Abstract
OBJECTIVE:
The present study aimed to investigate the mechanisms underlying the anti-inflammatory and anti-angiogenic effects of ethyl-p-methoxycinnamate isolated from Kaempferia galanga.
METHODS:
The anti-inflammatory effects of ethyl-p-methoxycinnamate were assessed using the cotton pellet granuloma assay in rats, whereby the levels of interleukin-1 and tumor necrosis factor-α were measured in the animals' blood. In addition, the levels of interleukin, tumor necrosis factor, and nitric oxide were measured in vitro using the human macrophage cell line (U937). The analgesic effects of ethyl-p-methoxycinnamate were assessed by the tail flick assay in rats. The anti-angiogenic effects were evaluated first by the rat aortic ring assay and, subsequently, by assessing the inhibitory effects of ethyl-p-methoxycinnamate on vascular endothelial growth factor, proliferation, migration, and tube formation in human umbilical vein endothelial cells.
RESULTS:
Ethyl-p-methoxycinnamate strongly inhibited granuloma tissue formation in rats. It prolonged the tail flick time in rats by more than two-fold compared with the control animals. The inhibition of interleukin and tumor necrosis factor by ethyl-p-methoxycinnamate was significant in both in vivo and in vitro models; however, only a moderate inhibition of nitric oxide was observed in macrophages. Furthermore, ethyl-p-methoxycinnamate considerably inhibited microvessel sprouting from the rat aorta. These mechanistic studies showed that ethyl-p-methoxycinnamate strongly inhibited the differentiation and migration of endothelial cells, which was further confirmed by the reduced level of vascular endothelial growth factor.
CONCLUSION:
Ethyl-p-methoxycinnamate exhibits significant anti-inflammatory potential by inhibiting pro-inflammatory cytokines and angiogenesis, thus inhibiting the main functions of endothelial cells. Thus, ethyl-p-methoxycinnamate could be a promising therapeutic agent for the treatment of inflammatory and angiogenesis-related diseases.
Keywords: Rat Aortic Ring Assay, Cotton Pellet Granuloma, Tail Flick Assay, Tube Formation Assay, Cell Migration Assay, Cytokine Inhibition Assay
INTRODUCTION
Inflammatory reactions involve the activation of a number of different intra-cellular pathways that lead to the induction of specific pro-inflammatory genes, including those encoding interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α). For instance, the attachment of a bacterial antigen to the toll-like receptors on the surface of macrophages and dendritic cells results in the activation of the mitogen-activated protein kinase pathway (MAPK/NF-kB pathway), which leads to the induction of pro-inflammatory genes encoding IL-1, TNF-α, cyclooxygenase-2 (COX-2), and inflammatory nitric oxide (iNO) 1. The release of IL-1 and TNF-α from the activated macrophages results in the further activation of immune cells that are in close proximity. This, in turn, provokes a positive feedback loop that promotes the synthesis of cytokines by the activated immune cells, thus aggravating the inflammatory reaction. iNO release leads to vasodilation in the vessels of the affected areas, thus facilitating the increased blood flow to the affected areas. In contrast, TNF-α induces the expression of attachment molecules, such as E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule (VCAM-1), on the surface of vascular endothelial cells 2-4. The circulating macrophages attach to the surface of the endothelial cells with the help of selectins, ICAM-1, and VCAM-1. The attached macrophages invade through the vascular walls and reach the affected site to engulf the invading bacteria. Together, all these factors cause inflammation in the affected area, which is manifested by redness and swelling (edema) due to the increased blood flow and permeability of vessels. Most of the phytoconstituents that are known to exert anti-inflammatory effects have been studied extensively for their potential to inhibit these pro-inflammatory cytokines 5,6. Accordingly, such investigations are mandatory for the mechanistic evaluation of newly reported anti-inflammatory constituents.
Kaempferia galanga is a valuable medicinal herb that is known for a number of its medicinal properties worldwide, particularly in Malaysia and Thailand 7,8. The herb has potential anti-inflammatory, analgesic, mosquito-repellent, larvicidal, nematicidal, vasorelaxant, and pro-apoptotic activities. The herb has been used for decades in traditional medicine in the management and treatment of swelling and pain, such as headaches, toothaches, stomachaches, and rheumatism 9. The crude extract of K. galanga has already been reported to possess anti-inflammatory and analgesic properties 9,10. However, the constituents responsible for these properties and the mechanisms underlying these properties have not been evaluated. In a recent study, we reported that the anti-inflammatory effect of K. galanga is mainly due to its active constituent, ethyl-p-methoxycinnamate (EPMC) 8. This was the first report on the anti-inflammatory effect of EPMC isolated from K. galanga. EPMC has been reported to have anti-tuberculosis 11, nematicidal 12,13, mosquito-repellent 14,15, larvicidal, antineoplastic, and anti-microbial potentials 7. Moreover, in an in vitro colorimetric assay, EPMC has been shown to inhibit the enzymatic activity of COX-1 and COX-2 in a cell-free system 8. In a recent study, the inhibitory effect of EPMC and its thiourea derivatives in a mouse fibrosarcoma model was reported 16. However, few scientific data validating the anti-inflammatory effects of EPMC in a chronic in vivo model are available, and its inhibitory action on pro-inflammatory cytokines has not yet been reported. Thus, the aim of the present study was to investigate the effect of EPMC in a sub-chronic in vivo model, particularly its inhibitory effect on potential cytokines. In a previous report, the inhibitory effect of EPMC on COX-1 and COX-2 encouraged the authors to focus on assessing its possible analgesic effect using an in vivo model. The inhibitors of cytokines, particularly TNF-α, have been shown to possess strong anti-angiogenic potential 12-14. Additionally, the inhibition of TNF-α synthesis has been shown to prevent the activation of the NF-kB pathway, which is necessary for the synthesis of angiogenic proteins 17-19. Therefore, the aim of this study was to further extend this knowledge and evaluate the possible anti-angiogenic effects of EPMC, with a primary focus on investigating its probable mechanism of action.
MATERIALS AND METHODS
Chemicals and equipment
The 1H-NMR Bruker 500-MHz Ultrashield (Billerica, Massachusetts, USA), TECAN Multi-mode microplate reader Model Infinite 200 (Mannedorf, Switzerland), and Buchi Rotavapor Model R-210/215 (Flawil, Switzerland) were used. The tail flick analgesia meter was purchased from IITC Life Sciences, CA, USA. Methylthiazolyldiphenyl-tetrazolium bromide reagent (MTT), Salmonella abortus lipopolysachharide (LPS), dimethyl sulfoxide (DMSO), phosphate-buffered saline (PBS), suramine, and penicillin/streptomycin (PS) solution were purchased from Sigma-Aldrich, Germany. Human IL-1, human TNF-α, human nitric oxide (NO), human vascular endothelial growth factor (VEGF), rat IL-1, and rat TNF-α ELISA kits were purchased from Cusabio, China. RPMI 1640, human umbilical vein endothelial cells (HUVEC), and endothelial cell medium (ECM) supplied with endothelial cell growth supplements (ECGS) were obtained from ScienCell, USA. Trypsin and heat-inactivated fetal bovine serum were purchased from GIBCO, UK. Matrigel matrix (10 mg/ml) was obtained from SABiosciences, USA. All other chemicals used in this study were analytical-grade or better.
Isolation of ethyl-p-methoxycinnamate (EPMC) from Kaempferia galanga
In our recent study, EPMC was isolated from the active sub-fraction of a chloroform extract of K. galanga 8, and the yield was low (0.026%). However, in the current study, the yield was increased to 1.04% using petroleum ether. The dried and powdered rhizomes (3 kg) were extracted with petroleum ether (4 l) in a Soxhlet apparatus at 40°C until the solvent in the Soxhlet column was nearly transparent. The extract was filtered, concentrated in a vacuum evaporator (Buchi Rotavapor Model R-210, Flawil, Switzerland) at 25°C, and freeze-dried. The dried extract (124 g) was carefully washed with n-hexane without stirring. The n-hexane supernatant was then decanted, and fresh n-hexane was added until the solvent was almost transparent. The residue that remained after washings consisted of pale yellow, needle-like crystals, which were collected in n-hexane in a conical flask, and a 1∶3 hexane-chloroform mixture was added drop-wise until the crystals completely dissolved. The solvent was allowed to evaporate slowly at room temperature, yielding pure crystals of EPMC (31.2 g). The compound had a sharp melting point of 49°C, as observed using a Toshniwal melting point apparatus. The molecular structure was elucidated using the nuclear magnetic resonance (NMR) spectroscopic technique [1H NMR (500 MHz, CDCl3) δ: 1.32 (3H, t, 1×CH3, J = 7.5 Hz), 3.82 (3H, s, 1×OCH3), 4.25 (2H, q, 1×CH2), 6.31 (1H, d, J = 16.0 Hz, 1×CH alkene), 6.90 (2H, d, J = 7.0 Hz, 2×CH benzylic), 7.42 (2H, d, J = 7.0 Hz, 2×CH benzylic), 7.65 (1H, d, J = 16.0 Hz, 1×CH alkene); 13C {1H} NMR (125.1 MHz, CDCl3) δ: 14.5 (CH3), 55.3 (OCH3), 60.3 (CH2), 114.3 (benzylic CH2), 115.7 (CH alkene), 127.3 (Ar-C), 129.7(Ar-C), 144.2 (CH alkene), 161.3 (Ar-C), 167.3 (carbonyl C)].
Evaluation of pharmacological activities
Animals
Male Sprague Dawley (SD) rats (200 to 250 g) were obtained from the Animal Research and Service Centre (ARSC), Universiti Sains Malaysia. The animals were kept in the animal transit room, School of Pharmaceutical Sciences, Universiti Sains Malaysia, at 23°C and 40-60% relative humidity in a 12-hour dark/light cycle. The experimental procedures were also performed in the same room. The animals were provided free access to water and food. However, food was withdrawn 12 hours before any experimental procedure was performed on the animals. The experimental procedure and the use of animals were approved by the Animal Ethics Committee of Universiti Sains Malaysia before the commencement of the experiments [Reference number: PPSG/07 (A)/044/(2011) (63)/; Approval number: USM/Animal Ethics Approval/2010/(63) (270)].
In vivo cotton pellet granuloma assay
The anti-inflammatory effect of EPMC was evaluated using the cotton pellet granuloma assay in rats, as described by Anosike and co-workers 20, with minor modifications. Briefly, rats were anaesthetized with an intra-peritoneal administration of pentobarbitone sodium (60 mg/kg). Two pouches were created using scissors, one on either side of the ventral abdominal area of each rat under the loosened skin, and a pre-autoclaved cotton pellet with a weight of 30 mg was implanted in each pouch. Thereafter, the pouches were stitched closed using surgical silk. Twenty-four hours after the implantation of the cotton pellets, the rats were given EPMC in three doses, specifically, 200, 400, and 800 mg/kg, once daily for 7 days through oral gavage. Reference group rats were given indomethacin (5 mg/kg) and dexamethasone (7 mg/kg), and negative control rats were given 1% tween 80. On the 8th day, the animals were anaesthetized by pentobarbitone sodium administration (60 mg/kg), and 3 ml of blood was withdrawn from each rat by cardiac puncture. The rats were euthanized by 100% CO2 using a CO2 chamber, and the cotton pellets were dissected from each rat. The pellets were dried in an oven at 40°C until they were of constant weight. The weight of each cotton pellet was recorded, and the percent inhibition of granuloma tissue formation was calculated using the following formula:
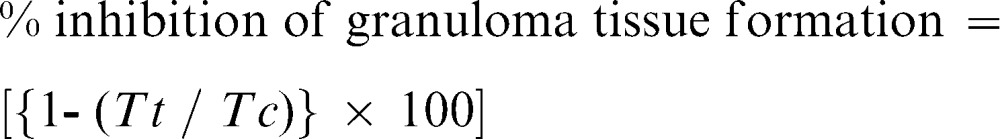 |
where Tc and Tt are the weights of the cotton pellets from the rats in the control group and treated group, respectively.
Enzyme-linked immunosorbant assay to measure TNF-α and IL-1
The blood samples collected from rats on the 8th day of the cotton pellet assay were centrifuged to obtain plasma, which was subjected to enzyme-linked immunosorbant assay (ELISA) for TNF-α and IL-1 assessment using rat TNF-α and rat IL-1 ELISA kits, respectively. The procedure was carried out according to the manufacturer's instructions (CUSABIO). The concentration of the respective cytokine in each sample was calculated using a standard curve equation. The percent inhibition of cytokine production was calculated using the following formula:
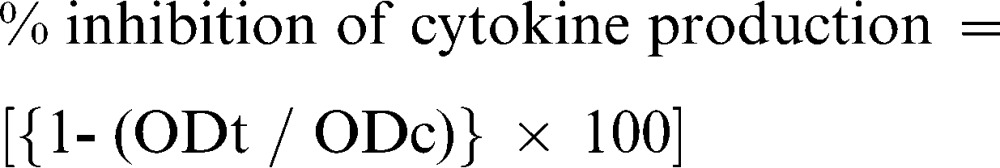 |
where ODt and ODc are the optical densities of the treatment and control group samples, respectively.
In vitro cytokine inhibition assay in human macrophage (U937) cells
Macrophage differentiation was induced in U937 human macrophage cells by adding 5 ng/ml PMA for 3 days, as described by Federica and co-workers 21. The cells were then starved overnight and activated to synthesize cytokines and NO by the addition of 5 ng/ml of Salmonella abortus LPS. Five concentrations of EPMC, namely, 200, 100, 50, 25, and 12.5 µg/ml, were added in the presence of PMA (5 ng/ml). The cells were incubated at 37°C for 48 hours in a CO2 incubator. The plate was then centrifuged and the cell culture supernatants were subjected to ELISA for TNF-α, IL-1, and NO assessment using human cytokine and NO ELISA kits according to the manufacturer's instructions. The percent inhibition of cytokine production was calculated using the following formula:
 |
where ODt and ODc are the optical densities of the wells of cells treated and not treated with EPMC, respectively.
The median inhibitory concentration (IC50) of EPMC in inhibiting cytokine synthesis was determined by nonlinear regression analysis of log-concentration-response curves.
Evaluation of analgesic activity of ethyl-p-methoxycinnamate
The analgesic effect of graded doses of EPMC was evaluated using the rat tail flick assay, as described by Dableh and co-workers 22, with minor modifications. A radiant heat source was used in this assessment. Light from the projector bulb of the analgesiometer was focused on the skin of the rat, at a distance of approximately 5 cm from the distal end of the tail. The area of the skin was blackened to facilitate maximum light absorption. The time taken by the rats to withdraw their tail was measured by a timer that was set to switch off automatically as soon as the rat moved its tail away from the light beam. The intensity of light was set to achieve a baseline time of 10-12 seconds. Each reading was taken 3 times, with a standard deviation of less than 10%. The animals were divided into five groups (n = 6). Groups 1, 2, and 3 were orally administered EPMC at a dose of 200, 400, and 800 mg/kg, respectively, whereas group 4 was given codeine phosphate (30 mg/kg). Group 5 rats were given 1% tween 80. The tail flick time for each rat was measured 30, 60, and 90 minutes after the administration of EPMC. An increase in the time taken by the rats to flick their tail away from the light beam was taken as a measure of the analgesic activity of EPMC. The percent inhibition of pain was calculated by the following formula:
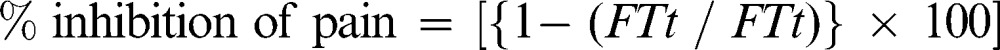 |
where FTt is the tail flick time recorded in treatment group rats and FTc is the tail flick time recorded in control group rats.
Assessment of the anti-angiogenic effect
Ex vivo rat aortic ring assay
The anti-angiogenic effect of EPMC was evaluated in vitro in rat aortic rings, as previously described 23. Briefly, two layers of cell culture medium, layer 1 (L1) and layer 2 (L2), were prepared. L1 contained 25 ml of M199 basal medium supplemented with fibrinogen (3 mg/ml), aprotinin (5 µg/ml), and L-glutamine (1% w/v). L2 contained 25 ml of M199 medium supplemented with FBS (20% v/v), L-glutamine (2 mM), aminocaproic acid (1 mg/ml), amphotericin B (2.5 µg/ml), and gentamicin (60 µg/ml). EPMC was added to L2 in 4 concentrations, specifically, 200, 100, 50, and 25 µg/ml. The thoracic aorta was dissected from each rat and placed in M199 medium in a petri dish. The tissue was cleaned of adipose tissue and cut into thin rings of approximately 1-mm thickness. Then, 500 µl of L1 was added to each well of a 48-well tissue culture plate, and 1 ring was placed in each well of the plate. Next, 10 µl of thrombin (50 NIH U/l) was added to each well, and after 90 minutes of incubation at 37°C and 5% CO2, 500 µl of L2 was overlaid on L1 in each well. After 4 days of incubation at 37°C and 5% CO2, the top layer, L2, was replaced with fresh L2 containing EPMC. On day 5, images of aortic rings were taken using an AMG EVOS f1 inverted microscope (40× magnification), and the growth of the sprouting blood vessels was quantified using Leica QWin software (Grand Island, NY, USA), as previously reported 24. Briefly, the distance of growth between at least 20 growth points was measured in each ring. The results were presented as the mean percent inhibition of five independent experiments. Dimethyl sulfoxide (DMSO) was used as a negative control, and suramin 100 µg/ml was used as a reference drug. The percent inhibition of blood vessels was calculated using the following formula:
where Ao is the length of blood vessel in treated rings in µm and A is the length of vessels in the control group. The IC50 of EPMC in inhibiting vascular growth was calculated from a linear regression equation obtained from a dose-response curve. Based on the results of this assay, the anti-angiogenic property of EPMC was subjected to further study.
Anti-proliferation assay in HUVECs
Human umbilical vein endothelial cells (HUVECs) were used as a model cell line for angiogenesis. The cells were harvested by trypsinization and re-suspended in 5 ml of fresh medium. The cell count was adjusted to 1.5×105/ml, and 100 µl was seeded in each well. The cells were allowed to attach overnight. Then, an additional 100 µl of fresh medium containing EPMC in 6 different concentrations, specifically, 200, 100, 50, 25, 12.5, and 6.25 µg/ml, was added, and the cells were further incubated at 37°C and 5% CO2 for 48 hours. The viability of the cells was then assessed by the MTT assay, as described previously 23. The percent inhibition was calculated using the following formula:
 |
Tube formation assay
The ability of EPMC to inhibit the formation of tube-like structures by HUVECs was evaluated in vitro in a Matrigel matrix, as described previously 23. Briefly, HUVECs were treated for 24-48 hours with three concentrations of EPMC, specifically, 200, 100, and 50 µg/ml. On the day of the experiment, Matrigel stock solution (10 mg/ml) was diluted at 1∶2 v/v in ice-cold, serum-free ECM. Then, 150 µl of the diluted Matrigel solution was added to each well of a 48-well culture plate. The gel was allowed to solidify for 45 minutes at 37°C in 5% CO2. After 6 hours, the tube-like structures were examined under light microscopy at 4× magnification. Images of each well were taken using an AMG EVOS fI inverted microscope at 4× magnification, and the length and width of the tube-like structures were quantified using the ScnImage software package. The results are presented as the mean percent inhibition of five replicates.
Determination of VEGF levels in HUVECs
HUVECs were seeded in endothelial cell medium at a concentration of 1×106 in a 6-well culture plate. After they had been allowed to attach overnight, the HUVECs were treated for 6 hours with EPMC at 3 different concentrations, specifically, 200, 100, and 50 µg/ml. The concentration of human VEGF 165 in the HUVEC lysate was estimated using a human VEGF ELISA kit (CUSABIO, China), according to the manufacturer's instructions.
Cell migration assay
The “scratch” wound assay was used to assess the effect of EPMC on HUVEC cell migration and was performed as described by Doleckova and co-workers 25. HUVEC cells were seeded in 6-cm dishes, and the cell monolayer was scraped with a disposable rubber policeman to create a cell-free zone. The cells were incubated overnight in M199 medium supplied with 3 concentrations of EPMC, specifically, 200, 100, and 50 µg/ml. Images of 6-8 microscopic fields were taken at the 0, 6th, and 12th hour of incubation using an AMG EVOS fI inverted microscope (4× magnification). The area of the cell-free zone was measured using Leica QWin software, and the percent of wound closure was calculated using the following formula:
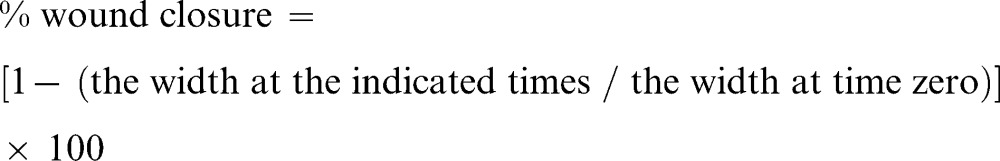 |
The results are presented as the percent of wound closure (n = 6).
Statistical analysis
Significant differences between the treatment and control groups were evaluated by one-way analysis of variance (ANOVA), followed by Tukey's multiple comparison test. Differences were considered significant at p<0.05, p<0.01, and p<0.001.
RESULTS
Ethyl-p-methoxycinnamate inhibits granuloma tissue formation
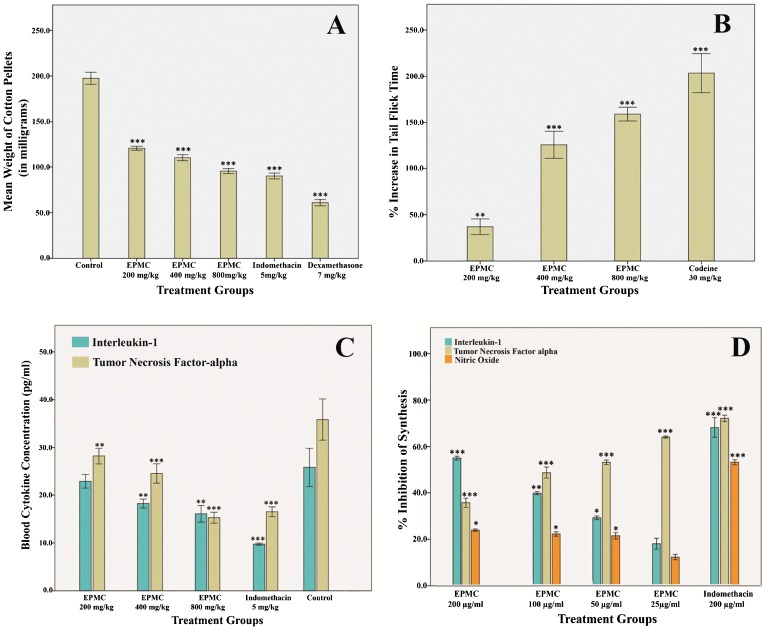
Figure 1A shows the mean dry weight of the cotton pellets dissected from the rats treated with graded doses of EPMC. At the doses of 200, 400, and 800 mg/kg, EPMC inhibited granuloma tissue formation by 38.98, 44.21, and 51.65% (p<0.001), respectively. The results were comparable with those derived from standard reference drugs. The percent inhibition of granuloma tissue formation by indomethacin (5 mg/kg) and dexamethasone (7 mg/kg) was found to be 54.35 and 69.12% (p<0.001), respectively.
Figure 1.
A) The mean weight of cotton pellets dissected from rats treated with ethyl-p-methoxycinnamate (EPMC) at doses of 200, 400, and 800 mg/kg. B) Percent increase in the time taken by rats to flick their tail away from heat, a measure of the analgesic effect of EPMC, at doses of 200, 400, and 800 mg/kg. C) Blood concentration of interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) in rats treated with EPMC at doses of 200, 400, and 800 mg/kg on the 8th day of the cotton pellet granuloma assay. D) Percent inhibition of IL-1, TNF-α, and nitric oxide (NO) synthesis by EPMC in U937 cells at concentrations of 25, 50, 100, and 200 µg/ml. The cell culture supernatants were collected after 48 hours and subjected to an enzyme-linked immunosorbant assay (ELISA) for the assessment of IL-1, TNF-α, and NO. All values are represented as the mean ± SEM (n = 6), and *, **, and *** indicate significant differences of p<0.05, p<0.01, and p<0.001, respectively, compared with the control-treated group.
Anti-analgesic effect of ethyl-p-methoxycinnamate
Figure 1B shows the percent delay in the tail flick time recorded in treatment group rats with respect to the control group rats. EPMC delayed the tail flick time up to 37, 125.6, and 159.1% at the doses of 200, 400, and 800 mg/kg, respectively (p<0.001). In comparison, the standard reference drug prolonged the tail flick response up to 204.6%.
In vivo inhibitory effect of EPMC on cytokine production
Figure 1C shows the mean concentrations of IL-1 and TNF-α in the blood samples of the rats used in the cotton pellet assay. The concentration of IL-1 in the blood was lowered by EPMC administration in a dose-dependent manner. The highest concentration of IL-1, 25.81 pg/ml, was found in the blood of negative-control rats, and the concentration of IL-1 in the blood samples of rats treated with 200, 400, and 800 mg/kg of EPMC was estimated to be 22.88, 20.42, and 16.09 pg/ml, respectively. The lowest concentration, 9.74 pg/ml, was recorded in the blood samples of indomethacin-treated rats. The median effective dose (ED50) of EPMC for the inhibition of IL-1 in vivo was estimated to be 1.079 g/kg. EPMC inhibited the synthesis of IL-1 by 11.35, 20.9, and 37.67% at the doses of 200, 400, and 800 mg/kg, respectively. However, the synthesis of IL-1 was inhibited by 62.25% by indomethacin.
The concentration of TNF-α in the blood samples of the control group rats was 35.85 pg/ml. Its concentration in rats treated with 200, 400, and 800 mg/kg of EPMC was estimated to be 27.09, 22.25, and 15.27 pg/ml, respectively. At 800 mg/kg, EPMC exhibited a more pronounced activity than that of the standard reference drug, indomethacin (16.51 pg/ml). EPMC inhibited the synthesis of TNF-α by 24.43, 37.95, and 57.40% at the doses of 200, 400, and 800 mg/kg, respectively.
In vitro inhibitory effect of EPMC on cytokine production in human macrophages
EPMC reduced the concentration of IL-1 in U937 cells in a dose-dependent manner. Figure 1D shows the percent inhibition of IL-1, TNF-α, and NO in macrophages by EPMC. The percent inhibition of IL-1 by 25, 50, 100, and 200 µg/ml of EPMC was estimated to be 18.09, 29.23, 39.84, and 55.04%, respectively, and the IC50 was 166.4 µg/ml. A strong inhibition of TNF-α (64.11%) was observed at the lowest concentration of EPMC (25 µg/ml) in vitro, and increasing doses further decreased the concentration of TNF-α in a dose-dependent manner. The percent inhibition of TNF-α at concentrations of 50, 100, and 200 µg/ml of EPMC was 53.25, 48.76, and 35.71%, respectively, and the IC50 was 96.84 µg/ml. The inhibitory effect of graded doses of EPMC on nitric oxide production was not very efficient. At the low dose of 25 µg/ml, the percent inhibition of NO synthesis was only 12.4%. The percent inhibition was significantly reduced further (p<0.05) to 21.4, 22.2, and 23.8% at 50, 100, and 200 µg/ml EPMC, respectively.
Anti-angiogenic effects of ethyl-p-methoxycinnamate
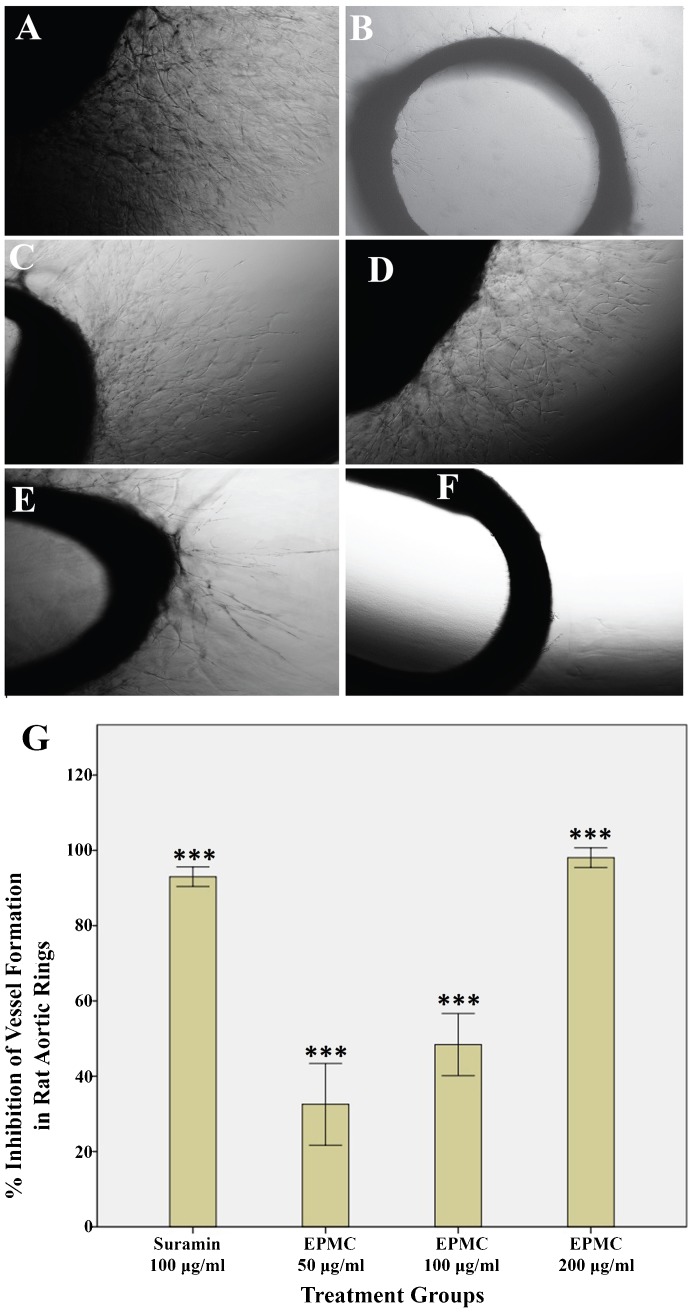
EPMC inhibited the sprouting of microvessels in rat aortic explants. Compared with the control treatment, EPMC significantly inhibited the growth of blood vessels in the aortic rings of rats. Figure 2A shows the prominent growth of vasculature in the untreated group (control). Figure 2B depicts the effect of the standard reference drug, suramin, which resulted in a 86.3% inhibition. In contrast, EPMC exhibited a dose-dependent inhibitory effect on microvessel formation. Figures 2C-2F show the inhibition of vascular growth in the aortic rings treated with different concentrations of EPMC. The inhibition of vessel growth by EPMC was estimated to be 18.2, 32.89, 53, and 99.3% at the concentrations of 25, 50, 100, and 200 µg/ml, respectively, and the IC50 91.90 was µg/ml. Figure 2G graphically illustrates the dose-dependent inhibition of the growth of microvessels from rat aortas, and the graph shows that the effect of EPMC was comparable to that of the standard reference drug, suramin.
Figure 2.
Photomicrograph images of vascular growth in rat aortic rings, which were taken on the 5th day of the experiment using an inverted phase-contrast microscope at 4× magnification. A) Control rings are shown. B) Rings treated with suramin 100 µg/ml are shown. C, D, E, and F) Rings treated with 25, 50, 100, and 200 µg/ml ethyl-p-methoxycinnamate (EPMC) are shown. G) Graphical representation of the inhibitory effect of EPMC on blood vessel growth in rat aortic rings at concentrations of 50, 100, and 200 µg/ml. The result is compared with the standard reference, suramin. All values are represented as the mean ± SEM (n = 10), and *** indicates significant differences of p<0.001 compared with the control-treated group.
Ethyl-p-methoxycinnamate inhibits the proliferation and migration of HUVECs
To investigate the possible mechanism by which EPMC inhibits the growth of aortic blood vessels, a series of in vitro experiments were performed to confirm the anti-angiogenic activity of EPMC. As angiogenesis begins with the local proliferation of endothelial cells in response to a pro-angiogenic stimulus, we first determined the anti-proliferative effect of EPMC on human endothelial cells. At concentrations of 12.5, 25, and 50 µg/ml, EPMC was not cytotoxic to HUVECs. However, at concentrations of 100 and 200 µg/ml, EPMC significantly inhibited the proliferation of HUVECs by 30 and 39.1%, respectively (p<0.05), compared with the control. The IC50 calculated for EPMC was 160 µg/ml.
The results showed that the standard reference, vincristine, exhibited a strong anti-proliferative effect, with an IC50 of 0.06 µg/ml. Figure 3A graphically summarizes the dose-dependent inhibitory effect of EPMC on the cell viability of HUVECs, as determined by the MTT assay.
Figure 3.
The effects of ethyl-p-methoxycinnamate (EPMC) on proliferation and migration of human umbilical vein endothelial cells (HUVECs). A) Graphical representation of the inhibitory effect of EPMC on the percent of viable HUVEC cells at concentrations of 25, 50, 100, and 200 µg/ml. B) The inhibitory effects of EPMC on cell migration in HUVECs. Images were taken under an inverted phase-contrast microscope at 4× magnification after 0, 6, and 12 hours of incubation. Straight lines show the width of the cell-free zone, whereas the circles represent the sites at which the cells completely filled the gap after migration. C) Graphical representation of the inhibitory effect of EPMC on the migration of endothelial cells. All values are represented as the mean ± SEM (n = 6), and *, **, and *** indicate significant differences of p<0.05, p<0.01, and p<0.001, respectively, compared with the control-treated group.
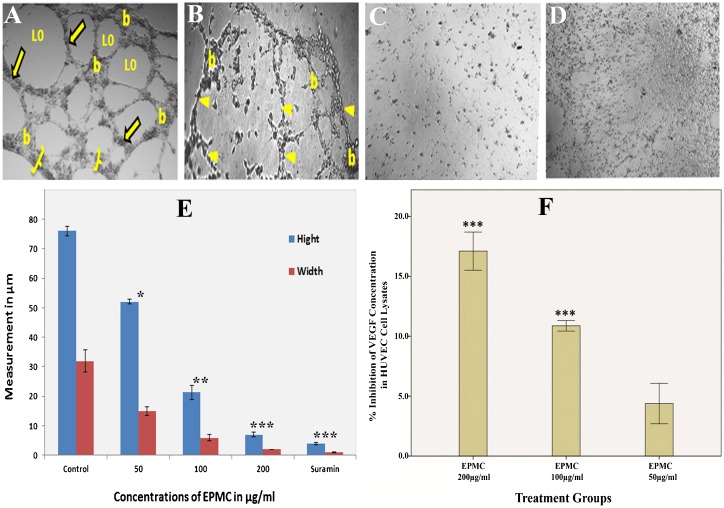
The HUVEC migration assay is representative of the ability of endothelial cells to form new blood vessels. The assay is also a straightforward and economical method by which to evaluate the migration ability of these cells 21. A scratch wound was created on the monolayer of cells (Figure 3B), and the effect of EPMC on the closure of the wound was assessed. Figure 3B shows a photomicrograph of endothelial cells migrating across the scratch wound after 6 and 12 hours of incubation. The analysis (Figure 3C) revealed that the percent closure of wounds in untreated cells was 93.4%. In contrast, the percent closure of wounds in cells treated with EPMC at concentrations of 50, 100, and 200 µg/ml was 72.8, 14.77, and 1.84%, respectively.
Ethyl-p-methoxycinnamate inhibits tube formation in HUVECs
To characterize the anti-angiogenic activity of EPMC, the tube formation assay, a well-established, three-dimensional in vitro angiogenesis assay, was conducted. HUVECs were cultured on Matrigel-formed, tube-like networks (Figure 4A) within 8 hours, reflecting, in part, the process of angiogenesis. The effects of EPMC were more pronounced than those of the standard drug, suramin, which inhibited the formation of tube-like structures by 76.8% at a dose of 100 µg/ml (Figure 4B). EPMC showed a dose-dependent inhibitory effect, with 87.6 and 98.2% inhibition observed at concentrations of 100 and 200 µg/ml, respectively (Figure 4C and 4D). It is noteworthy that 100 µg/ml EPMC completely abrogated the integrity of the endothelial tube network, reducing the tube-like structures both in width and length. This concentration is lower than the IC50 value of EPMC for inhibiting HUVEC proliferation. The figure clearly shows that the endothelial cells lost their pseudopodia-like cellular extensions and that the network structures were disrupted following EPMC treatment. Figure 4E depicts the dose-dependent inhibitory effect of EPMC on the width and length of the endothelial cell tube network.
Figure 4.
Effects of ethyl-p-methoxycinnamate (EPMC) on tube formation and VEGF content in HUVECs. For the tube-formation assay, HUVECs (2×104 cells/well) were plated on Matrigel-precoated 96-well plates and treated with different concentrations of EPMC for 24 hours. Phase-contrast micrographs showing the effects of EPMC and suramin on the differentiation of HUVECs are presented. A) Control group (vehicle treated), showing that the endothelial cells grown on the three-dimensional Matrigel media differentiated into branching structures to form capillary tube-like structures composed of multiple cells with intercellular spaces or lumens. The image clearly shows prominent areas covered with thick cells (brackets), tubes (arrows), loops (Lo), and branching points (b). B) Treatment with EPMC (50 µg/ml) resulted in a slight disruption of bridges (arrow head) and branching points. C) Treatment with EPMC (100 µg/ml) caused notable disorganization in the tube-like structures. Specifically, the improper development of lumens in the cell-cell connections can be seen. The apparent inhibitory effect of EPMC on the differentiation of HUVECs is also noticeable, and the affected tubes and network can be clearly seen. D) Treatment with suramin (100 µg/ml) caused the complete abrogation of the bridges in the tube-like structures, which is evident by the absence of cell-covered areas (brackets in Figure A), tubes, and branching points. E) Graphical depiction of the dose-dependent inhibitory effect of EPMC on the height and width of the capillary-like structures in HUVECs. F) Graphical representation of the percent inhibition of vascular endothelial growth factor (VEGF) level in HUVEC lysates by EPMC. All values are represented as the mean ± SEM (n = 6); ** and *** indicate significant differences of p<0.01 and p<0.001, respectively, compared with the control-treated group.
In vitro inhibitory effect of EPMC on VEGF level in HUVECs
The level of VEGF in treated and untreated HUVECs was determined using a human VEGF 165 ELISA kit. EPMC treatment caused a significant (p<0.01) decrease in VEGF content in cell lysates compared with the control (p<0.01). EPMC resulted in a dose-dependent reduction in VEGF levels (Figure 4F). Specifically, at 100 µg/ml EPMC, the VEGF level was 10.8%, and at 200 µg/ml EPMC, the level of VEGF was further drastically reduced to 17.1%.
DISCUSSION
In a recent study, we reported the anti-inflammatory effect of K. galanga 8. It has been shown that plant extracts, many of which have potent anti-inflammatory constituents, can inhibit inflammation by effectively suppressing the production of granulation tissue in a rat model 26. Although EPMC, the most dominant anti-inflammatory constituent of K. galanga, has been found to inhibit COX-1 and COX-2 in a cell-free system, the effects of EPMC on pro-inflammatory nitric oxide and the cytokines IL-1 and TNF-α have not been elucidated. Thus, this study sought to evaluate the anti-inflammatory mechanisms of EPMC in addition to any other potential pharmacological activities, such as analgesic and anti-angiogenic effects. Therefore, the present study was designed to investigate the potential inhibitory effect of EPMC on IL-1, TNF-α, and NO in both in vitro and in vivo models. Accordingly, the cotton pellet-induced rat granuloma assay was conducted to evaluate the anti-inflammatory potential of EPMC in a sub-chronic model. EPMC inhibited the formation of granuloma tissue in a dose-dependent manner, and these results were in agreement with those from our previous report, thus confirming EPMC as an active principle component of K. galanga. Moreover, the inhibition of IL-1, TNF-α, and NO levels in macrophages suggests that EPMC exerts its anti-inflammatory effects by suppressing the ability of macrophages to produce these proinflammatory mediators.
The significantly low median inhibitory concentration (IC50) of EPMC for IL-1, TNF-α, and NO, which was estimated in human monocytic macrophage cells (U937), corroborates the finding that EPMC is a potent cytokine inhibitor. The pattern of IL-1 inhibition obtained in the in vivo model was similar. However, the inhibition of TNF-α in macrophages (U-937 cell line) was found to be decreased with increasing concentrations of EPMC. It is evident from some previous studies that EPMC has cytotoxic and pro-apoptotic effects in cancer cell lines 27,28. Accordingly, the decreased inhibition of TNF-α at high doses of EPMC is likely due to the cytotoxic effects of EPMC on U937 cells.
The analgesic effect of EPMC was evaluated by the tail flick method in rats. Similar to its dose-dependent anti-inflammatory effects, EPMC also significantly increased the tail flick time in a dose-dependent manner. The suppression of the pro-inflammatory cytokines investigated blocks the induction of genes that encode COX-2. In addition to IL-1, COX-2 is also necessary for the synthesis of prostaglandins from arachidonic acid. Accordingly, blocking COX-2 synthesis may account for the significant analgesic effect of EPMC that was observed. It has been reported that EPMC inhibits the enzymatic active sites on COX-1 and COX-2 8, which may contribute to its analgesic effect. Although this is the first report of an analgesic effect of EPMC, a previous report on the analgesic effect of a water extract of K. galanga suggested that the effect involved the opioid analgesic pathway 10. Therefore, in addition to the inhibition of the cyclooxygenase pathway, the analgesic effect of EPMC may also be associated with its regulation of the opioid pathway. However, further investigation is required to confirm this hypothesis.
Angiogenesis has been strongly implicated in the progression of chronic inflammation 29, as it aids in the maintenance of tissue perfusion and increases the cellular traffic required for inflammation 30. Thus, the inhibition of angiogenesis is a suitable target for treating chronic inflammatory diseases. In addition, cytokines, particularly IL-1 and TNF-α, have been found to be produced at high levels in rapidly growing granulation tissue 31,32, which has been suggested to promote excessive angiogenesis 33,34. It has been reported that macrophages enhance angiogenesis in inflammatory granulation tissue through cytokines via the production of VEGF 35. The present study revealed that while EPMC suppressed the production of granulation tissue in cotton pellets, it also significantly inhibited the production of VEGF in human endothelial cells.
Cytokines from infiltrating macrophages play a significant role in angiogenesis in granulation tissue 35. Macrophages predominate in the chronic inflammation phase and are generally considered to be the primary inflammatory cells involved in the formation of granulation tissue 36. In addition, macrophages appear to be the principal source of the cytokines, such as IL-1 and TNF-α, that are required for the formation of granulation tissue 36.
Previous reports and experimental evidence have shown that EPMC has a significant ability to suppress cytokine production, suggesting that it could be useful for inhibiting angiogenesis. However, its antiangiogenic properties had not been investigated using standard experimental protocols. Accordingly, in this study, a series of antiangiogenic assays were performed to evaluate the antiangiogenic potential of EPMC, and it was observed that EPMC inhibited the sprouting and growth of microvessels in rat aortas.
In the present work, EPMC exerted a considerable inhibitory effect on the sprouting of microvessels from rat aortas. Further investigation revealed that the anti-angiogenic effect of EPMC was mediated by the blocking of vital functions of endothelial cells. It is well known that antiangiogenic agents can act via direct or indirect inhibitory mechanisms. Direct angiogenesis inhibitors interfere with endothelial cell functions, such as proliferation, migration, and differentiation. During the process of angiogenesis, a single layer of endothelial cells lining the inside of blood vessels divides and breaks off from the vessel membrane, forming the tubes and eventually becoming new capillaries 26. EPMC effectively inhibited the principal features of angiogenesis in endothelial cells, including proliferation and migration. After migrating into the perivascular space in blood vessels, HUEVCs undergo differentiation. During this process, the cells assume a characteristic shape, which facilitates intercellular adherence and the formation of a lumen or tube-like structure 27. EPMC remarkably inhibited the formation of such tube-like structures in a dose-dependent manner (Figure 4). These results are also in agreement with those of a recent study that showed that trans-ethyl-p-methoxycinnamate was a potent antiangiogenic constituent 37. Additionally, EPMC inhibited VEGF, which is a key factor required for angiogenesis 21. Like its anti-inflammatory and analgesic effects, the anti-angiogenic property of EPMC can also be attributed to its suppression of cytokines (IL-1 and TNF-α) and angiogenic mitogens (VEGF), which are important for the activation of pro-angiogenic pathways.
The results of the present study indicate that ethyl-p-methoxycinnamate (EPMC) isolated from K. galanga possesses significant anti-inflammatory and analgesic effects. The main mechanism of this activity involves inhibition of the de novo synthesis of pro-inflammatory cytokines, including TNF-α and IL-1. EPMC exhibited a profound anti-angiogenic effect in the rat aortic ring assay. This effect was found to involve the inhibition of vital functions of endothelial cells, such as proliferation, migration, and tube formation, and was due to the inhibition of the synthesis of vascular endothelial growth factor in the cells. Thus, ethyl-p-methoxycinnamate could be a potential precursor for the development of therapeutic agents with the potential to treat diseases involving inflammation and angiogenesis.
ACKNOWLEDGMENTS
The authors are grateful to Universiti Sains Malaysia (USM-IPS) for financial support [fellowship: USM.IPS/JWT/1/19 (JLD 6): P-FD0017/10].
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13(4-5):413–21. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 2.Meager A. Cytokine regulation of cellular adhesion molecule expression in inflammation. Cytokine Growth Factor Rev. 1999;10(1):27–39. doi: 10.1016/s1359-6101(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 3.Makondo K, Kimura K, Kitamura T, Yamaji D, Jung BD, Shibata H, et al. Hepatocyte growth factor/scatter factor suppresses TNF-a-induced E-selectin expression in human umbilical vein endothelial cells. Biochim Biophys Acta. 2004;1644(1):9–15. doi: 10.1016/j.bbamcr.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Choi K-W, Park H-J, Jung DH, Kim T-W, Park Y-M, Kim B-O, et al. Inhibition of TNF-α-induced adhesion molecule expression by diosgenin in mouse vascular smooth muscle cells via downregulation of the MAPK, Akt and NF-κB signaling pathways. Vascul Pharmacol. 2010;53(5-6):273–80. doi: 10.1016/j.vph.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269(2):199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Kundu JK, Surh Y-J. Breaking the relay in deregulated cellular signal transduction as a rationale for chemoprevention with anti-inflammatory phytochemicals. Mutat Res. 2005;591(1-2):123–46. doi: 10.1016/j.mrfmmm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Umar MI, Asmawi MZB, Sadikun A, Altaf R, Iqbal MA. Phytochemistry and medicinal properties of Kaempferia galanga L. (Zingiberaceae) extracts. Afr J Pharm Pharmacol. 2011;5(14):1638–47. [Google Scholar]
- 8.Ihtisham UM, Zaini AM, Amirin S, Justin AI, Fei YM, Rabia A, et al. Bioactivity-Guided Isolation of Ethyl-p-methoxycinnamate, an Anti-inflammatory Constituent, from Kaempferia galanga L. Extracts. Molecules. 2012;17(7):8720–34. doi: 10.3390/molecules17078720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridtitid W, Sae-Wong C, Reanmongkol W, Wongnawa M. Antinociceptive activity of the methanolic extract of Kaempferia galanga Linn. in experimental animals. J Ethnopharmacol. 2008;118(2):225–30. doi: 10.1016/j.jep.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Sulaiman MR, Zakaria ZA, Daud IA, Ng FN, Ng YC, Hidayat MT. Antinociceptive and anti-inflammatory activities of the aqueous extract of Kaempferia galanga leaves in animal models. J Nat Med. 2008;62(2):221–7. doi: 10.1007/s11418-007-0210-3. [DOI] [PubMed] [Google Scholar]
- 11.Lakshmanan D, Werngren J, Jose L, Suja KP, Nair MS, Varma RL, et al. Ethyl p-methoxycinnamate isolated from a traditional anti-tuberculosis medicinal herb inhibits drug resistant strains of Mycobacterium tuberculosis in vitro. Fitoterapia. 2011;82(5):757–61. doi: 10.1016/j.fitote.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 12.In-Ho C, Ju-Yong P, Sang-Chul S, Il-Kwon P. Nematicidal activity of medicinal plant extracts and two cinnamates isolated from Kaempferia galanga L. (Proh Hom) against the pine wood nematode, Bursaphelenchus xylophilus. Nematology. 2006;8(3):359–65. [Google Scholar]
- 13.Tae-Kyun H, Jae-Kook LEE, Jae-Won HEO, Soon-Il KIM, Dong-Ro C, Young-Joon AHN. Toxicity of Kaempferia galanga rhizome-derived extract and steam distillate to Meloidogyne incognita juveniles and eggs, and their effects on Lycopersicon esculentum germination and growth. Nematology. 2010;12(5):775–82. [Google Scholar]
- 14.Sutthanont N, Choochote W, Tuetun B, Junkum A, Jitpakdi A, Chaithong U, et al. Chemical composition and larvicidal activity of edible plant-derived essential oils against the pyrethroid-susceptible and -resistant strains of Aedes aegypti (Diptera: Culicidae) J Vector Ecol. 2010;35(1):106–15. doi: 10.1111/j.1948-7134.2010.00036.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim N-J, Byun S-G, Cho J-E, Chung K, Ahn Y-J. Larvicidal activity of Kaempferia galanga rhizome phenylpropanoids towards three mosquito species. Pest Management Sci. 2008;64(8):857–62. doi: 10.1002/ps.1557. [DOI] [PubMed] [Google Scholar]
- 16.Ekowati J, Tejo BA, Sasaki S, Highasiyama K, Sukardiman Siswandono, Budiati T. Structure modification of ethyl p-methoxycinnamate and their bioassay as chemopreventive agent against mice's fibrosarcoma. Int J Pharm Pharmaceut Sci. 2012;4(3):528–32. [Google Scholar]
- 17.Kim NH, Jung HJ, Shibasaki F, Kwon HJ. NBBA, a synthetic small molecule, inhibits TNF-α-induced angiogenesis by suppressing the NF-κB signaling pathway. Biochem Biophys Res Commun. 2010;391(3):1500–5. doi: 10.1016/j.bbrc.2009.12.101. [DOI] [PubMed] [Google Scholar]
- 18.Araújo FA, Rocha MA, Mendes JB, Andrade SP. Atorvastatin inhibits inflammatory angiogenesis in mice through down regulation of VEGF, TNF-α and TGF-β1. Biomed Pharmacother. 2010;64(1):29–34. doi: 10.1016/j.biopha.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Thejass P, Kuttan G. Allyl isothiocyanate (AITC) and phenyl isothiocyanate (PITC) inhibit tumour-specific angiogenesis by downregulating nitric oxide (NO) and tumour necrosis factor-α (TNF-α) production. Nitric Oxide. 2007;16(2):247–57. doi: 10.1016/j.niox.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Anosike CA, Obidoa O, Ezeanyika LUS. The anti—inflammatory activity of garden egg (Solanum aethiopicum) on egg albumin—induced oedema and granuloma tissue formation in rats. Asian Pac J Trop Med. 2012;5(1):62–6. doi: 10.1016/S1995-7645(11)60247-2. [DOI] [PubMed] [Google Scholar]
- 21.Alciato F, Sainaghi PP, Sola D, Castello L, Avanzi GC. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leuko Biol. 2010;87(5):869–75. doi: 10.1189/jlb.0909610. [DOI] [PubMed] [Google Scholar]
- 22.Dableh LJ, Yashpal K, Henry JL. Physiological evidence of a postsynaptic inhibition of the tail flick reflex by a cannabinoid receptor agonist. Eur J Pharmacol. 2009;602(1):36–40. doi: 10.1016/j.ejphar.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Salahi OSA, Chan KL, Majid AMSA, Al-Suede FSR, Saghir SAM, Abdullah WZ, et al. Anti-angiogenic quassinoids-rich fraction from Eurycoma longifolia modulates endothelial cell function. Microvascular Res. 2013 doi: 10.1016/j.mvr.2013.07.007. (in press) [DOI] [PubMed] [Google Scholar]
- 24.Nassar ZD, Aisha AF, Ahamed MB, Ismail Z, Abu-Salah KM, Alrokayan SA, et al. Antiangiogenic properties of Koetjapic acid, a natural triterpene isolated from Sandoricum koetjaoe Merr. Cancer Cell Int. 2011;11(1):12. doi: 10.1186/1475-2867-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolečková I, Rárová L, Grúz J, Vondrusová M, Strnad M, Kryštof V. Antiproliferative and antiangiogenic effects of flavone eupatorin, an active constituent of chloroform extract of Orthosiphon stamineus leaves. Fitoterapia. 2012;83(6):1000–7. doi: 10.1016/j.fitote.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Khadeer ABM, Krishna V, Malleshappa K. In vivo wound healing activity of the methanolic extract and its isolated constituent, gulonic acid gamma-lactone, obtained from Grewia tiliaefolia. Planta Med. 2009;75(5):478–82. doi: 10.1055/s-0029-1185315. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Liu F, Chen C, Gao H. Supercritical carbon dioxide extraction of ethyl p-methoxycinnamate from Kaempferia galanga L. rhizome and its apoptotic induction in human HepG2 cells. Nat Prod Res. 2010;24(20):1927–32. doi: 10.1080/14786419.2010.490913. [DOI] [PubMed] [Google Scholar]
- 28.Vimala S, Norhanom AW, Yadav M. Anti-tumour promoter activity in Malaysian ginger rhizobia used in traditional medicine. Br J Cancer. 1999;80(1/2):110. doi: 10.1038/sj.bjc.6690329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11(6):457–65. [PubMed] [Google Scholar]
- 30.Colville-Nash PR, Alam CA, Appleton I, Brown JR, Seed MP, Willoughby DA. The pharmacological modulation of angiogenesis in chronic granulomatous inflammation. J Pharmacol Exp Ther. 1995;274(3):1463–72. [PubMed] [Google Scholar]
- 31.Kahlson G, Rosengren E. New approaches to the physiology of histamine. Physiol Rev. 1968;48(1):155–96. doi: 10.1152/physrev.1968.48.1.155. [DOI] [PubMed] [Google Scholar]
- 32.Hirasawa N, Watanabe M, Mue S, Tsurufuji S, Ohuchi K. Downward regulation of neutrophil infiltration by endogenous histamine without affecting vascular permeability responses in air-pouch-type carrageenin inflammation in rats. Inflammation. 1991;15(2):117–26. doi: 10.1007/BF00917506. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen HJ, Kikuchi Y. Histamine-2 receptor antagonists as potential adjuvant treatment of malignant diseases. Advances Biosci. 1993;89:319–34. [Google Scholar]
- 34.Suonio E, Tuomisto L, Kangas L. The role of histamine in cancer growth as assessed by the mouse subrenal capsule assay. Adv. Biosci. 89:349–374. [Google Scholar]
- 35.Ghosh AK, Hirasawa N, Ohtsu H, Watanabe T, Ohuchi K. Defective angiogenesis in the inflammatory granulation tissue in histidine decarboxylase-deficient mice but not in mast cell-deficient mice. J Exp Med. 2002;195(8):973–82. doi: 10.1084/jem.20011782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tellechea A, Leal E, Veves A, Carvalho E. Inflammatory and Angiogenic Abnormalities in Diabetic Wound Healing: Role of Neuropeptides and Therapeutic Perspectives. Open Circulation Vascular J. 2010;3:43–55. [Google Scholar]
- 37.He Z-H, Yue GG-L, Lau CB-S, Ge W, But PP-H. Antiangiogenic effects and mechanisms of trans-ethyl p-methoxycinnamate from Kaempferia galanga L. J Agric Food Chem. 2012;60(45):11309–17. doi: 10.1021/jf304169j. [DOI] [PubMed] [Google Scholar]