Abstract
OBJECTIVE:
High genistein doses have been reported to induce fluid accumulation in the uteri of ovariectomised rats, although the mechanism underlying this effect remains unknown. Because genistein binds to the oestrogen receptor and the cystic fibrosis transmembrane regulator mediates uterine fluid secretion, we hypothesised that this genistein effect involves both the oestrogen receptor and cystic fibrosis transmembrane regulator.
METHODS:
Ovariectomised adult female Sprague-Dawley rats were treated with 25, 50, or 100 mg/kg/day genistein for three consecutive days with and without the ER antagonist ICI 182780. One day after the final drug injection, the animals were humanely sacrificed, and the uteri were removed for histology and cystic fibrosis transmembrane regulator mRNA and protein expression analysis using real-time polymerase chain reaction and Western blotting, respectively. The cystic fibrosis transmembrane regulator protein distribution was analysed visually by immunohistochemistry.
RESULTS:
The histological analysis revealed an increase in the circumference of the uterine lumen with increasing doses of genistein, which was suggestive of fluid accumulation. Moreover, genistein stimulated a dose-dependent increase in the expression of cystic fibrosis transmembrane regulator protein and mRNA, and high-intensity cystic fibrosis transmembrane regulator immunostaining was observed at the apical membrane of the luminal epithelium following 50 and 100 mg/kg/day genistein treatment. The genistein-induced increase in uterine luminal circumference and cystic fibrosis transmembrane regulator expression was antagonised by treatment with ICI 182780.
CONCLUSION:
Genistein-induced luminal fluid accumulation in ovariectomised rats' uteri involves the oestrogen receptor and up-regulation of cystic fibrosis transmembrane regulator expression, and these findings reveal the mechanism underlying the effect of this compound on changes in fluid volume in the uterus after menopause.
Keywords: Genistein, Cystic Fibrosis Transmembrane Regulator, Oestrogen Receptor
INTRODUCTION
Genistein, a phyto-oestrogen, is capable of binding to oestrogen receptor (ER)-α and β, which are expressed in the uterus 1. This compound has also been reported to induce morphological changes 2, luminal fluid secretion 3 and proliferation of the endometrium, as evidenced by the increased expression of proliferative markers 3,4 in the uteri of ovariectomised adult rats. These genistein-mediated effects may have various implications on the female reproductive system. For example, genistein-induced changes may help to restore some uterine functions and reduce uterine atrophy after menopause 5. At high doses, however, genistein may produce harmful effects because it can stimulate the development of endometrial hyperplasia 3,6. There is also evidence that genistein can affect female fertility by interfering with the normal development of the reproductive system 7,8 and the normal pattern of the reproductive cycle 7.
The reported effects of genistein on fluid secretion may affect the volume of fluid in the uterus. Under conditions in which a low amount of fluid is present, such as in menopause 9, genistein may help to restore the uterine fluid volume. Although changes in morphology and fluid secretion have been reported in rodent uteri, genistein has been shown to have no significant effect on restoring normal endometrial morphology in humans 10 and primates 11 after menopause. The presence of fluid within the uterine cavity in the post-menopausal period is typically associated with endometrial pathology, although increased fluid accumulation has also been observed in healthy post-menopausal women 9. Therefore, the function of fluid in the uterus post-menopause remains unknown. In females of reproductive age, a normal amount of uterine fluid is important to sustain fertility 12, as any interference with the fluid volume can result in infertility 13. Before menopause, uterine fluid volume regulation is under the control of sex steroids such as oestrogen (E2) and progesterone (P4) 14.
The cystic fibrosis transmembrane regulator (CFTR), a cAMP-dependent chloride channel, plays an important role in mediating uterine fluid secretion 15,16. CFTR mutations lead to cystic fibrosis, a disease associated with defective Cl- and fluid secretion 17. CFTR has been reported to be expressed at the apical membrane of the endometrial epithelium 14, and its function is dependent on cyclic AMP (cAMP) 18. In addition to secreting fluid and Cl-, CFTR has also been reported to participate in cAMP-dependent HCO3- secretion 19. CFTR expression is under the influence of sex-steroids; this protein is down-regulated by P4 and up-regulated by E2, during the proestrus and estrus stages of the oestrous cycle in rats 15. The effect of genistein on uterine CFTR expression is unknown, although this compound has been reported to affect CFTR expression in the intestine 20,21, kidney 22, airways 23, and epididymis 24.
Genistein is widely consumed as a supplement to prevent or alleviate menopause-related symptoms such as hot flashes, night sweats, and headaches 25. In addition, this compound has also been shown to assist in overcoming post-menopausal-related complications such as osteoporosis 26 and cardiovascular diseases such as hypertension and ischaemic heart disease 27. Despite its wide use, the effect of genistein on the post-menopausal uterus remains poorly characterised. However, limited data indicate that this compound can cause fluid accumulation in the uteri of ovariectomised rats 3. Because the underlying mechanism is unknown, the present study investigated the involvement of the ER and CFTR in regulating this genistein-mediated effect. This study is of clinical significance because the presence of fluid in the uterine cavity of post-menopausal women could be due to high genistein consumption in addition to other factors known to cause fluid accumulation in the uterus during this period.
METHODS
Animal preparation and hormone treatment
Three-month-old adult female Sprague-Dawley (SD) rats weighing approximately 225 g were housed in a clean and well-ventilated animal room with standardised housing conditions (lights on for 12 h from 06:00 h to 18:00 h; room temperature of 24°C; 5-6 animals per cage). Post-weaning, the animals were provided free access to a soy-free diet (Gold-Coin Pellets) and water free of dissolved endocrine-disrupting chemicals (EDCs). All procedures were approved by the Faculty of Medicine, Animal Care and Use Committee, University of Malaya, under ethics number FIS/01/12/2008 (NFK). Genistein (G-6055) was purchased from LC Laboratories (Woburn, MA, USA) at greater than 99% purity; this compound is a crystalline powder with a light yellowish colour.
Bilateral ovariectomy was performed under isoflurane anaesthesia at least ten days prior to drug treatment to eliminate the effect of endogenous sex steroids 14. After surgery, the animals were given intramuscular injections of 0.1 ml Kombitrim antibiotic to prevent post-surgical wound infection. The animals were divided into eight groups (n = 6 per group). Group 1 was treated for three days with peanut oil (vehicle); groups 2 to 7 received subcutaneous injections of genistein at doses of 25, 50, and 100 mg/kg/day for three consecutive days with and without the ER antagonist ICI 182780 at 100 µg/kg/day; and group 8 received ICI 182780 only at 100 µg/kg/day. The drugs were dissolved in peanut oil and were then administered via subcutaneous injection behind the scruff of the neck.
Uterine morphological analyses
At the end of the 3-day period, the rats were sacrificed via anaesthetic overdose followed by cervical dislocation 24 h after the last drug treatment. The right uterine horns were immediately harvested and fixed in 4% paraformaldehyde (PFD) at 4°C for 4 to 5 h. A standardised region of the uterine horn for all animals (mid-portion) was processed using an automated tissue processing machine (Leica, Germany). Tissues were then embedded in paraffin wax using conventional methods, and sections with a thickness of 5 µm were prepared and mounted onto glass slides. The sections were then stained with haematoxylin and eosin (H&E) and visualised under a light microscope (Olympus, Japan). The circumference of the uterine lumen was measured using the NIS-Elements AR program. All images were captured using a Nikon Eclipse 80i camera that was attached to the light microscope.
Quantification of CFTR mRNA expression by real-time PCR
Immediately after the animals were sacrificed, the uteri were removed and placed into RNAlater solution (Ambion, USA), which maintains RNA integrity prior to extraction. In this study, the absolute quantification method, which correlates the PCR signal to a standard curve, was used to determine the copy number of RNA 28. Absolute quantification provides the exact copy number 29 and can be used to compare various treatment groups. In brief, the protocol for PCR included the following steps. First, total RNA was freshly isolated from rat uteri using the RNeasy Plus Mini Kit (Qiagen, USA). The total RNA concentration in the tissue samples was then determined using a Nanodrop (Fisher-Scientific). Reverse transcription into cDNA was performed using a High-capacity RNA-to-cDNA Kit (Applied Biosystems, USA). Taqman® real-time PCR was used to evaluate gene expression, with B-actin and GAPDH serving as endogenous controls. The PCR program consisted of 2 min at 50°C for UNG activity, 20 s at 95°C for activation of the ampliTaq gold DNA polymerase, 1 min of denaturation at 95°C, 20 s for annealing, and extension at 60°C for 1 min. Denaturing and annealing were performed for 40 cycles. All TaqMan assays were purchased from Applied Biosystem, USA. Control reactions included amplifications performed on samples identically prepared with no reverse transcriptase (-RT) or no added substrate (water control). The CFTR Primer was obtained from Roche (USA) with id number Rn014559. Each reaction was performed in duplicate on RNA isolated from three separate animals. The average crossover point (CT) was determined using Roche software and was then converted into copy number per ng of total RNA by comparison to the standard curve. For absolute quantification, standard cDNA (Roche, USA) was diluted to different concentrations, and a standard curve was generated. Quantitative PCR was then performed as described above.
Quantification of CFTR protein expression by Western blotting
Snap-frozen uterine tissues were homogenised using a sonicator with PRO-PREP (Intron) extraction solution in the presence of protease inhibitors. Total cell protein was obtained by centrifugation at 13,000 g for 15 min at 4°C. After determination of the protein concentration, an equal amount of protein was loaded onto a 12% SDS-PAGE gel. The protein was then transferred onto a PVDF membrane and incubated in 5% BSA for 90 min. The blot was then exposed to the primary antibody at a 1:1,000 dilution. After incubation with a CFTR goat polyclonal antibody obtained from Santa Cruz, USA, the blot was incubated with HRP-conjugated secondary antibody and was finally visualised using Optic 4C (Bio RAD). B-actin (abCam, UK) was used as a loading control. Photos of the blots were captured, and the density of each band was determined using Image J software. The ratio of each band/β-actin was determined and was considered the expression level of each of the target proteins.
Localisation of CFTR protein distribution by immunohistochemistry (IHC)
Immunohistochemistry was performed to examine the CFTR protein distribution in the uterus. Uterine paraffin blocks from the various treatment groups were sectioned into 5 µm thick sections and mounted onto glass slides. The tissue sections were incubated in 10% H2O2 in methanol to quench the activity of endogenous peroxidase and were then incubated overnight with primary antibody (rat polyclonal IgG) against CFTR at a 1:100 dilution at 4°C in a humidified chamber. The tissues were then incubated with secondary antibody, i.e., biotinylated rabbit anti-rat IgG (Amersham, UK), at 1:500 for 1 h at room temperature followed by tertiary antibody, i.e., streptavidin-horseradish peroxidase (Amersham, UK), at 1:500 for 1 h at room temperature. The sites of antibody binding were visualised using DAB (diaminobenzidine HCl), which provided a dark brown stain. The sections were then counterstained with haematoxylin for nuclear staining.
Evaluation of immunostaining
The relative intensity of the immunoreactions at the luminal and glandular epithelium was evaluated and graded blindly by three independent observers using a light microscope (Olympus, Japan) at 40X and 100X magnifications. The staining intensity was estimated semi-quantitatively on a scale of 0–3+ (+++) as follows: −, no detectable stain; −/+, faint; +, moderate; ++, strong and +++, very intense staining, as previously described 30.
Statistical analyses
The results are expressed as the mean ± SEM, with n indicating the number of rats. Comparisons between groups were performed using one-way ANOVA. P-values <0.05 were considered statistically significant. Data assist v3 was used to analyse the real-time PCR (qPCR) results.
RESULTS
Effect of genistein treatment on uterine luminal circumference
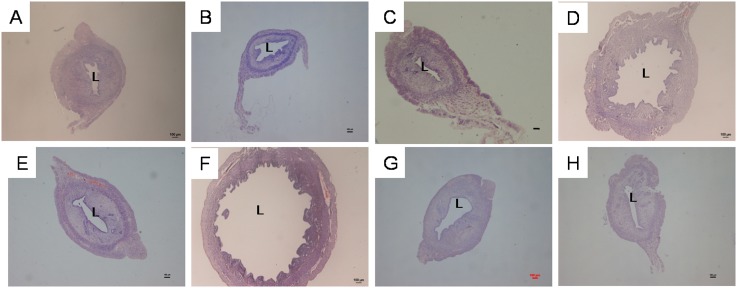
Figure 1 shows images of uterine sections from ovariectomised rats pre-treated with genistein at various doses with and without the presence of the ER antagonist ICI 182780, whereas Table 1 presents the circumference of the uterine lumen from these treatment groups. Treatment with 25, 50, and 100 mg/kg/day genistein resulted in 1.19-, 3.94-, and 4.76-fold increases in uterine circumference, respectively, compared with the control. Administration of ICI 182780 significantly reduced the genistein-related increase in uterine luminal circumference. In particular, ICI 182780 administration resulted in 1.1-, 3.06-, and 2.17-fold decreases in uterine circumference in the groups receiving 25, 50, and 100 mg/kg/day genistein, respectively. ICI 182780 treatment alone did not cause any significant changes in uterine circumference compared with the control treatment.
Figure 1.
Representative images of uterine sections from ovariectomised SD rats treated with (A) peanut oil (control), (B) 25G (25 mg/kg/day genistein), (C) 25G (25 mg/kg/day genistein) + ICI 182780, (D) 50G (50 mg/kg/day genistein), (E) 50G (50 mg/kg/day genistein + ICI 182780), (F) 100G (100 mg/kg/day genistein), (G) 100G (100 mg/kg/day genistein) + ICI 182780, or (H) ICI 182780 (100 μg/kg/day) only for 3 consecutive days. There was a clear increase in the luminal circumference following treatment with 50 and 100 mg/kg/day genistein, although these changes were antagonised by treatment with ICI 182780. Sections were stained with H&E, and the magnification is X4. L indicates the uterine lumen. (G-genistein), n = 6 per treatment group.
Table 1.
Measurement of the circumference of the uterine lumen in ovariectomised rats treated with peanut oil (control) and different doses of genistein (25, 50 and 100 mg/kg/day) with and without ICI 182780. The values represent the mean ± SEM, with n = 6 per treatment group. The circumference was increased with increasing doses of genistein. Administration of ICI 182780 reduced the uterine circumference in all genistein-treated groups.
| Uterine luminal circumference (mm) | ||
| Control | With ICI 182780 | |
| Vehicle | 1,693±78.98 | 1,622.97±69.11 |
| 25G | 2,019.97±60.13 *,† | 1,837.95±75.44 |
| 50G | 6,674.11±100.32 *,† | 2,182±99.18 |
| 100G | 8,062.84±125.76 *,† | 3,721±102.49 |
CFTR mRNA expression analysis by real-time PCR (qPCR)
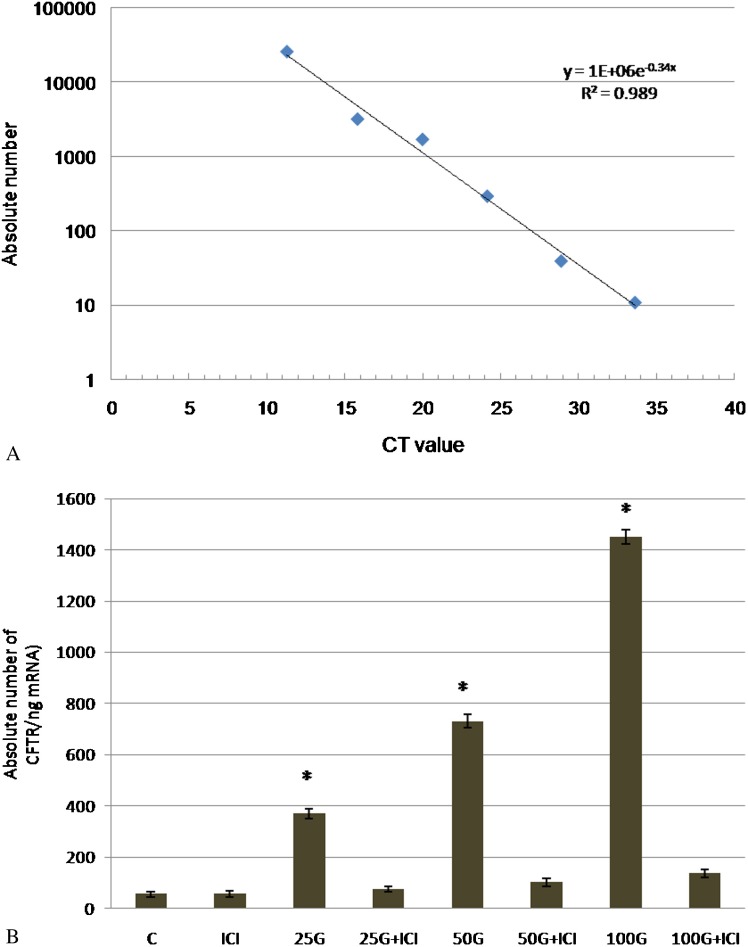
Based on the CT values obtained for each sample, CFTR mRNA expression was quantified using the absolute quantification method. Absolute and relative quantifications have been shown to produce comparable results 31. Figure 2(a) shows the standard curve generated using standard cDNA. As shown in Figure 2(b), the absolute CFTR mRNA expression was determined according to the standard curve from CT values obtained in each treatment group. CFTR mRNA expression was markedly increased following 25, 50, and 100 mg/kg/day genistein treatment, and these levels were 6.8-, 13.5-, and 26.75-fold greater, respectively, than the control. Administration of ICI 182780 resulted in a significant inhibition of mRNA expression in all groups receiving different doses of genistein.
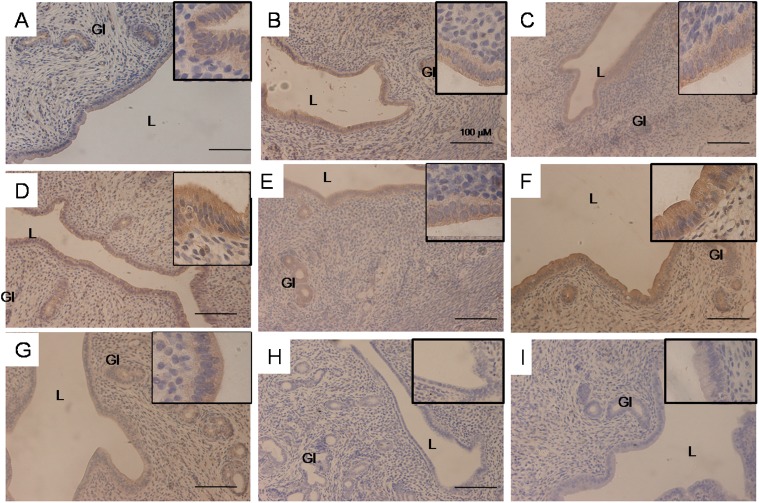
Figure 2.
Representative images of the uterine glandular and luminal epithelium under 20X magnification and the luminal epithelium under 100X magnification (in the upper right-hand corner) from ovariectomised SD rats treated with (A) control (vehicle-treated) (B) 25G (25 mg/kg/day genistein), (C) 25G (25 mg/kg/day genistein) + ICI 182780, (D) 50G (50 mg/kg/day), (E) 50G (50 mg/kg/day genistein) + ICI 182780, (F) 100G (100 mg/kg/day genistein), (G) 100G (100 mg/kg/day genistein) + ICI 182780, or (H) ICI 182780 (100 μg/kg/day) only for 3 consecutive days or (I) incubation with a non-immune peptide. There was a clear increase in the intensity of immunostaining in the group treated with 100 mg/kg/day genistein. L- uterine lumen, Gl– uterine gland, G-genistein. Sections were stained with H&E for nuclear staining; n = 6 per treatment group.
CFTR protein expression analysis by Western blotting
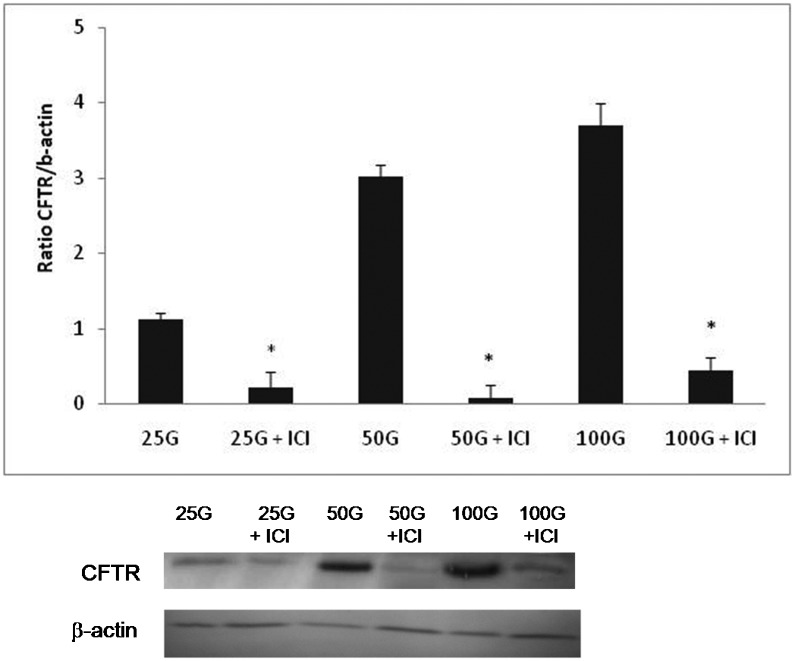
As shown in Figure 3, the expression of CFTR protein was increased with increasing doses of genistein. The ratio of CFTR/B-actin was 2.92- and 3.35-fold higher in the groups treated with 50 and 100 mg/kg/day genistein, respectively, compared with the group receiving 25 mg/kg/day genistein treatment. Meanwhile, ICI 182780 administration led to a significant decrease in genistein-induced CFTR protein expression in all treatment groups.
Figure 3.
(a) Standard curve obtained from cDNA dilution. The y axis represents the absolute nuclear DNA copy number, and the x axis represents the CT value. The linear regression equation was given by y = 1E+06e-0.34x, R2 = 0.989. (b) CFTR mRNA expression evaluated by real-time PCR. The absolute copy number (per 1 ng mRNA used for real-time PCR) was calculated from the standard curve (a). The expression of CFTR mRNA was 26 times higher following treatment with 100 mg/kg/day genistein compared with the control. The absolute copy number obtained from each sample represents the mean of four assays with the standard deviation. C = control, ICI = ICI 182780, 25G = 25 mg/kg/day genistein, 25G (25 mg/kg/day genistein) + ICI 182780, 50G = 50 mg/kg/day genistein, 50G (50 mg/kg/day genistein) + ICI 182780, 100G = 100 mg/kg/day genistein, 100G (100 mg/kg/day genistein) + ICI 182780.
Analysis of CFTR protein distribution by IHC
Figure 4 shows images of the endometrium at 20× magnification and the luminal epithelium at 100× magnification, whereas Table 2 presents a semi-quantitative analysis of the staining intensity of CFTR in various treatment groups with and without ICI 182780. Our findings indicated that the intensity of immunostaining was highest following treatment with 50 and 100 mg/kg/day genistein, predominantly at the luminal and glandular epithelium, and these intensity values were decreased following ICI 182780 administration. CFTR immunostaining was highest at the apical membrane of the luminal epithelium following treatment with 50 and 100 mg/kg/day genistein. Mild to moderate staining could be observed following treatment with 25 mg/kg/day genistein, which was also reduced following ICI 182780 administration. Incubation with non-immune peptide resulted in no staining (negative control).
Figure 4.
The level of CFTR protein expression in ovariectomised rats treated with different doses of genistein (25, 50, and 100 mg/kg/day) with and without ICI 182780. The expression of CFTR protein was increased in a dose-dependent manner with increasing doses of genistein, and this increase was significantly antagonised by ICI 182780. *p<0.05 compared with the respective treatment group without the presence of ICI 182780; n = 6 per group. The data are presented as the mean ± SEM. 25G = 25 mg/kg/day genistein, 25G (25 mg/kg/day genistein) + ICI 182780, 50G = 50 mg/kg/day genistein, 50G (50 mg/kg/day genistein) + ICI 182780, 100G = 100 mg/kg/day genistein, 100G (100 mg/kg/day genistein) + ICI 182780.
Table 2.
Semi-quantitative analysis of the intensity of CFTR immunostaining following different doses of genistein treatment (25, 50, and 100 mg/kg/day) with and without ICI 182780. The staining intensity was graded as follows: −, negative; −/+, faint; +, moderate; ++ strong and +++, very intense. The intensity was confirmed by 3 independent observers.
| Apical | Basolateral | |
| Control | + | + |
| ICI 182 780 | - | - |
| 25G | ++ | +/- |
| 25G + ICI 182 780 | +/- | - |
| 50G | +++ | + |
| 50G + 1CI 182 780 | + | + |
| 100G | +++ | + |
| 100G + ICI 182780 | + | + |
25G = 25 mg/kg/day genistein, 50G = 50 mg/kg/day genistein, 100G = 100 mg/kg/day genistein.
DISCUSSION
To the best of our knowledge, this study was the first to report the mechanism underlying genistein-induced fluid accumulation in the uteri of ovariectomised rats. Our study confirmed the previous observation that genistein at high doses (50 and 100 mg/kg/day) leads to a significant increase in fluid volume in the uteri of ovariectomised rats 3. Although the significance of this effect is unknown, it could help to restore fluid in the uterus after menopause. Because high-dose genistein has also been reported to cause an increase in the thickness of the endometrium and myometrium in ovariectomised rats 3, this compound may therefore be used to block uterine atrophy resulting from a lack of oestrogen. In other studies involving ovariectomised rats, high-dose genistein (100 mg/kg/day) was reported to increase bone mineral density (BMD) 32 and partially regress atrophied mammary glands 33. In humans, consumption of a genistein-rich diet, including approximately 50 mg/day of this compound, by women in the post-menopausal period has been reported to reduce the incidence of vasomotor episodes such as hot flashes 34. Genistein supplementation has also been found to decrease the total cholesterol 35 and low density lipoprotein (LDL) 36 levels and to reduce the risk for breast cancer development 37 in women after menopause.
Our findings revealed that genistein-induced fluid accumulation in the uteri of ovariectomised rats involves the ER, as evidenced from the antagonising effect of ICI 182780. The effect of genistein on ER expression in the uterus has been previously reported. Administration of selective soy extract (SSE) containing genistein at 50 and 100 mg/kg/day to ovariectomised rats was found to enhance the expression of ER-β in the uterine stroma. This increase in ER-β expression was shown to negatively modulate the expression of ER-α in the luminal epithelia 38. Down-regulation of ER-α has also been reported in the uteri of intact non-ovariectomised rats receiving chronic genistein treatment 39, suggesting that this effect may be due to the up-regulation of ER-β expression by genistein. In view of these findings, we speculated that the effect of genistein as observed in our study may be mediated via ER-β, although further studies are needed to confirm this hypothesis.
In humans, the presence of fluid within the uterine cavity of post-menopausal women has been associated with endometrial malignancy 40 and cervical stenosis 41. In most cases, however, there is no obvious relationship between the neoplastic lesions and the amount of fluid within the uterus after menopause 42. In women who have no pathological lesions either in the uterus or cervix, increased uterine fluid amount after menopause is almost always associated with advancing age 43 and the co-existence of medical conditions such as diabetes and hypertension 9. The association between the use of hormone replacement therapy (HRT), i.e., oestrogen or selective ER modulators (SERMs), and post-menopausal uterine fluid accumulation remains unknown. Because our study found that genistein induced fluid accumulation in the uteri of ovariectomised rats, it is possible that this compound may lead to increased fluid accumulation in the uterus during the post-menopausal period. High-dose genistein intake should therefore be considered as one of the causes for increased fluid levels in the uteri of post-menopausal women.
Our findings also revealed that CFTR is involved in mediating the genistein-induced increase in the fluid amount in ovariectomised rats' uteri. In particular, we found that the expression of CFTR mRNA and protein was markedly increased following treatment with 50 and 100 mg/kg/day genistein. Up-regulation of CFTR mRNA and protein expression was dependent on the ER, suggesting that this effect was mediated via a genomic pathway. Under the effect of genistein, CFTR was expressed mainly at the apical membrane of the luminal epithelium, which suggests that it may participate in fluid, Cl-, and HCO3- secretion into the uterine lumen. The importance of CFTR in mediating uterine fluid secretion was demonstrated by Navis et al. 44, in which deletion of the CFTR gene was found to inhibit luminal fluid secretion. Our hypothesis that CFTR is involved in uterine fluid secretion is also supported by the observation that a marked increase in uterine CFTR expression occurs in diseases related to excessive fluid accumulation in the uterus, such as hydrosalpinx 45. Apart from the uterus, CFTR has been reported to mediate fluid secretion in the small intestine 46, submucosal glands 47, and salivary glands 48. Therefore, it is likely that the observed increase in fluid accumulation within the uterine lumen was due to genistein-induced CFTR up-regulation. In addition to mediating fluid secretion, CFTR has also been reported to be involved in Cl- and HCO3- secretion in the uterus 49, small intestine 20, and airways 50. As genistein has been reported to induce CFTR-mediated Cl- secretion in the small intestine 20 and jejunum 51, it is likely that this compound may also stimulate Cl- secretion in the uterus via CFTR.
This study investigated the acute effect of genistein on fluid accumulation and CFTR protein and mRNA expression in the uterus of ovariectomised rats. Three days of genistein treatment was sufficient to cause an increase in CFTR expression in the uterus. A similar duration of genistein treatment in ovariectomised rats was also found to induce changes in the morphology of the uterus and vagina 52 and affect the expression of several other proteins, including proliferative cell nuclear antigen (PCNA) 2,3 and complement C3 53 in the uterus and blood, respectively. In this study, the route of genistein administration could represent the limiting factor for extrapolating these findings to humans. In humans, this compound is usually consumed orally as a dietary supplement or as a component of the diet. Moreover, the level of genistein in the blood is expected to be higher following subcutaneous administration compared with oral administration, as orally administered genistein is metabolised by the gut flora to 5-hydroxy-equol 54. In addition, oral genistein administration also undergoes metabolism in the liver 55. Thus, the lack of uterotopic responses observed in humans following oral genistein consumption 10 is likely due to low plasma bioavailability as the consequence of these first-pass effects.
In conclusion, genistein-induced fluid accumulation in the uteri of ovariectomised rats was shown to involve the ER and CFTR, and these findings reveal the underlying effect of this compound on the uterus after menopause. Although the significance of this effect is unknown, this compound may help to restore fluid within the uterine cavity and inhibit uterine atrophy. Finally, high-dose genistein consumption, rather than the presence of malignancy, should be considered a potential cause for fluid in the uteri of post-menopausal women.
ACKNOWLEDGMENTS
This study was supported by the UMRG grant (RG404/12HTM) from the University of Malaya, Kuala Lumpur.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Norrby M, Madej A, Ekstedt E, Holm L. Effects of genistein on oestrogen and progesterone receptor, proliferative marker Ki-67 and carbonic anhydrase localisation in the uterus and cervix of gilts after insemination. Anim Reprod Sci. 2013 doi: 10.1016/j.anireprosci.2013.01.011. Feb 6. [DOI] [PubMed] [Google Scholar]
- 2.Diel P, Geis R-B, Caldarelli A, Schmidt S, Leschowsky UL, Voss A, et al. The differential ability of the phytoestrogen genistein and of estradiol to induce uterine weight and proliferation in the rat is associated with a substance specific modulation of uterine gene expression. Molecular and Cellular Endocrinology. 2004;221((1–2)):21–32. doi: 10.1016/j.mce.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Salleh N, Helmy MM, Fadila KN, Yeong SO. Isoflavone genistein induces fluid secretion and morphological changes in the uteri of post-pubertal rats. Int J Med Sci. 2013;10((6)):665–75. doi: 10.7150/ijms.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rimoldi G CJ, Seidlova-Wuttke D, Jarry H, Wuttke W. Effects of chronic genistein treatment in mammary gland, uterus, and vagina. Environ Health Perspect. 2007;115((Suppl 1)):62–8. doi: 10.1289/ehp.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos E, Sampaio M, Cecon P, Jesus Simões M, Sartori M, Girão M. Effects of soy isoflavones on the uterus and urethra of ovariectomized rats. Int Urogynecol J. 2010;21((1)):111–6. doi: 10.1007/s00192-009-0995-6. [DOI] [PubMed] [Google Scholar]
- 6.Chandrareddy A, Muneyyirci-Delale O, McFarlane SI, Murad OM. Adverse effects of phytoestrogens on reproductive health: A report of three cases. Complementary Therapies in Clinical Practice. 2008;14((2)):132–5. doi: 10.1016/j.ctcp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the female reproductive system by the phytoestrogen genistein. Reprod Toxicol. 2007;23:308–16. doi: 10.1016/j.reprotox.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Jefferson WN, Padilla-Banks E, Phelps JY, Cantor AM, Williams CJ. Neonatal Phytoestrogen Exposure Alters Oviduct Mucosal Immune Response to Pregnancy and Affects Preimplantation Embryo Development in the Mouse. Biol Reprod. 2012;87((1)):10,1–10. doi: 10.1095/biolreprod.112.099846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inceboz U, Uyar Y, Baytur Y, Kandiloglu AR. Endometrial fluid in postmenopausal women. Int J Gynaecol Obstet. 2009;107((2)):154–5. doi: 10.1016/j.ijgo.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 10.D'Anna R, Cannata ML, Marini H, Atteritano M, Cancellieri F, Corrado F, et al. Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: a 2-year randomized, double-blind, placebo-controlled study. Menopause. 2009;16((2)):301–6. doi: 10.1097/gme.0b013e318186d7e2. [DOI] [PubMed] [Google Scholar]
- 11.Wood CE, Appt SE, Clarkson TB, Franke AA, Lees CJ, Doerge DR, et al. Effects of High-Dose Soy Isoflavones and Equol on Reproductive Tissues in Female Cynomolgus Monkeys. Biol Reprod. 2006;75((3)):477–86. doi: 10.1095/biolreprod.106.052142. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Ajonuma LC, Tsang LL, Lam SY, Rowlands DK, Ho LS, et al. Differential expression and localization of CFTR and ENaC in mouse endometrium during pre-implantation. Cell Biol Int. 2004;28((6)):433–9. doi: 10.1016/j.cellbi.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Naftalin R, Thiagarajah, Pedley K, Pocock V, Milligan S. Progesterone stimulation of fluid absorption by the rat uterine gland. Reproduction. 2002. 2002;123((5)):633–8. doi: 10.1530/rep.0.1230633. [DOI] [PubMed] [Google Scholar]
- 14.Salleh N, Baines DL, Naftalin RJ, Milligan SR. The Hormonal Control of Uterine Luminal Fluid Secretion and Absorption. J Membr Biol. 2005;206((1)):17–28. doi: 10.1007/s00232-005-0770-7. [DOI] [PubMed] [Google Scholar]
- 15.Gholami K, Muniandy S, Salleh N. Progesterone downregulates oestrogen-induced expression of CFTR and SLC26A6 proteins and mRNA in rats' uteri. J Biomed Biotechnol. 2012;2012:596084. doi: 10.1155/2012/596084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan L, Tsang LL, Rowlands DK, Rochelle LG, Boucher RC, Liu CQ, et al. Distribution and regulation of ENaC subunit and CFTR mRNA expression in murine female reproductive tract. J Membr Biol. 2002;185((2)):165–76. doi: 10.1007/s00232-001-0117-y. [DOI] [PubMed] [Google Scholar]
- 17.Marson FA BC, Secolin R, Ribeiro AF, Ribeiro JD. Genetic interaction of GSH metabolic pathway genes in cystic fibrosis. BMC Med Genet. 2013;10((14(1))):60. doi: 10.1186/1471-2350-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bargon J, Trapnell BC, Chu CS, Rosenthal ER, Yoshimura K, Guggino WB, et al. Down-regulation of cystic fibrosis transmembrane conductance regulator gene expression by agents that modulate intracellular divalent cations. Mol Cell Biol. 1992;12((4)):1872–8. doi: 10.1128/mcb.12.4.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang XF, Zhou CX, Shi QX, Yuan YY, Yu MK, Ajonuma LC, et al. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat Cell Biol. 2003;5((10)):902–6. doi: 10.1038/ncb1047. [DOI] [PubMed] [Google Scholar]
- 20.Al-Nakkash L, Batia L, Bhakta M, Peterson A, Hale N, Skinner R, et al. Stimulation of Murine Intestinal Secretion by Daily Genistein Injections: Gender-dependent Differences. Cell Physiol Biochem. 2011;28((2)):239–50. doi: 10.1159/000331736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuo B, Wen G, Seidler U. Differential activation of the HCO3- conductance through the cystic fibrosis transmembrane conductance regulator anion channel by genistein and forskolin in murine duodenum. Br J Pharmacol. 2009;158((5)):1313–21. doi: 10.1111/j.1476-5381.2009.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt A, Hughes LK, Cai Z, Mendes F, Li H, Sheppard DN, et al. Prolonged treatment of cells with genistein modulates the expression and function of the cystic fibrosis transmembrane conductance regulator. Br J Pharmacol. 2008;153((6)):1311–23. doi: 10.1038/sj.bjp.0707663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson C, Servetnyk Z, Roomans GM. Activation of CFTR by genistein in human airway epithelial cell lines. Biochem Biophys Res Commun. 2003;308((3)):518–22. doi: 10.1016/s0006-291x(03)01436-0. [DOI] [PubMed] [Google Scholar]
- 24.Leung GPH, Wong PYD. Activation of Cystic Fibrosis Transmembrane Conductance Regulator in Rat Epididymal Epithelium by Genistein. Biol Reprod. 2000;62((1)):143–9. doi: 10.1095/biolreprod62.1.143. [DOI] [PubMed] [Google Scholar]
- 25.Reed SD, Lampe JW, Qu C, Gundersen G, Fuller S, Copeland WK, et al. Self-reported menopausal symptoms in a racially diverse population and soy food consumption. Maturitas. 2013;75((2)):152–8. doi: 10.1016/j.maturitas.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Tsuang Y-H, Chen L-T, Chiang C-J, Wu L-C, Chiang Y-F, Chen P-Y, et al. Isoflavones prevent bone loss following ovariectomy in young adult rats. J Orthop Surg Res. 2008;3((1)):12. doi: 10.1186/1749-799X-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Squadrito F, Altavilla D, Squadrito G, Saitta A, Cucinotta D, Minutoli L, et al. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc Res. 2000;45((2)):454–62. doi: 10.1016/s0008-6363(99)00359-4. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25((4)):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Watzinger F, Suda M, Preuner S, Baumgartinger R, Ebner K, Baskova L, et al. Real-Time Quantitative PCR Assays for Detection and Monitoring of Pathogenic Human Viruses in Immunosuppressed Pediatric Patients. J Clin Microbiol. 2004;42((11)):5189–98. doi: 10.1128/JCM.42.11.5189-5198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrot-Applanat M, Deng M, Fernandez H, Lelaidier C, Meduri G, Bouchard P. Immunohistochemical localization of estradiol and progesterone receptors in human uterus throughout pregnancy: expression in endometrial blood vessels. J Clin Endocrinol Metab. 1994;78((1)):216–24. doi: 10.1210/jcem.78.1.8288707. [DOI] [PubMed] [Google Scholar]
- 31.Wong ML MJ. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39((1)):75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- 32.Gallo D, Zannoni GF, Apollonio P, Martinelli E, Ferlini C, Passetti G, et al. Characterization of the pharmacologic profile of a standardized soy extract in the ovariectomized rat model of menopause: effects on bone, uterus, and lipid profile. Menopause. 2005;12((5)):589–600. doi: 10.1097/01.GME.0000156348.61767.D5. [DOI] [PubMed] [Google Scholar]
- 33.Gallo D, Zannoni GF, Martinelli E, Ferlini C, Fabrizi M, Riva A, et al. Estradiol and phytoestrogens differently influence the rodent postmenopausal mammary gland. Menopause. 2006;13((1)):72–9. doi: 10.1097/01.gme.0000191208.05491.94. [DOI] [PubMed] [Google Scholar]
- 34.Jenks B, Iwashita S, Nakagawa Y, Ragland K, Lee J, Carson W. A pilot study on the effects of S-equol compared to soy isoflavones on menopausal hot flash frequency. J Womens Health (Larchmt) 2012;21((6)):674–82. doi: 10.1089/jwh.2011.3153. [DOI] [PubMed] [Google Scholar]
- 35.Yang T-S, Wang S-Y, Yang Y-C, Su C-H, Lee F-K, Chen S-C, et al. Effects of standardized phytoestrogen on Taiwanese menopausal women. Taiwan J Obstet Gynecol. 2012;51((2)):229–35. doi: 10.1016/j.tjog.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Hwang J, Sevanian A, Hodis HN, Ursini F. Synergistic inhibition of LDL oxidation by phytoestrogens and ascorbic acid. Free Radic Biol Med. 2000;29((1)):79–89.37. doi: 10.1016/s0891-5849(00)00322-1. [DOI] [PubMed] [Google Scholar]
- 37.Boucher B, Cotterchio M, Anderson LN, Kreiger N, Kirsh VA, Thompson LU. Use of isoflavone supplements is associated with reduced postmenopausal breast cancer risk. Int J Cancer. 2013;132((6)):1439–50. doi: 10.1002/ijc.27769. [DOI] [PubMed] [Google Scholar]
- 38.Gallo D ZG, Fabrizi M, De Stefano I, Mantuano E, Scambia G. Comparative effects of 17beta-estradiol and phytoestrogens in the regulation of endometrial functions in the rodent uterus. J Endocrinol Invest. 2008;31((1)):48–56. doi: 10.1007/BF03345566. [DOI] [PubMed] [Google Scholar]
- 39.Zin S, Omar SZ, Khan NL, Musameh NI, Das S, Kassim NM. Effects of the phytoestrogen genistein on the development of the reproductive system of Sprague Dawley rats. Clinics. 2013;68((2)):253–62. doi: 10.6061/clinics/2013(02)OA21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zalel Y, Tepper R, Cohen I, Goldberger S, Beyth Y. Clinical significance of endometrial fluid collections in asymptomatic postmenopausal women. J Ultrasound Med. 1996;15((7)):513–5. doi: 10.7863/jum.1996.15.7.513. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein SR. The presence of endometrial fluid in asymptomatic postmenopausal women is associated with clinically relevant cervical stenosis. J Ultrasound Med. 1997;16((3)):208. doi: 10.7863/jum.1997.16.3.208. [DOI] [PubMed] [Google Scholar]
- 42.Bedner R, Rzepka-Górska I. Diagnostic value of uterine cavity fluid collection in the detection of pre-neoplastic lesions and endometrial carcinoma in the asymptomatic post-menopausal women. Ginekol Pol. 1998;69((5)):237–40. [PubMed] [Google Scholar]
- 43.Gull B, Karlsson B, Wikland M, Milsom I, Granberg S. Factors influencing the presence of uterine cavity fluid in a random sample of asymptomatic postmenopausal women. Acta Obstet Gynecol Scand. 1998;77((7)):751–7. [PubMed] [Google Scholar]
- 44.Navis A, Marjoram L, Bagnat M. Cftr controls lumen expansion and function of Kupffer's vesicle in zebrafish. Development. 2013;140((8)):1703–12. doi: 10.1242/dev.091819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang JZ, Jiang X, Dong J, Guo J, Chen H, Tsang LL, et al. Abnormally enhanced cystic fibrosis transmembrane conductance regulator-mediated apoptosis in endometrial cells contributes to impaired embryo implantation in controlled ovarian hyperstimulation. Fertil Steril. 2011;95((6)):2100–6.e2. doi: 10.1016/j.fertnstert.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 46.Patanayindee J, Muanprasat C, Soodvilai S, Chatsudthipong V. Antidiarrheal efficacy of a quinazolin CFTR inhibitor on human intestinal epithelial cell and in mouse model of cholera. Indian J Pharmacol. 2012;44((5)):619–23. doi: 10.4103/0253-7613.100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee RJ, Foskett JK. Why mouse airway submucosal gland serous cells do not secrete fluid in response to camp stimulation. J Biol Chem. 2012;287((45)):38316–26. doi: 10.1074/jbc.M112.412817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med. 2012;2((6)): doi: 10.1101/cshperspect.a009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan HC, Shi QX, Zhou CX, Wang XF, Xu WM, Chen WY, et al. Critical role of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Mol Cell Endocrinol. 2006;250((1-2)):106–13. doi: 10.1016/j.mce.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 50.Derichs N, Mekus F, Bronsveld I, Bijman J, Veeze HJ, von der Hardt H, et al. Cystic fibrosis transmembrane conductance regulator (CFTR)-mediated residual chloride secretion does not protect against early chronic Pseudomonas aeruginosa infection in F508del homozygous cystic fibrosis patients. Pediatr Res. 2004;55((1)):69–75. doi: 10.1203/01.PDR.0000100758.66805.CE. [DOI] [PubMed] [Google Scholar]
- 51.Baker MJ, Hamilton KL. Genistein stimulates electrogenic Cl− secretion in mouse jejunum. Am J Physiol Cell Physiol. 2004;287((6)):C1636–45. doi: 10.1152/ajpcell.00236.2003. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt S, Degen G, Seibel J, Hertrampf T, Vollmer G, Diel P. Hormonal activity of combinations of genistein, bisphenol A and 17&bgr;-estradiol in the female Wistar rat. Arch Toxicol. 2006;80((12)):839–45. doi: 10.1007/s00204-006-0102-4. [DOI] [PubMed] [Google Scholar]
- 53.Diel P, Smolnikar K, Schulz T, Laudenbach-Leschowski U, Michna H, Vollmer G. Phytoestrogens and carcinogenesis—differential effects of genistein in experimental models of normal and malignant rat endometrium. Human Reprod. 2001;16((5)):997–1006. doi: 10.1093/humrep/16.5.997. [DOI] [PubMed] [Google Scholar]
- 54.Matthies A, Loh G, Blaut M, Braune A. Daidzein and genistein are converted to equol and 5-hydroxy-equol by human intestinal slackia isoflavoniconvertens in gnotobiotic rats. J Nutr. 2012;142((1)):40–6. doi: 10.3945/jn.111.148247. [DOI] [PubMed] [Google Scholar]
- 55.Coldham N, Zhang AQ, Key P, Sauer MJ. Absolute bioavailability of [14C] genistein in the rat; plasma pharmacokinetics of parent compound, genistein glucuronide and total radioactivity. Eur J Drug Metab Pharmacokinet. 2002;27((4)):249–58. doi: 10.1007/BF03192335. [DOI] [PubMed] [Google Scholar]