were wrong stage, cell type, or primary cancer in nine of 23 patients. The demographic and tumor characteristics of the enrolled patients are summarized in Table 1. Patients were primarily white, non-Hispanic women with a good performance status with either FIGO stage IIB or IIIb squamous cell cancer of the cervix.
Fig 1.
CONSORT diagram. CIS, cisplatin; TPZ, tirapazamine.
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | CIS Plus RT (n = 194) |
CIS, TPZ, and RT (n = 185) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| ≤ 30 | 7 | 3.6 | 10 | 5.4 |
| 31-40 | 41 | 21.1 | 37 | 20.0 |
| 41-50 | 65 | 33.5 | 61 | 33.0 |
| 51-60 | 53 | 27.3 | 48 | 25.9 |
| 61-70 | 20 | 10.3 | 23 | 12.4 |
| > 70 | 8 | 4.1 | 6 | 3.2 |
| Median | 48.0 | 47.0 | ||
| Race | ||||
| Black | 43 | 22.2 | 30 | 16.2 |
| American Indian | 8 | 4.1 | 2 | 1.1 |
| Pacific Islander | 1 | 0.5 | 2 | 1.1 |
| Asian | 6 | 3.1 | 10 | 5.4 |
| White | 129 | 66.5 | 134 | 72.4 |
| Unknown | 7 | 3.6 | 7 | 3.8 |
| Ethnicity | ||||
| Hispanic | 20 | 10.3 | 24 | 13.0 |
| Non-Hispanic | 161 | 83.0 | 138 | 74.6 |
| Unknown/not specified | 13 | 6.7 | 23 | 12.4 |
| Performance status | ||||
| 0 | 147 | 75.8 | 141 | 76.2 |
| 1 | 46 | 23.7 | 41 | 22.2 |
| 2 | 1 | 0.5 | 2 | 1.1 |
| 3 | 0 | 0.0 | 1 | 0.5 |
| Tumor grade | ||||
| 1 | 8 | 4.1 | 12 | 6.5 |
| 2 | 105 | 54.1 | 103 | 55.7 |
| 3 | 78 | 40.2 | 67 | 36.2 |
| Not graded | 3 | 1.5 | 3 | 1.6 |
| Disease stage | ||||
| IB | 33 | 17.0 | 32 | 17.3 |
| IIA | 11 | 5.7 | 12 | 6.5 |
| IIB | 93 | 47.9 | 82 | 44.3 |
| IIIB | 51 | 26.3 | 52 | 28.1 |
| IVA | 6 | 3.1 | 7 | 3.8 |
| Cell type | ||||
| Squamous cell carcinoma | 164 | 84.5 | 158 | 85.4 |
| Adenocarcinoma, unspecified | 15 | 7.7 | 18 | 9.7 |
| Adenosquamous carcinoma | 11 | 5.7 | 5 | 2.7 |
| Other | 4 | 2.1 | 4 | 2.2 |
| Para-aortic lymph node | ||||
| Not sampled | 25 | 12.9 | 33 | 17.8 |
| Sampled | 169 | 87.1 | 152 | 82.2 |
| Brachytherapy | ||||
| None | 4 | 2.1 | 5 | 2.7 |
| Low-dose rate | 52 | 26.8 | 51 | 27.6 |
| High-dose rate | 138 | 71.1 | 129 | 69.7 |
| Cooperative group | ||||
| GOG | 167 | 86.1 | 167 | 90.3 |
| NCIC | 24 | 12.4 | 17 | 9.2 |
| Other | 3 | 1.5 | 1 | 0.5 |
Abbreviations: CIS, cisplatin; GOG, Gynecologic Oncology Group; NCIC, National Cancer Institute of Canada; RT, radiotherapy; TPZ, tirapazamine.
Of the 379 evaluable patients, 324 completed study therapy without dose-limiting toxicity or progression. Discontinuation for toxicity or refusal of study therapy was slightly higher in the experimental versus control arm (6.5% v 2.6% and 9.2% v 5.2%, respectively). Completion of all prescribed cycles was higher in the control versus experimental arm (93.8% v 82.2%). The differences in these factors were not statistically significant.
There was no impact of treatment arm on the ability to deliver radiation therapy; a detailed summary of treatment length is shown in Appendix Table A1 (online only). A majority of patients (283 [74.7%] of 379) were able to complete both external irradiation and intracavitary brachytherapy in ≤ 52 days.
Detailed toxicities for the planned interim safety analyses are listed in Table 2. There was a statistically significant increase in grade 3 or 4 leukopenia; GI toxicities manifested mainly as nausea, vomiting, and/or diarrhea; and metabolic abnormalities (renal or hepatic function) in the experimental arm at the first interim safety analysis. On the basis of the difference in toxicities between the control and experimental arms, a planned dose reduction was initiated. Analysis of the toxicities after the dose reduction revealed no additional differences between the two arms. The final toxicity analyses for all patients treated at the initial dose level and for all treated patients are summarized in Table 3 and Appendix Table A2 (online only).
Table 2.
Interim Safety Analysis
| Adverse Event (grade 3 or 4) | Early Safety Analysis (%) |
|||
|---|---|---|---|---|
| First |
Second |
|||
| CIS Plus RT (n = 29) | CIS, TPZ, and RT (n = 32) | CIS Plus RT (n = 28) | CIS, TPZ, and RT (n = 31) | |
| Leukopenia | 24 | 34 | 25 | 23 |
| GI | 14 | 19 | 21 | 3 |
| Metabolic/laboratory* | 17 | 28 | 14 | 10 |
Abbreviations: CIS, cisplatin; RT, radiotherapy; TPZ, tirapazamine.
Renal or hepatic function.
Table 3.
Treatment-Related Adverse Events
| Adverse Event | Grade |
P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CIS Plus RT (n = 187) |
CIS, TPZ, and RT (n = 179) |
||||||||||
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | ||
| Leukopenia | 48 | 30 | 60 | 43 | 6 | 53 | 26 | 47 | 47 | 6 | |
| Anemia | 37 | 60 | 75 | 16 | 0 | 46 | 58 | 65 | 10 | 0 | |
| Thrombocytopenia | 115 | 57 | 8 | 5 | 2 | 113 | 52 | 8 | 3 | 3 | |
| ANC | 103 | 25 | 31 | 20 | 8 | 101 | 22 | 30 | 23 | 3 | |
| Other hematologic | 163 | 2 | 3 | 11 | 8 | 160 | 2 | 1 | 9 | 7 | |
| Allergy/immunology | 182 | 4 | 1 | 0 | 0 | 171 | 4 | 2 | 2 | 0 | |
| Auditory/ear | 161 | 2 | 11 | 0 | 0 | 68 | 0 | 19 | 0 | 1 | |
| Cardiac | 179 | 5 | 2 | 1 | 0 | 152 | 1 | 25 | 0 | 0 | |
| Fatigue | 49 | 66 | 58 | 14 | 0 | 49 | 61 | 46 | 20 | 3 | |
| Fever, without neutropenia | 176 | 8 | 3 | 0 | 0 | 171 | 3 | 5 | 0 | 0 | |
| Other constitutional symptoms | 139 | 38 | 10 | 0 | 0 | 120 | 39 | 18 | 2 | 0 | |
| Dermatologic* | 137 | 36 | 14 | 0 | 0 | 102 | 35 | 26 | 15 | 1 | < .001 |
| Endocrine | 174 | 8 | 4 | 1 | 0 | 175 | 3 | 1 | 0 | 0 | |
| Nausea | 60 | 77 | 37 | 13 | 0 | 46 | 63 | 58 | 12 | 0 | |
| Vomiting* | 113 | 41 | 21 | 12 | 0 | 74 | 44 | 49 | 12 | 0 | < .001 |
| Diarrhea* | 72 | 81 | 23 | 11 | 0 | 70 | 52 | 38 | 19 | 0 | .007 |
| Constipation | 149 | 29 | 9 | 0 | 0 | 143 | 23 | 11 | 1 | 1 | |
| Other GI | 108 | 43 | 28 | 8 | 0 | 91 | 38 | 41 | 8 | 1 | |
| Renal/genitourinary | 135 | 30 | 20 | 2 | 0 | 142 | 26 | 9 | 2 | 0 | |
| Hemorrhage/bleeding | 169 | 16 | 1 | 1 | 0 | 165 | 10 | 2 | 2 | 0 | |
| Hepatobiliary/pancreas | 187 | 0 | 0 | 0 | 0 | 178 | 0 | 0 | 1 | 0 | |
| Infection | 166 | 0 | 9 | 11 | 1 | 153 | 0 | 17 | 8 | 1 | |
| Lymphatics | 181 | 6 | 0 | 0 | 0 | 164 | 10 | 4 | 1 | 0 | |
| Metabolic/laboratory | 92 | 45 | 25 | 19 | 6 | 76 | 47 | 19 | 33 | 4 | |
| Musculoskeletal/soft tissue | 176 | 7 | 4 | 0 | 0 | 159 | 8 | 7 | 5 | 0 | |
| Neuropathy, motor* | 186 | 0 | 1 | 0 | 0 | 168 | 6 | 5 | 0 | 0 | .003 |
| Neuropathy, sensory | 159 | 24 | 3 | 1 | 0 | 152 | 20 | 6 | 1 | 0 | |
| Other neurologic | 157 | 20 | 8 | 1 | 1 | 137 | 25 | 9 | 8 | 0 | |
| Ocular/visual | 179 | 8 | 0 | 0 | 0 | 163 | 15 | 1 | 0 | 0 | |
| Pain* | 118 | 42 | 24 | 3 | 0 | 76 | 36 | 38 | 29 | 0 | < .001 |
| Pulmonary | 174 | 10 | 1 | 2 | 0 | 164 | 8 | 3 | 4 | 0 | |
| Sexual/reproductive | 168 | 18 | 1 | 0 | 0 | 166 | 9 | 4 | 0 | 0 | |
| Vascular | 182 | 2 | 0 | 3 | 0 | 175 | 0 | 1 | 2 | 1 | |
NOTE. There was one treatment-related death.
Abbreviations: ANC, absolute neutrophil count; CIS, cisplatin; RT, radiotherapy; TPZ, tirapazamine.
Distribution of toxicity grade significantly different across treatment arms (Fisher's exact test).
As of November 30, 2011, there were 136 events among 379 eligible and evaluable patients in the study. Median follow-up was 28.3 months (interquartile range, 22.2 to 39.1 months). PFS and OS at 3 years (product time estimate) in the control and experimental arms were 64.4% versus 63.0% (unadjusted hazard ratio [HR], 1.047; 95% CI, 0.748 to 1.466; P = .7869) and 70.6% versus 70.5% (unadjusted HR, 1.042; 95% CI, 0.710 to 1.531; P = .8333), respectively (Figs 2 and 3). In multivariable analysis adjusting for known prognostics factors (age and stage) and enrollment period (pre– v post–dose modification amendment), the treatment effects on PFS (HR, 1.063; 95% CI, 0.649 to 1.708; P = .8344) and OS (HR, 1.174; 95% CI, 0.652 to 2.112; P = .5929) were insignificant. Furthermore, there was no significant interaction between treatment and enrollment period relative to either PFS (P = .3609) or OS (P = .6158).
Fig 2.
Overall survival by treatment (log-rank P = .8333). CIS, cisplatin; HR, hazard ratio; RT, radiotherapy; TPZ, tirapazamine.
Fig 3.
Progression-free survival by treatment (log-rank P = .7869). CIS, cisplatin; HR, hazard ratio; RT, radiotherapy; TPZ, tirapazamine.
Patterns of recurrence in those patients whose disease recurred are summarized in Table 4. Of note, there was a statistically significant increase in the rate of distant failure at the time of first recurrence in the control arm (Fisher's exact P = .0381). When analyzed by dosing before and after the dose reduction amendment, there was no significant difference noted, although there was a greater trend toward a higher distant failure rate in the control arm before the dose reduction.
Table 4.
Site of First Recurrence
| Treatment Group | Local | Locoregional | Distant |
|---|---|---|---|
| CIS plus RT | 29 | 13 | 20 |
| CIS, TPZ, and RT | 32 | 17 | 7 |
| P | .0381* | ||
Abbreviations: CIS, cisplatin; RT, radiotherapy; TPZ, tirapazamine.
Difference in rate of distant metastasis (Fisher's exact test).
DISCUSSION
For women with locally advanced cervical cancer, no regimen has been shown to be superior to CIS chemoradiotherapy. Despite theoretic advantages of adding TPZ to CIS in this patient population, this trial did not demonstrate the superiority of the TPZ/CIS regimen compared with CIS chemoradiotherapy. We conclude that this regimen is tolerable, and in devising an interim safety analysis structurally as part of the statistical design, a dose reduction ameliorated significant toxicity differences between the treatment arms. This type of design was critical given the lack of significant experience with the combination of TPZ and CIS with irradiation in this patient population. It is possible that the inability to complete all designed systemic therapy courses at optimal doses in the experimental arm prevented differences between the arms being demonstrated.
Since the initiation of this study, randomized trials in head and neck cancer have been published evaluating the role of TPZ in addition to standard chemoradiotherapy. A randomized phase II trial designed to assess the efficacy of adding TPZ to FU/CIS chemoradiotherapy in resectable stage IV head and neck cancer demonstrated no improvement in survival for the TPZ arm at the cost of increased hematologic toxicity.33 A phase III trial of the Trans-Tasman Radiation Oncology Group assessed the addition of TPZ to standard CIS chemoradiotherapy in stages III and IV head and neck cancer. In 861 patients, the addition of TPZ did not demonstrate an improvement in PFS, time to locoregional failure, or quality of life.34 Our study was not able to meet its planned accrual goal because of a lack of available study drug. The cause of this was multifactorial. However, the lack of a survival advantage and concerns about toxicity in the head and neck cancer population that became known during the course of this study may have been contributing factors working against further development of TPZ. Study accrual was slower than predicted. The complexity of the trial design and associated toxicities may have contributed to the pace of accrual. Given the low overall incidence of locally advanced cervical cancer relative to other cancer types in the United States and Canada and a historically low rate of clinical trial participation among patients with cancer overall, international cooperation may be essential to conduct studies such as this in the future.
Theoretically, treating patients with cancers expressing higher levels of tissue hypoxia with TPZ should be advantageous. This study was not able to demonstrate such an advantage clinically. However, translational science studies using tissue from this study are pending, and whether evidence of hypoxia as a biomarker for TPZ success allows for further development of this agent remains to be seen. In the future, use of hypoxia as a biomarker for treatment success may be beneficial with drugs that function in a fashion similar to that of TPZ.
The observation of a lower distant failure rate in the TPZ arm at initial progression is difficult to explain from a hypothetic perspective, because the potential benefit of TPZ would be in enhancing irradiation efficacy at the primary tumor site. This was not evident in this study. The clinical implications of a lower distant metastatic rate should be improved survival outcomes, again not seen in this study. Further investigation of this finding was negatively affected by the early closure of the trial.
Looking to the future, studies in the treatment of locally advanced cervical cancer may have to move toward questions outside the chemoradiotherapy paradigm because of the limited length of the treatment being studied. Given the evidence for a potential survival benefit with extended therapy beyond chemoradiotherapy seen in both the Peters et al study7 and a more recent study by Dueñas-González et al,35 the impact of extended therapy is one area warranting further evaluation. The role of adjuvant chemotherapy after initial chemoradiotherapy is the focus of two large-scale international trials. The Australia New Zealand Gynaecological Oncology Group 0902/GOG 0274 trial is evaluating the role of adjuvant paclitaxel and carboplatin after chemoradiotherapy in locally advanced disease, and the Radiation Therapy Oncology Group 0724 trial is evaluating the same intervention in early-stage, high-risk disease after radical hysterectomy. Of note, both of these are international, intergroup trials, fulfilling the need for international cooperation.
Acknowledgment
We thank all the patients who enrolled onto this trial, because it is their commitment that enables investigators to improve cancer care now and in the future.
Presented at the 43rd Annual Meeting of the Society of Gynecologic Oncology, Austin, TX, March 24-27, 2012.
Appendix
The following Gynecologic Oncology Group member institutions participated in this protocol: Roswell Park Cancer Institute; University of Alabama at Birmingham; Duke University Medical Center; Abington Memorial Hospital; Walter Reed National Military Medical Center; Wayne State University; University of Minnesota Medical School; University of Mississippi; University of Colorado–Anschutz Cancer Pavilion; University of California at Los Angeles; Fred Hutchinson Cancer Research Center; University of Pennsylvania Cancer Center; Penn State Milton S. Hershey Medical Center; University of Cincinnati Medical Center; University of North Carolina; University of Iowa Hospitals and Clinics; Southwestern Medical Center of Texas; Indiana University Cancer Center; Wake Forest University School of Medicine; University of California Medical Center at Irvine; Rush University Medical Center; Magee Women's Hospital; State University of New York at Brooklyn; University of New Mexico Health Sciences Center; Cleveland Clinic Foundation; State University of New York at Stony Brook; Washington University School of Medicine; Memorial Sloan-Kettering Cancer Center; Cooper Hospital University Medical Center; Columbus Cancer Council/Ohio State; MD Anderson Cancer Center; University of Massachusetts Memorial Health Care; Fox Chase Cancer Center; Women's Cancer Center of Nevada; University of Oklahoma; University of Virginia Health Sciences Center; University of Chicago; Mayo Clinic; Case Western Reserve University; Moffitt Cancer Center and Research Institute; Yale University; Cancer Trials Support Unit; Women and Infants Hospital; Georgia Core; Aurora Women's Pavilion of West Allis Memorial Hospital; and the Community Clinical Oncology Program.
Additionally, the following National Cancer Institute of Canada Clinical Trials Group member institutions participated in this protocol: Alberta: Tom Baker Cancer Centre, Calgary; British Columbia: British Columbia Cancer Agency (BCCA) –Vancouver Cancer Centre, BCCA–Fraser Valley Cancer Centre, Surrey; Ontario: Odette Cancer Centre–Sunnybrook Health Sciences Centre, Toronto, Northeast Cancer Center Health Sciences North/Horizon Sante-Nord, Sudbury, Thunder Bay Regional Health Science Centre; Quebec: Le Centre Hospitalier Universitaire de Québec–Pavillon Hotel-Dieu de Quebec, Quebec City, Centre Hospitalier de l'Université de Montréal–Hopital Notre-Dame, Montreal; Saskatchewan: Allan Blair Cancer Centre, Regina.
Table A1.
Radiotherapy
| Irradiation Span (days) | CIS Plus RT (n = 194) |
CIS, TPZ, and RT (n = 185) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| ≤ 52 | 142 | 73.2 | 141 | 76.2 |
| 53-60 | 31 | 16.0 | 21 | 11.4 |
| > 60 | 17 | 8.8 | 18 | 9.7 |
| Not treated | 4 | 2.1 | 5 | 2.7 |
Abbreviations: CIS, cisplatin; RT, radiotherapy; TPZ, tirapazamine.
Table A2.
Detailed Toxicities in Initial Dose Cohort
| Adverse Event | Grade |
P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CIS Plus RT (n = 92) |
CIS, TPZ, and RT (n = 88) |
||||||||||
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | ||
| Leukopenia | 20 | 15 | 28 | 26 | 3 | 25 | 10 | 18 | 29 | 6 | |
| Anemia | 14 | 31 | 39 | 8 | 0 | 25 | 23 | 37 | 3 | 0 | |
| Thrombocytopenia | 49 | 37 | 5 | 1 | 0 | 51 | 28 | 5 | 3 | 1 | |
| ANC | 48 | 13 | 14 | 12 | 5 | 47 | 8 | 16 | 14 | 3 | |
| Other hematologic | 78 | 2 | 1 | 3 | 8 | 79 | 0 | 0 | 4 | 5 | |
| Allergy/immunology* | 92 | 0 | 0 | 0 | 0 | 82 | 3 | 2 | 1 | 0 | .023 |
| Auditory/ear* | 79 | 2 | 11 | 0 | 0 | 68 | 0 | 19 | 0 | 1 | .045 |
| Cardiac | 88 | 2 | 1 | 1 | 0 | 82 | 3 | 3 | 0 | 0 | |
| Fatigue | 25 | 32 | 30 | 5 | 0 | 22 | 32 | 25 | 8 | 1 | |
| Fever, without neutropenia | 87 | 4 | 1 | 0 | 0 | 82 | 3 | 3 | 0 | 0 | |
| Other constitutional symptoms | 70 | 16 | 6 | 0 | 0 | 59 | 18 | 9 | 2 | 0 | |
| Dermatologic* | 74 | 16 | 2 | 0 | 0 | 52 | 15 | 15 | 6 | 0 | < .001 |
| Endocrine | 85 | 4 | 3 | 0 | 0 | 87 | 1 | 0 | 0 | 0 | |
| Nausea* | 36 | 32 | 18 | 6 | 0 | 14 | 33 | 33 | 8 | 0 | < .001 |
| Vomiting* | 55 | 21 | 11 | 5 | 0 | 26 | 22 | 31 | 9 | 0 | < .001 |
| Diarrhea* | 46 | 34 | 7 | 5 | 0 | 29 | 26 | 23 | 10 | 0 | < .001 |
| Constipation | 74 | 12 | 6 | 0 | 0 | 71 | 10 | 5 | 1 | 1 | |
| Other GI | 54 | 22 | 11 | 5 | 0 | 45 | 19 | 18 | 5 | 1 | |
| Renal/genitourinary | 65 | 15 | 10 | 2 | 0 | 69 | 13 | 5 | 1 | 0 | |
| Hemorrhage/bleeding | 84 | 7 | 0 | 1 | 0 | 84 | 2 | 2 | 0 | 0 | |
| Hepatobiliary/pancreas | 92 | 0 | 0 | 0 | 0 | 87 | 0 | 0 | 1 | 0 | |
| Infection | 81 | 0 | 3 | 7 | 1 | 73 | 0 | 9 | 5 | 1 | |
| Lymphatics | 89 | 3 | 0 | 0 | 0 | 83 | 2 | 2 | 1 | 0 | |
| Metabolic/laboratory | 39 | 22 | 19 | 9 | 3 | 33 | 20 | 12 | 22 | 1 | |
| Musculoskeletal/soft tissue | 86 | 3 | 3 | 0 | 0 | 76 | 4 | 6 | 2 | 0 | |
| Neuropathy, motor * | 92 | 0 | 0 | 0 | 0 | 80 | 5 | 3 | 0 | 0 | .005 |
| Neuropathy, sensory | 80 | 10 | 2 | 0 | 0 | 75 | 9 | 3 | 1 | 0 | |
| Other neurologic | 76 | 10 | 5 | 1 | 0 | 73 | 8 | 3 | 4 | 0 | |
| Ocular/visual | 88 | 4 | 0 | 0 | 0 | 80 | 7 | 1 | 0 | 0 | |
| Pain* | 65 | 13 | 13 | 1 | 0 | 42 | 16 | 16 | 14 | 0 | < .001 |
| Pulmonary | 86 | 5 | 1 | 0 | 0 | 79 | 5 | 2 | 2 | 0 | |
| Sexual/reproductive | 87 | 5 | 0 | 0 | 0 | 86 | 5 | 0 | 0 | 0 | |
| Vascular | 91 | 1 | 0 | 0 | 0 | 86 | 0 | 1 | 1 | 0 | |
Abbreviations: ANC, absolute neutrophil count; CIS, cisplatin; RT, radiotherapy; TPZ, tirapazamine.
Distribution of toxicity grade significantly different across treatment arms (Fisher's exact test).
Footnotes
Supported by National Cancer Institute Grants No. CA 27469 (Gynecologic Oncology Group), No. CA 37517 (Gynecologic Oncology Group Statistical and Data Center), and No. CA 077202 (National Cancer Institute of Canada Clinical Trials Group [NCIC CTG]) and by the Canadian Cancer Society Research Institute Grant No. 21039 (NCIC CTG).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00262821.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Paul A. DiSilvestro, Shamshad Ali, Peter S. Craighead, Joseph A. Lucci, Walter H. Gajewski, Bradley J. Monk
Administrative support: Bradley J. Monk
Provision of study materials or patients: Paul A. DiSilvestro, Joseph A. Lucci, Carolyn Muller, Bradley J. Monk
Collection and assembly of data: Shamshad Ali, Joseph A. Lucci, Yi-Chun Lee, David E. Cohn, Nicola M. Spirtos, Krishnansu S. Tewari, Carolyn Muller, Margaret M. Steinhoff, Bradley J. Monk
Data analysis and interpretation: Shamshad Ali, Joseph A. Lucci, Krishnansu S. Tewari, Bradley J. Monk
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.DiSaia PJ, Creasman WT. Clinical Gynecologic Oncology. eds 1-7. St Louis, MO: Mosby; 1993. p. 76. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Whitney CW, Sause WT, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIb-IVa carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 4.Rose PG, Bundy B, Watkins EB, et al. Concurrent CIS-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 5.Keys HM, Bundy BN, Stehman FB, et al. CIS, radiation and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 6.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 7.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 8.Lanciano R, Calkins A, Bundy B, et al. Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2005;23:8289–8295. doi: 10.1200/JCO.2004.00.0497. [DOI] [PubMed] [Google Scholar]
- 9.Thomas G, Ali S, Hoebers FJ, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dl with erythropoietin versus above 10.0 g/dl without erythropoietin in anemic patients receiving radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108:317–325. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown JM, Lemmon MJ. Potentiation by the hypoxic cytotoxin SR 4233 of cell killing produced by fractionated irradiation of mouse tumors. Cancer Res. 1990;50:7745–7749. [PubMed] [Google Scholar]
- 11.Brown JM, Wang LH. Tirapazamine: Laboratory data relevant to clinical activity. Anticancer Drug Des. 1998;13:529–539. [PubMed] [Google Scholar]
- 12.Koch CJ. Unusual oxygen concentration dependence of toxicity of SR-4233, a hypoxic cell toxin. Cancer Res. 1993;53:3992–3997. [PubMed] [Google Scholar]
- 13.Brown JM. Exploiting the hypoxic cancer cell: Mechanisms and therapeutic strategies. Mol Med Today. 2000;6:157–162. doi: 10.1016/s1357-4310(00)01677-4. [DOI] [PubMed] [Google Scholar]
- 14.Moulder JE, Rockwell S. Hypoxic fractions of solid tumors: Experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984;10:695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- 15.Dorie MJ, Brown JM. Tumor-specific, schedule-dependent interaction between tirapazamine (SR 4233) and CIS. Cancer Res. 1993;53:4633–4636. [PubMed] [Google Scholar]
- 16.Gatzemeier U, Rodriguez G, Treat J, et al. Tirapazamine-cisplatin: The synergy. Br J Cancer. 1998;77(suppl 4):15–17. doi: 10.1038/bjc.1998.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fyles A, Milosevic M, Hedley D, et al. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol. 2002;20:680–687. doi: 10.1200/JCO.2002.20.3.680. [DOI] [PubMed] [Google Scholar]
- 18.Treat J, Johnson E, Langer C, et al. Tirapazamine with cisplatin in patients with advanced non–small-cell lung cancer: A phase II study. J Clin Oncol. 1998;16:3524–3527. doi: 10.1200/JCO.1998.16.11.3524. [DOI] [PubMed] [Google Scholar]
- 19.von Pawel J, von Roemeling R, Gatzemeier U, et al. Tirapazamine plus cisplatin versus cisplatin in advanced non–small-cell lung cancer: A report of the international CATAPULT I study group—Cisplatin and tirapazamine in subjects with advanced previously untreated non–small-cell lung tumors. J Clin Oncol. 2000;18:1351–1359. doi: 10.1200/JCO.2000.18.6.1351. [DOI] [PubMed] [Google Scholar]
- 20.Rischin D, Peters L, Hicks R, et al. Phase I trial of concurrent tirapazamine, cisplatin, and radiotherapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:535–542. doi: 10.1200/JCO.2001.19.2.535. [DOI] [PubMed] [Google Scholar]
- 21.Shulman LN, Buswell L, Riese N, et al. Phase I trial of the hypoxic cell cytotoxin tirapazamine with concurrent radiation therapy in the treatment of refractory solid tumors. Int J Radiat Oncol Biol Phys. 1999;44:349–353. doi: 10.1016/s0360-3016(99)00016-4. [DOI] [PubMed] [Google Scholar]
- 22.Maluf FC, Leiser AL, Aghajanian C, et al. Phase II study of tirapazamine plus cisplatin in patients with advanced or recurrent cervical cancer. Int J Gynecol Cancer. 2006;16:1165–1171. doi: 10.1111/j.1525-1438.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith HO, Jiang CS, Weiss GR, et al. Tirapazamine plus CIS in advanced or recurrent carcinoma of the uterine cervix: A Southwest Oncology Group study. Int J Gynecol Cancer. 2006;16:298–305. doi: 10.1111/j.1525-1438.2006.00339.x. [DOI] [PubMed] [Google Scholar]
- 24.Craighead PS, Pearcey R, Stuart G. A phase I/II evaluation of tirapazamine administered intravenously concurrent with cisplatin and radiotherapy in women with locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2000;48:791–795. doi: 10.1016/s0360-3016(00)00720-3. [DOI] [PubMed] [Google Scholar]
- 25.Schoenfeld D. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39:499–503. [PubMed] [Google Scholar]
- 26.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 27.Wieand S, Schroeder G, O'Fallon JR. Stopping when the experimental regimen does not appear to help. Stat Med. 1994;13:1453–1458. doi: 10.1002/sim.4780131321. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 29.Cantor AB. Sample size calculations for the log-rank test: A Gompertz model approach. J Clin Epidemiol. 1992;45:1131–1136. doi: 10.1016/0895-4356(92)90153-e. [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. The logrank test. BMJ. 2004;328:1073. doi: 10.1136/bmj.328.7447.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox DR. Regression model and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 32.Agresti A. A survey of exact inference for contingency tables. Stat Sci. 1992;7:131–177. [Google Scholar]
- 33.Le Q, Taira A, Budenz S, et al. Mature results from a randomized phase II trial of cisplatin plus 5-fluorouracil and radiotherapy with or without tirapazamine in patients with resectable stage IV head and neck squamous cell carcinomas. Cancer. 2006;106:1940–1949. doi: 10.1002/cncr.21785. [DOI] [PubMed] [Google Scholar]
- 34.Rischin D, Peters LJ, O'Sullivan B, et al. Tirapazamine, cisplatin and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): A phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010;28:2989–2995. doi: 10.1200/JCO.2009.27.4449. [DOI] [PubMed] [Google Scholar]
- 35.Dueñas-González A, Zarbá JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29:1678–1685. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]