Abstract
A 42-year-old man diagnosed with pseudohypoparathyroidism and Albright's hereditary osteodystrophy as an infant was lost to follow-up and remained, unmonitored, on calcitriol and calcium for over 20 years. He presented after having an ST-elevation myocardial infarction. In addition to coronary artery calcifications, he was found to have diffuse subcutaneous and joint calcifications. His calcium, phosphate and parathyroid hormone (PTH) levels were normal, and given the lack of prior documentation in the diagnosis he was instructed to discontinue calcitriol and calcium until further investigations were completed. Despite stopping the medication, his serum calcium remained normal for over 1 year. It was not until 18 months later, when his soft tissue calcium stores were depleted, that he finally developed symptomatic hypocalcaemia and an elevated PTH. This case not only emphasises the importance of long-term follow-up for patients with pseudohypoparathyroidism, but also highlights the potential complications of long-term, unmonitored, calcitriol use.
Background
Pseudohypoparathyroidism (PHP) is a rare genetic disorder characterised by end-organ resistance (kidney and bone) to parathyroid hormone (PTH) action. Treatment is supplementation with calcium and potent vitamin D compounds to maintain normocalcaemia. Hypercalcaemia, hypercalciuria and hyperphosphataemia are known potential complications of this treatment so monitoring of these patients is critical to avoid soft tissue calcium deposition and renal injury from nephrocalcinosis. The patient we describe was lost to follow-up during adolescence and suffered severe complications related to calcitriol therapy that were likely preventable. This case provides further evidence that patients with PHP require life-long biochemical follow-up and that long-term unmonitored calcitriol therapy can lead to significant complications.
Case presentation
A 42-year-old man was seen in follow-up at the cardiac function clinic after having a defibrillator placed for cardiac arrhythmia causing syncope. One year prior he had suffered an ST-elevation myocardial infarction that was treated with stents to the proximal left anterior descending and right circumflex artery. During the evaluation it was noted that the patient had a reported history of PHP that was not being followed so he was referred to endocrinology. The patient's medical history was otherwise remarkable for: educational performance difficulties as a child, hypertension, atrial fibrillation, a remote left nephrectomy for an unknown diagnosis, chronic kidney disease of unknown origin, hypothyroidism, gout and obesity.
When reviewed by endocrinology, there were no records of the patient's diagnosis of PHP. He reported being diagnosed at age 3 at the same time as he underwent a left nephrectomy for an unknown diagnosis, possibly a congenital atretic kidney. He was followed by an internist until he was 22 years of age, but had not seen an endocrinologist for over 20 years. He had been taking calcium and calcitriol since his diagnosis, stopping infrequently on his own for short periods without any change in symptoms, and had been off both medications for 3 months at presentation to endocrinology. He denied knowledge of nephrocalcinosis or kidney stones but had been troubled by numerous subcutaneous dystrophic calcifications, many of which had been surgically excised.
Little was known about his paternal family history but his maternal history revealed an extensive cardiac history and type 2 diabetes mellitus. His social history was unremarkable.
On physical examination the patient was noted to be of short stature with a height of 162 cm (<3rd centile) and weight 78.5 kg (75th centile). He had macrocephaly with a head circumference of 59 cm (>98th centile), an elongated forehead, but otherwise normal facial features. There was no corneal arcus, Chvostek's and Trousseau's signs were negative and he had normal secondary sexual characteristics. There were several areas of subcutaneous calcifications, mainly over the extensor surfaces of his hands bilaterally, bilateral arms and abdomen. There was a café-au-lait patch over the volar aspect of the right forearm measuring 6.5×3.75 cm. The absence of brachydactyly was noted. The rest of the physical examination was unremarkable.
The patient's blood work revealed normal levels of calcium (2.33 mmol/L, reference range 2.1–2.6 mmol/L), phosphate (1.30 mmol/L, reference range 0.8–1.45 mmol/L) and an almost suppressed PTH (14 ng/L, reference range 13–54 ng/L). He had a borderline low 25-OH vitamin D level (48 nmol/L, reference range 80–200 nmol/L) and an elevated creatinine (161 mmol/L, reference range 50–115 mmol/L).
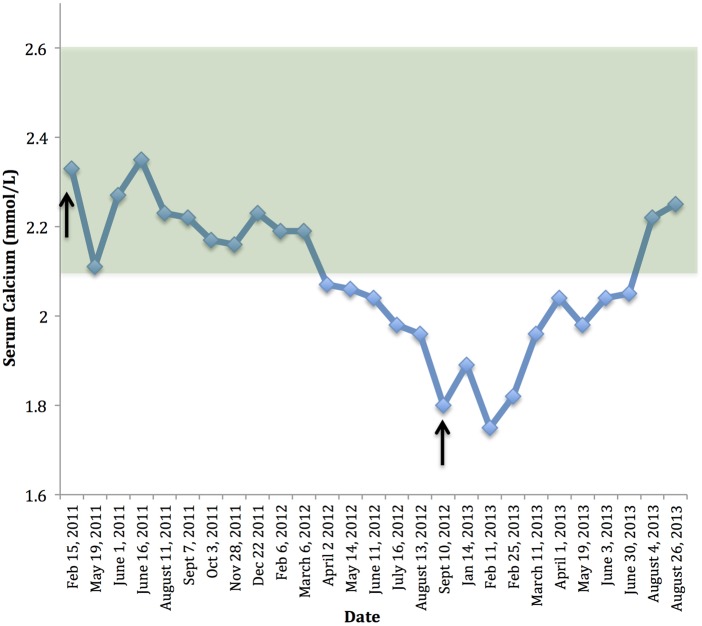
Given the lack of records and uncertainty of the diagnosis the patient was instructed to remain off calcium and calcitriol and his serum calcium, creatinine, PTH and phosphate were followed. His calcium remained normal for over a year (see figure 1). This was not expected with PHP so the diagnosis seemed uncertain. However, given his extensive subcutaneous calcifications it was probable that after so many years of calcium and calcitriol use he had accumulated sufficient soft tissue stores of calcium to maintain normal serum values due to slow resorption of calcium deposits, also causing nearly suppressed serum PTH levels in contrast to the expected high levels typically seen in PHP.
Figure 1.
Time plot of serum calcium values by date. The normal range for calcium is depicted by the green shadowed area and critical clinical interventions are noted with arrows. First arrow: calcium and calcitriol stopped. Second arrow: patient presented with symptoms of hypocalcaemia and was restarted on calcium and calcitriol.
Investigations
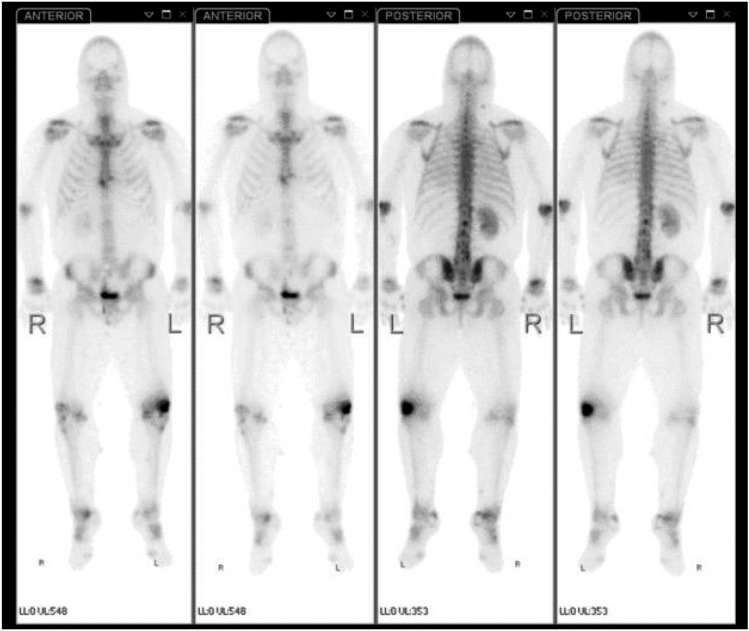
A PTH stimulation test was performed which failed to show a rise in urinary phosphate excretion, urinary cyclic AMP (c-AMP) or calcitriol and failed to show a decrease in tubular phosphate resportion (maximum)/glomerular filtration rate (TmP/GFR; table 1). These results are typical of classical resistance to PTH (PHP type 1A). He went on to have a whole-body technetium-bisphosphonate bone scan that showed several areas of low-grade increased uptake within the soft tissues, mainly in the neck and right tibia, as well as increased periarticular uptake throughout the hands, wrists, elbows, knees, ankles, mid feet, bilateral first metatarsophalangeal joints as well as increased uptake at the bilateral calcaneal tuberosities (see figure 2). Given these findings the patient was evaluated by rheumatology who diagnosed periarticular calcifications and calcific periarthritis.
Table 1.
PTH (teriparatide) stimulation test results
| Parameter | 0 min | 30 min | 60 min | 90 min | 150 min |
|---|---|---|---|---|---|
| Serum creatinine (µmol/L) | 135 | 131 | |||
| Serum calcium (mmol/L) | 2.11 | 2.23 | |||
| Serum PO4 (mmol/L) | 1.43 | 1.43 | 1.45 | ||
| Spot urine creatinine (mmol/L) | 5.22 | 2.55 | 4.29 | 5.44 | 7.78 |
| Spot urine PO4 (mmol/L) | 5.4 | 2.2 | 4.1 | 8.2 | 15.6 |
| FePO4 | 9.77% | 9.8% | 9.0% | ||
| TmP/GFR | 1.31 | 1.19 | |||
| Urine c-AMP | 1.2 | 1.6 | 2.4 | 2.7 | 2.4 |
| 1,25 vitamin D (pmol/L) | 32 | 24 |
c-AMP, cyclic AMP; PTH, parathyroid hormone; TmP/GFR, tubular phosphate resportion (maximum)/glomerular filtration rate.
Figure 2.
Images from a whole body technetium-bisphosphonate bone scan showing several areas of low-grade increased uptake within soft tissues, mainly the neck and right tibia, as well as increased periarticular uptake throughout the hands, wrists, elbows, knees, ankles, mid feet, bilateral first metatarsophalangeal joints and at bilateral calcaneal tuberosities.
Fractional excretion of phosphate (FePO4), TmP/GFR, urine c-AMP expressed in nmol/dL glomerular filtrate, normal range (1.3–3.7). Calcitriol normal range was 55–120 pmol/L.
Outcome and follow-up
After being off calcitriol and calcium for a total of 18 months the patient developed leg cramps and was found to have hypocalcaemia (figure 1), a normal creatinine (123 µmol/L, reference range 65–125 µmol/L) and a PTH that was elevated at almost 600% of the upper limit of the assay. At that point it was felt to be confirmation that he did in fact have PHP type 1A, but that he had developed such extensive soft tissue calcification from long-term calcitriol use that had remained eucalcaemic by resorbing his soft tissue stores, and when these had finally been exhausted he became hypocalcaemic with a high PTH and improved renal function. He was restarted on calcitriol and calcium, and instructed to have monthly blood work for calcium, PTH and phosphate. His calcium and PTH have since normalised and the patient remains clinically well.
Discussion
PHP refers to a group of disorders characterised by end-organ resistance to PTH. Patients typically present with hypocalcaemia, hyperphosphataemia and an elevated PTH. PHP is an uncommon genetic disorder that is further subdivided into PHP types 1A, 1B and pseudopseudohypoparathyroidism based on the clinical and biochemical findings. Two further variants, PHP types 1C and 2, are genotypically distinct from the other forms.1
PHP type 1A is due to loss of function of one allele of the gene encoding the stimulatory G protein (Gs). The α-subunit of the Gs (Gsa) is a signalling protein essential for the actions of PTH and many other hormones through its interactions with adenylyl cyclase. Abnormalities in this signalling pathway lead to resistance to PTH and other hormones, including thyroid stimulating hormone, gonadotropins and growth hormone releasing hormone. The hormonal resistance leads to hypocalcaemia, hyperphosphataemia, elevated PTH levels, and in some, thyroid and gonadal dysfunction. Type 1A PHP is often, but not always, associated with Albright's hereditary osteodystrophy (AHO) which is characterised by short stature, round facies, brachydactyly, brachymetacarpia, central obesity, subcutaneous ossifications and, in some cases, mental or developmental delay.1 Type 1B PHP is characterised by resistance to PTH in renal tissue and other tissues, but does not have the AHO phenotype.1 Type 1C PHP is characterised by AHO, resistance to PTH and other hormones with normal Gsa activity.1 Patients with PHP type 2 have hypocalcaemia, hyperphosphataemia and increased serum PTH, but they lack the physical features associated with AHO.1
Because the PHP syndromes are rare, little is know about the long-term effects and possible complications from these conditions. However, there are a number of case reports describing possible complications from PHP, of which practitioners should be aware.
Life threatening complications
Many patients with PHP come for medical attention with acute complications of hypocalcaemia, including life-threatening manifestations such as QTc interval prolongation on ECG, stridor, seizures or other neurological sequelae.2 It is important to identify seizures that are a consequence of hypocalcaemia, as certain anticonvulsant medications can further decrease calcium levels and worsen symptoms, and this complication has been documented in patients with PHP.3 There are patients who have presented with syncope and ventricular arrhythmias secondary to QTc prolongation.1 4––6 Another report describes a case of transient neonatal hyperparathyroidism in an infant born to a mother with undiagnosed, and hence untreated PHP.7
Neurological complications
Seizures or convulsions are the most frequently described neurological complications from hypocalcaemia and one report describes a case where a young man was misdiagnosed for years despite suffering from recurrent atypical seizures and cataracts.6 Another case describes a woman in her 50s who presented with symptoms of Parkinson's (decreased mobility, pill-rolling tremor, mask-like facies and cog-wheel rigidity). She was found to be hypocalcaemic and later diagnosed with PHP. Her Parkinsonism symptoms improved dramatically with correction of serum calcium.8 One patient presented with recurrent polyneuropathy and was found to have distal dominant depletion of myelinated fibres as well as widespread calcification of the medial walls of brain arteries and veins and was later diagnosed with PHP.9 A further complication that has been reported is papilloedema from long-term, untreated hypocalcaemia.10
Musculoskeletal complications
Others have reported patients presenting with severe skeletal abnormalities resembling rickets that respond to treatment with 1,25 dihydroxycholecalciferol.11 In rare cases surgical intervention has been required to fix extreme skeletal abnormalities.12 A number of reports describe spinal cord compression and spinal stenosis secondary to narrowed spinal canals, excessive bony formation in the spinal canal or ossification of paravertebral ligaments in patients with PHP.13––16 Surgical intervention was required in all of these cases to relieve the devastating consequences of spinal cord compression. Also, numerous dental abnormalities have been reported including delayed tooth eruption, malocclusion, enamel aplasia and hypoplasia as well as heterotopic ossification in the maxillofacial region.17
Common presentations and follow-up
Since PHP can present with many varied symptoms, patients may present to practitioners for various consequences of the disease, including dermatology for cutaneous ossifications, paediatrics for childhood obesity and poor growth, endocrinology for hypothyroidism or hypogonadism, dentistry for dental abnormalities or neurology for neurological manifestations, so it is important that practitioners have a high level of suspicion for the disease, given the right clinical context.18––20 There are very few long-term studies examining patients with PHP. Since the condition may be associated with hyporesponsiveness to other hormones one retrospective cohort followed 12 patients with PHP type 1A overtime and identified that hypothyroidism and subcutaneous ossifications were generally the first manifestations of the disease. PTH resistance with hypocalcaemia and hyperphospataemia developed later along with resistance to other hormones, emphasising the need for long-term biochemical follow-up.21
Complications of therapy
Levels of 1,25 dihydroxycholecalciferol are low in patients with PHP and replacement of this vitamin D derivative in the form of calcitriol can prevent the hypocalcemic complications. Little is described in the literature regarding possible risks of calcitriol treatment, and what is known mainly comes from patients with secondary or tertiary hyperparathyroidism secondary to chronic renal failure, but may still be applicable to patients with PHP.
It has been shown that adynamic renal osteodystrophy and decreased bone formation can develop or worsen in patients on intermittent calcitriol therapy and that calcitriol may directly suppress osteoblastic activity in patients with secondary hyperparathyroidism undergoing peritoneal dialysis. These changes can be associated with skeletal complications, including fractures.22––24
Vascular calcifications have been described from calcitriol therapy in patients with secondary hyperparathyroidism and research has demonstrated that calcitriol treatment leads to aortic calcifications, aneurysms and ventricular hypertrophy in animal models.25 26 Emerging data suggest that treatment of renal osteodystrophy with calcitriol may increase vascular calcifications and the risk of death from cardiovascular causes among patients undergoing dialysis.24 It is interesting to note that our patient also presented with cardiac calcifications in the setting of long-term calcitriol therapy and chronic renal failure.
Although rare, central nervous system and brain calcifications have been described in patients undergoing dialysis being treated with calcitriol.27 There is also conflicting evidence that treatment with calcitriol may lead to a more rapid decline in renal function in patients with chronic renal failure, and that doses greater than 0.5 µg/day seem to be more highly associated with this decline.22 It has been shown that calcitriol impairs creatinine secretion by the renal tubules leading to increased serum creatinine and reduced creatinine clearance in patients with mild to moderate renal failure, which may have been relevant to our patient's chronic renal failure.22
Another subset of patients requiring long-term calcitriol therapy is the one with surgically induced hypoparathyroidism whose complications from long-term therapy have also been described in this group. The most commonly documented complication is hypercalciuria and there have been case reports of calcium-alkali syndrome developing in these patients.28 There is also documentation of these patients developing nephrocalcinosis after long-term calcium and calcitriol therapies.29
Related to pregnancy, it has been noted that women with hypoparathyroidism on calcitriol treatment often require an increased dose during the third trimester to maintain normal calcium levels but that breastfeeding is associated with an increased risk of hypercalcaemia and thus monitoring of calcium levels during pregnancy and the postpartum period is extremely important for these patients.30
Another uncommon side effect that has been documented and could affect any patient taking calcitriol is an anaphylactic allergic reaction to the medication. In certain instances successful desensitisation to the medication has been performed so that the treatment could continue.31
Learning points.
Pseudohypoparathyroidism type 1A is a rare genetic disorder characterised by end-organ resistance to parathyroid hormone (PTH) and other hormones leading to varied clinical manifestations including hypocalcaemia, an elevated PTH and in some, Albright's hereditary osteodystrophy.
Long-term studies examining patients with pseudohypoparathyroidism are lacking but given the potential complications of the disease and its treatment, long-term follow-up and biochemical monitoring is crucial.
Few studies have strictly examined the potential side effects of long-term calcitriol plus calcium therapy, but soft tissue deposition of calcium may result in serious vascular or renal consequences.
Resorption of soft tissue calcium deposits may take longer than 12 months after calcitriol therapy is stopped.
Footnotes
Contributors: EEC wrote the first draft of the manuscript. GK provided clinical care of the patient and critically revised the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Donghi V, Mora S, Zamproni I, et al. Pseudohypoparathyroidism, an often delayed diagnosis: a case series. Cases J 2009;2:6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sunder RA, Singh M. Pseudohypoparathyroidism: a series of three cases and an unusual presentation of ocular tetany. Anaesthesia 2006;61:394–8 [DOI] [PubMed] [Google Scholar]
- 3.Glyne A, Hunter I, Thomson J. Pseudohypoparathyroidism with paradoxical increase in hypocalcemic seizuresdue to long term anti-convulsant therapy. Postgrad Med J 1972;48:632–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang TC, Cecchin FC, Mahoney P, et al. Corrected QT interval (QTC) prolongation and syncope associated with pseudohypoparathyroidism and hypocalcemia. J Pediatr 2000;136:404–7 [DOI] [PubMed] [Google Scholar]
- 5.Isikay S, Akdemir I, Yilmaz K. Pseudohypoparathyroidism presenting with ventricular arrhythmia: a case report. J Clin Res Pediatr Endocrinol 2012;4:42–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faig JC, Kalinyak J, Marcus R, et al. Chronic atypical seizure disorder and cataracts due to delayed diagnosis of pseudohypoparathyroidism. West J Med 1992;157:64–5 [PMC free article] [PubMed] [Google Scholar]
- 7.Glass EJ, Barr DG. Transient neonatal hyperparathyroidism secondary to maternal pseudohypoparathyroidism. Arch Dis Child 1981;56:565–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson DW, Durward WF, Fogelman I, et al. Pseudohypoparathyroidism presenting as severe Parkinsonism. Postgrad Med J 1981;57:445–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanda T, Nagashima T, Oda M, et al. Pseudopseudohypoparathyroidism with recurrent polyneuropathy: an autopsy report with special reference to the peripheral nervous system. J Neurol Sci 1991;103:42–7 [DOI] [PubMed] [Google Scholar]
- 10.Asplund J. Pseudotumor cerebri in pseudohypoparathyroidism. Acta Med Scand 1980;208:331–2 [DOI] [PubMed] [Google Scholar]
- 11.Bajpai A, Sharma J, Haft P, et al. Pseudohypoparathyroidism presenting with bony deformities resembling rickets. Indian J Pediatr 2004;71:345–8 [DOI] [PubMed] [Google Scholar]
- 12.Cho TJ, Choi IH, Chung CY, et al. Humerus varus in a patient with pseudohypoparathyroidism. J Korean Med Sci 2005;20:158–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alam SM, Kelly W. Spinal cord compression associated with pseudohypoparathyroidism. J R Soc Med 1990;83:50–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li P, Huang L, Zhao Z, et al. Spinal-cord compression related to pseudohypoparathyroidism. J Clin Neurosci 2011;18:143–5 [DOI] [PubMed] [Google Scholar]
- 15.Okada K, Iida K, Sakusabe N, et al. Pseudohypoparathyroidism-associated spinal stenosis. Spine 1994;19:1186–9 [DOI] [PubMed] [Google Scholar]
- 16.Van Dop C, Wang H, Mulaikal RM, et al. Pseudopseudohypoparathyroidism with spinal cord compression. Pediatr Radiol 1988;18:429–31 [DOI] [PubMed] [Google Scholar]
- 17.DuVal MG, Davidson S, Ho A, et al. Albright's hereditary osteodystrophy with extensive heterotopic ossification of the oral and maxillofacial region: how fetuin research may help a seemingly impossible condition. J Can Dent Assoc 2007;73:845–50 [PubMed] [Google Scholar]
- 18.Barranco VP. Cutaneous ossification in pseudohypoparathyroidism. Arch Dermatol 1971;104:643–7 [PubMed] [Google Scholar]
- 19.Poomthavorn P, Zacharin M. Early manifestation of obesity and calcinosis cutis in infantile pseudohypoparathyroidism. J Paediatr Child Health 2006;42:821–3 [DOI] [PubMed] [Google Scholar]
- 20.Dudley AW, Jr, Hawkins H. Mineralization of the central nervous system in pseudopseudohypoparathyroidism (PPH). J Neurol Neurosurg Psychiatry 1970;33:147–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelfand IM, Eugster EA, DiMeglio LA. Presentation and clinical progression of pseudohypoparathyroidism with multi-hormone resistance and Albright hereditary osteodystrophy: a case series. J Pediatr 2006;149:877–80 [DOI] [PubMed] [Google Scholar]
- 22.Goodman WG, Coburn JW. The use of 1,25-dihydroxyvitamin D3 in early renal failure. Annu Rev Med 1992;43:227–37 [DOI] [PubMed] [Google Scholar]
- 23.Goodman WG, Ramirez JA, Belin TR, et al. Development of adynamic bone in patients with secondary hyperparathyroidism after intermittent calcitriol therapy. Kidney Int 46:1160–6 [DOI] [PubMed] [Google Scholar]
- 24.Tend M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis and paricalcitol or calcitriol therapy. N Engl J Med 2003;349:446–56 [DOI] [PubMed] [Google Scholar]
- 25.Henley C, Colloton M, Cattley RC, et al. 1,25-Dihydroxyvitamin D3 but not cinacalcet HCl (Sensipar/Mimpara) treatment mediates aortic calcification in a rat model of secondary hyperparathyroidism. Nephrol Dial Transplant 2005;20:1370–7 [DOI] [PubMed] [Google Scholar]
- 26.Zebger-Gong H, Muller D, Diercke M, et al. 1,25-Dihydroxyvitamin D3-induced aortic calcifications in experimental uremia: up-regulation of osteoblast markers, calcium-transporting proteins and osterix. J Hypertens 2011;29:339–48 [DOI] [PubMed] [Google Scholar]
- 27.Bilge I, Sadıkoğlu B, Emre S, et al. Brain calcification due to secondary hyperparathyroidism in a child with renal failure. Turk J Pediatr 2005;47:287–90 [PubMed] [Google Scholar]
- 28.Fernandez-Garcia M, Vazquez L, Hernandez JL. Calcium-alkali syndrome in post-surgical hypoparathyroidism. QJM 2012;105:1209–12 [DOI] [PubMed] [Google Scholar]
- 29.Kocak G, Kocak E, Azak A, et al. An unusual cause of acute renal failure in a patient with surgical hypoparathyroidism: nephrocalcinosis. Endocrine 2012;41:162–3 [DOI] [PubMed] [Google Scholar]
- 30.Caplan RH, Beguin EA. Hypercalcemia in a calcitriol-treated hypoparathyroid woman during lactation. Obstet Gynecol 76(3 Pt 2):485–9 [PubMed] [Google Scholar]
- 31.Amandeep S, Lomaestro B, Meuwissen HJ. Hypersensitivity to intravenous and oral calcitriol with successful desensitization. J Allergy Clin Immunol 103(1 Pt 1):176. [DOI] [PubMed] [Google Scholar]