Abstract
Denosumab, a fully humanised monoclonal antibody, is licensed for treatment of postmenopausal osteoporosis, hormone ablation-induced bone loss and for prevention of skeleton-related events in patients with bone metastases from solid tumours. In pivotal phase 3 randomised trials, denosumab caused profound hypocalcaemia in patients with normocalcaemia despite oral calcium and vitamin D supplementation. This significant hypocalcaemic effect can be exploited to treat hypercalcaemia of malignancy (HCM). Recent reports from the USA suggest that denosumab is an effective treatment of HCM. According to our knowledge, we report the first two cases in UK with bisphosphonate refractory hypercalcaemia who responded to denosumab injections. Our first case gained 7 months of stabilisation of hypercalcaemia following prolonged admissions with life-threatening levels, while our second case achieved rapid normalisation of serum calcium levels for the first time in 14 months. We conclude that denosumab should be the treatment of choice for patients with bisphosphonate refractory hypercalcaemia.
Background
Hypercalcaemia is an oncological emergency with an estimated incidence of 10–20% in adult patients with cancer. Hypercalcaemia can cause neurological, gastrointestinal and cardiac symptoms such as drowsiness, confusion, personality change, cognitive dysfunction, disorientation, incoherent speech and psychotic symptoms such as hallucinations and delusions, dizziness, anorexia, nausea, vomiting, and in severe cases, cardiac arrhythmias, coma and death.1 Chronic hypercalcaemia can also cause disabling symptoms such as bone pain, lethargy and constipation, which in turn significantly affects the quality of life of patients with advanced cancer with limited life expectancy. Hypercalcaemia is also a poor prognostic factor for patients with advanced cancer.
The current standard of care for patients with cancer with severe hypercalcaemia is rehydration with intravenous fluids and intravenous bisphosphonates. Zoledronic acid, a potent bisphosphonate, is the current standard of care for hypercalcaemia of malignancy.2 A hospital admission for aggressive intravenous hydration and intravenous bisphosphonate therapy offers only a temporary solution. Hence, treatment of hypercalcaemia of malignancy also includes control of the underlying cancer with systemic treatment. Unfortunately in those patients where systemic therapies have failed, chronic hypercalcaemia usually necessitates frequent inpatient stays, during a time when quality of life at home is a premium. Management of patients with bisphosphonate refractory hypercalcaemia is even more challenging with no highly effective therapy available. Calcitonin (subcutaneous injection) and steroids have an adjunct role for their modest calcium-lowering effect but there is no widely accepted second-line therapy for refractory hypercalcaemia.
Receptor activator of nuclear factor κ-B ligand (RANKL) is a cell surface molecule and plays an important role in bone resorption and bone remodelling through its effect on osteoclasts.3 Denosumab, a fully humanised monoclonal antibody, binds and inhibits RANKL with high affinity and has beneficial effect on bone remodelling (see figure 1). Following randomised phase 3 trials, denosumab is now licensed for the treatment of postmenopausal osteoporosis, hormone ablation-induced bone loss and for the prevention of skeleton-related events (SRE) in patients with bone metastases from solid tumours.4 5
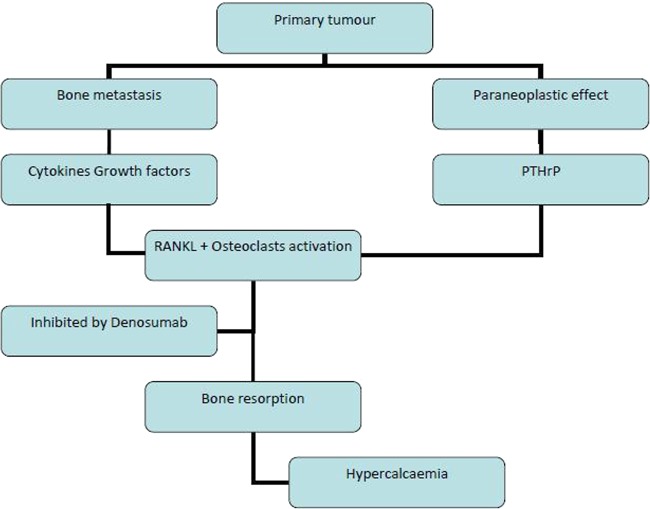
Figure 1.
Mechanism of hypercalcaemia from paraneoplastic syndrome and bone metastasis.
In pivotal phase 3 randomised trials, denosumab not only reduced the incidence of hypercalcaemia, but also caused profound hypocalcaemia in some patients with normocalcaemia in spite of oral calcium supplementation. The incidence of hypocalcaemia in these trials of denosumab was more pronounced than zoledronic acid, which is currently the treatment of choice for hypercalcaemia of malignancy.2 6 This better hypocalcaemic potency of denosumab can be exploited to treat hypercalcaemia and recent reports from the USA suggest that denosumab is effective in treating hypercalcaemia caused by cancer.7–9 According to our knowledge, we report the first two cases in UK with bisphosphonate refractory hypercalcaemia which responded to denosumab injections.
Case presentation
Case 1
Our first case is a middle-aged woman in her 40s, who originally presented with a 2-month history of left hip pain to her general practitioner in June 2011. An X-ray revealed lytic lesions to the left ischial and superior iliac bone. She had no significant medical history, family history, and apart from taking over-the-counter analgesia, she was fit and was in fulltime work. Staging CT revealed large left renal primary (89×78 mm) and subcentimetre lung nodules. Bone scan revealed pelvic bone metastasis and sternal metastasis. Baseline serum calcium was slightly elevated at 2.76 mmol/L (normal limit 2.20–2.60 mmol/L).
Following consultations with the urologists and oncologists, she had palliative radiotherapy to her sternum and left hemipelvis (20 Gy in 5 fractions). She also subsequently underwent cytoreductive nephrectomy. Surgery confirmed a primary renal cell carcinoma. Following recovery from surgery, restaging CT and repeat bone scan were conducted prior to consideration of systemic therapy. Both the scans showed progression of bone disease with new right rib, and right proximal femoral shaft lesions. Shortly after the scan, she fell and sustained a pathological fracture through the right femoral/trochanteric region. On admission for her fracture, she was found to have adjusted serum calcium level of 3.55 mmol/L, which was corrected with intravenous hydration with serum calcium falling down to 2.31 mmol/L over 4 days.
The patient underwent pinning of the fracture and also received further radiotherapy (20 Gy in 5 fractions) to her right hip following recovery from the operation. Four weeks later, her serum calcium increased to 3.13 mmol/L while parathyroid hormone (PTH) remained physiologically suppressed at <3 ng/L. She was readmitted, given intravenous hydration and zoledronic acid, and discharged after 3 days of inpatient stay.
She started first-line systemic therapy with sunitinib (at a dose of 50 mg) with a standard 4 weeks on and 2 weeks off treatment schedule. Her repeat CT scan after 3 months of treatment showed a mixed response. There was resolution of lung nodules, but she had progressive disease in bones. Scans demonstrated numerous bone metastases in the spine with moderate spinal encroachment of tumour at T11 vertebrae level. Her serum calcium during this period remained high (median 2.88, range 2.47–3.15 mmol/L), but was particularly high during the 2-weeks off sunitinib treatment. Hence, she was switched to continuous schedule of sunitinib at a daily dose of 37.5 mg but continuous schedule had only limited efficacy in suppressing serum calcium levels.
The patient received more radiotherapy to her cervical spine and T9-L4 (8 Gy in single fractions to both areas), but her disease continued to progress in the bones and her 6-month scan showed further progression. A further 6 months later, MRI confirmed progression at L2-L4 with cauda equina compression and hence she had a single fraction of radiotherapy to T9-L4 vertebrae. Her serum calcium at this time was high at 3.30 mmol/L and hence she was admitted for intravenous fluids for 3 days. Her systemic therapy was then switched to everolimus, a standard second-line systemic therapy for kidney cancers.
Only 11 days later, her serum calcium went up to 3.24 mmol/L. She was readmitted to hospital and spent 14 of the next 17 days in hospital, receiving intravenous fluids, intravenous zoledronic acid and subcutaneous calcitonin three times a day to maintain her calcium between 3.05 and 3.34 mmol/L. Despite the new systemic therapy (everolimus) and the patient maintaining high oral fluid intake and daily calcitonin injections, her calcium level rose to 3.98 mmol/L after discharge from hospital.
The patient was very symptomatic with hypercalcaemia at this stage and reported generalised bone pain and constipation. This is in contrast to her condition in which whenever her serum calcium levels were in the lower range, she was able to work when out of hospital. Thus, the bisphosphonate refractory hypercalcaemia affected her quality of life significantly.
Funding for denosumab, a human monoclonal antibody to RANKL inhibitor, was sought through individual drug request September 2012 (prior to National Institute for Health and Care Excellence (NICE) UK approval for SRE in October 2012) on the basis of phase 3 evidence of reduction in SRE.10
On starting denosumab subcutaneous injections, her adjusted serum calcium levels decreased from 3.62 to a nadir of 3.12 mmol/L in 11 days and calcitonin was subsequently stopped. Her median serum calcium following the nadir was 3.19 (range 2.97–3.40 mmol/L). More importantly, she had an excellent symptomatic response with partial resolution of bone aches and constipation.
Despite subsequent CTs showing continued cancer progression in the bone, following initiation of denosumab (4 weekly as subcutaneous injection at her outpatient clinic visits), she has avoided further hospital admissions for hypercalcaemia for 7 months. She also received further radiotherapy to L5 and sacrum in December 2012 as an outpatient.
Of note, following her first injection of denosumab, she had a tooth abscess and required tooth extraction and oral antibiotics. A review by the maxillofacial team ruled out osteonecrosis. She had problems with dentition previously and had a further dental extraction 25 weeks after starting denosumab without any problems. Despite the risk of denosumab-related osteonecrosis of jaw, a well-known side effect of denosumab, further injections were continued because of potential benefits outweighing the risks. She continues to tolerate denosumab injections very well in spite of reduced glomerular filtration rate due to her nephrectomy. She continues to be clinically well, symptomatically and biochemically from hypercalcaemia point of view. Figure 2 maps the patient's adjusted calcium levels during the treatment.
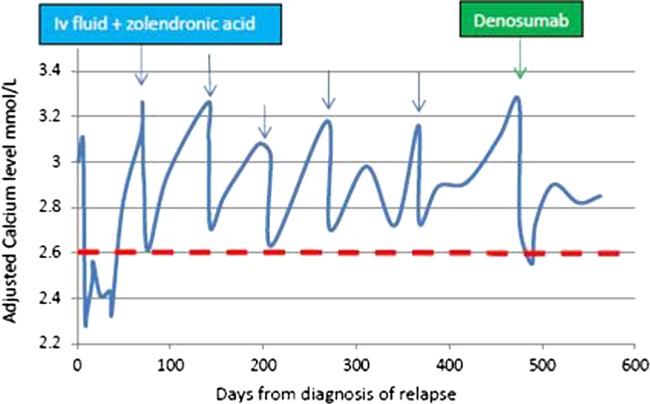
Figure 2.
Case 1: Adjusted calcium levels from diagnosis (day 1) until present day. Note blue arrows denote intravenous fluids±zoledronic acid. Green arrow indicates treatment with denosumab. Dashed red line shows upper limit of normal for adjusted serum calcium. Orange arrow denotes where patient received calcitonin 200 units subcutaneous injection as inpatient three times daily increased to four times daily, but stopped on starting denosumab.
Case 2
Our second case is a fit woman in her 60s, who presented with a suprapubic mass in February 2012. She had a medical history of stage 1c ovarian mixed papillary and clear cell carcinoma for which she had a total abdominal hysterectomy with bilateral salpingo-oopherectomy in January 2001, with negative peritoneal washings. The adherent cyst had ruptured perioperatively, and hence she postoperatively received six cycles of adjuvant carboplatin in 2001. She had no other comorbidities, was taking no medications, and had no family history of cancer. She lived alone and had a performance status of zero. A CT staging scan in February 2012 showed a 64×65×60 mm pelvic mass with pelvic lymphadenopathy. She had a biopsy which confirmed relapse of her previous ovarian cancer. Her CA125, as previously, was within normal limits. She was offered palliative chemotherapy for her relapsed ovarian cancer.
The patient had raised serum calcium at time of relapse with levels of 3 mmol/L, which, 1 week later, went up further to 3.11 mmol/L. She was admitted to hospital, and after hydration with intravenous fluids and treatment with intravenous zoledronic acid, her calcium levels normalised in 48 h. Her PTH was found to be physiologically suppressed at <3 ng/L. A bone scan performed shortly afterwards showed no evidence of bone metastasis. She was diagnosed with paraneoplastic hypercalcaemia due to cancer relapse, and started on first-line chemotherapy with carboplatin and paclitaxel, shortly after discharge. After three cycles of chemotherapy, there was no response and the cancer was found to have progressed. She also had to be admitted 9 weeks later with raised serum calcium of 3.26 mmol/L and symptoms of lethargy and constipation. These symptoms improved with intravenous fluids and intravenous zoledronic acid with serum calcium reaching a nadir of 2.71 mmol/L on discharge.
Subsequently, she started second-line therapy with liposomal doxorubicin and a CT scan reassessment after four cycles showed further disease progression. Over the next 7 months, she received third-line chemotherapy with topotecan, which then was changed to etoposide due to toxicity. In spite of multiple lines of chemotherapy, the cancer progressed and hence chemotherapy was stopped.
Because of persistently raised serum calcium levels, she received multiple infusions of zoledronic acid with limited efficacy. She had to be admitted to hospital three times with hypercalcaemia over 3 mmol/L, which on treatment reached a nadir of 2.70 mmol/L. On her last admission (16 months after the diagnosis of relapse), she was treated with subcutaneous denosumab. Even though pelvic bone metastasis was suspected clinically, a subsequent bone scan a few days showed evidence of no bone metastasis, thereby confirming the diagnosis of paraneoplastic hypercalcaemia. Despite her more advanced disease and lack of response to systemic cancer therapy, her serum calcium dramatically fell with denosumab injections. She also had an excellent symptomatic response to denosumab. Within 13 days of denosumab injection, the serum calcium normalised for the first time in 14 months to 2.55 mmol/L. She tolerated the denosumab very well without any significant side effects. She continues to be symptomatically and biochemically well from hypercalcaemia point of view. Figure 3 maps her adjusted calcium since relapse of her cancer.
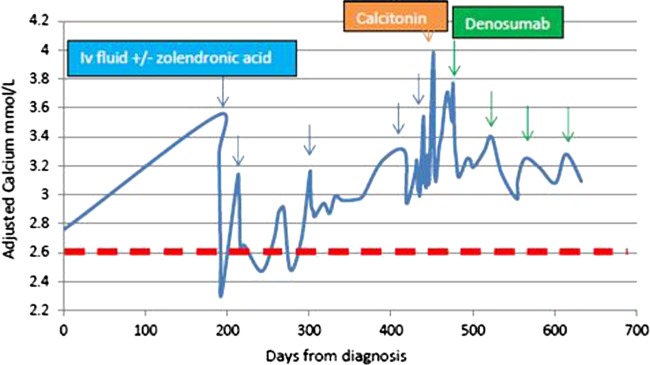
Figure 3.
Case 2: Adjusted calcium levels from diagnosis of relapse (day 1) until present day. Note blue arrows denote hospital admissions for intravenous fluids and zoledronic acid. Green arrow indicates treatment with denosumab. Dashed red line shows upper limit of normal for adjusted serum calcium.
Discussion
RANKL is a cytokine normally expressed in health and is integral to osteoclast formation, activation, adherence and survival. RANKL expressed by osteoblasts and released by activated T cells, binds to RANK expressed by osteoclasts. Its expression is modulated by PTH and calcitriol. Its overall effect is bone resorption.3
Hypercalcaemia of malignancy occurs as a result of osteoclast/RANKL activation either due to local effects of tumour (bone secondaries) or distant effects of a tumour (paraneoplastic syndrome).
When metastatic tumour cells are present in the bone, local cytokines and growth factors in the bone induce osteoblast to release RANKL and activate osteoclasts. Furthermore, bone resorption may release growth factors promoting tumour proliferation and survival enforcing a vicious cycle.11 12 This vicious cycle due to metastasis causes SRE and hypercalcaemia of malignancy.
In the absence of bone metastasis, paraneoplastic hypercalcaemia is driven by PTH-related peptide (PTHrP) secreted by tumour. PTHrP, an osteotrophic factor, mimics the effect of physiological PTH and increases bone resorption, enhances renal calcium retention and consequently leads to hypercalcaemia of malignancy.13 Figure 1 summarises the mechanisms of hypercalcaemia.
Denosumab, a fully humanised monoclonal antibody, binds and inhibits RANKL with high affinity, and has beneficial effects on bone remodelling. In three phase 3 double-blind trials, denosumab was compared with zoledronic acid.11 14 15 Denosumab was found to significantly reduce SRE which are defined as fracture of bones, radiotherapy to bone, spinal cord compression or surgery to bone. These phase 3 trial results led to denosumab being licensed by European Medicines agency for the prevention of SRE in patients with bone metastases from solid tumours. Following randomised phase 3 trials, denosumab is also licensed for the treatment of postmenopausal osteoporosis and hormone ablation-induced bone loss.4 5 Denosumab is now NICE approved for use in most solid malignancies for reduction of SRE.10
In pivotal phase 3 randomised trials, denosumab caused profound hypocalcaemia in patients with cancer with normocalcaemia.6 All three phase 3 registration studies focusing on SREs in comparison with zoledronic acid showed higher rates of hypocalcaemia (grade III or worse toxicity) in the denosumab arm despite the trial recommendation of oral supplementation with calcium and vitamin D. Zoledronic acid is currently the standard of care for hypercalcaemia of malignancy due to its hypocalcaemic effects.2 Hence, this higher hypocalcaemic potency of denosumab can be exploited to treat hypercalcaemia of malignancy. In particular, denosumab can be useful in refractory hypercalcaemia.
According to our knowledge, we report the first two cases in UK with bisphosphonate refractory hypercalcaemia who clinically responded to denosumab injections. Our first case gained 7 months of stabilisation of hypercalcaemia with denosumab having been previously subjected to prolonged admissions with life-threatening levels, while our second case achieved normalisation of serum calcium levels for the first time in 14 months with the help of denosumab.
Recently published reports from the USA confirm the effectiveness of denosumab in treating hypercalcaemia of malignancy.7–9
A case of paraneoplastic hypercalcaemia treated by denosumab has been previously reported from the USA.7 A male patient, with a new diagnosis of renal cell cancer, had bisphosphonate refractory hypercalcaemia. The patient initially improved with intravenous bisphosphonate infusion, intravenous fluids and calcitonin, but relapsed within a week. Subsequently, denosumab was administered which reduced the patient’s serum calcium within 2 days and the calcium-lowering effect was reported to be durable for a month. The patient died of disease progression before further treatments. Even though the patient did not demonstrate definite long-term bisphosphonate resistance, he benefited biochemically from subcutaneous denosumab injections.
A second case of bisphosphonate-resistant hypercalcaemia published recently also had metastatic renal carcinoma.8 Hypercalcaemia was resistant to intravenous pamidronate and the patient needed two hospital admissions in the span of a month. Denosumab injections led to prompt correction of hypercalcaemia. The response to denosumab was reported to be durable for 10 months. However, the role of denosumab in maintaining normocalcaemia could not be ascertained fully due to the fact that the patient started systemic therapy with sunitinib and gemcitabine concurrently. The response to systemic therapy is not well documented, and hence the calcium-lowering effect may have been due to the systemic anticancer treatment effect.16
A single proof of concept trial involving 15 patients was published recently by authors from MD Anderson Cancer Centre, USA.9 The patient population was a heterogeneous group in terms of tumour type, performance status and other baseline characteristics. There was a significant imbalance in sex with 80% of the patients being males in the study. Denosumab was administered following previous bisphosphonate therapy and response was defined in the study as serum calcium levels below 2.88 mmol/L within 10 days of injection. Duration of response was defined as time until serum calcium returned above 2.88 mmol/L. Denosumab subcutaneous injections was administered in the study on days 1, 8, 15 and 29 and 4 weekly thereafter. This study showed excellent response to denosumab. By day 10, 80% (12 patients) had responded and the median time to response was 8 days. The median response duration in the study was 26 days. This study confirms the clinical effectiveness of denosumab for treatment of hypercalcaemia of malignancy.
In contrast to the proof of principle study from the USA, which had predominately male patients (n=12), the two cases in this report are women. Both cases had poor response to systemic treatment and there was a clear treatment effect, with denosumab being superior to previous long-term zoledronic acid use. The first patient, with definite bisphosphonate refractory hypercalcaemia demonstrated prolonged clinical response and control. Although her serum calcium never normalised, stabilisation in a particularly challenging case, where otherwise life-threatening consequences would inevitably pursue, is a success. The ability to give full dose of denosumab in the presence of a single kidney and as an outpatient procedure was an added benefit for our patient with renal cell cancer. Our second case has very long periods of hypercalcaemia in spite of regular zoledronic infusions. The dramatic fall in serum calcium with just one subcutaneous injection of denosumab adds to the evidence that denosumab is highly active in paraneoplastic hypercalcaemia, independent of bone metastasis.
We conclude, based on our experience and published reports that denosumab should be the treatment of choice for patients with bisphosphonate refractory hypercalcaemia of malignancy.7–9
Denosumab, a potent hypocalcaemic agent, is also an effective alternative for patients with hypercalcaemia, who are intolerant of bisphosphonates, for instance acute phase reactions are more common with zolendronic acid than with denosumab.6 Zoledronic acid administration involves intravenous access in a day-case unit whereas denosumab can be conveniently given as a subcutaneous outpatient injection. Zoledronic acid is also contraindicated in patients with a creatinine clearance less than 30 mL/min and requires dose modification at moderate levels of impairment.11 14 15 Denosumab is not cleared by the kidneys and therefore requires no dose modification in renal impairment.6 17 Hence denosumab would be a better treatment option for patients with poor venous access or impaired renal function.
Learning points.
Denosumab should be the treatment of choice for patients with bisphosphonate refractory hypercalcaemia.
Denosumab is also a highly effective alternative for patients with hypercalcaemia if bisphosphonates are contraindicated or not tolerated.
Denosumab is effective in hypercalcaemia due to bone metastasis as well as in hypercalcaemia due to paraneoplastic effect of cancer.
Denosumab, a human monoclonal antibody, can be conveniently administered as a subcutaneous injection in the outpatient setting and is very well tolerated.
Footnotes
Contributors: JA is the main author of manuscript. YN and SS contributed to the writing of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.National Cancer Institute: PDQ Hypercalcemia Bethesda, MD: National Cancer Institute; Date last modified 01/09/2013. [Internet]. [cited 28 Oct 2013]. http://www.cancer.gov/cancertopics/pdq/supportivecare/hypercalcemia/HealthProfessional [Google Scholar]
- 2.Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001;19:558–67 [DOI] [PubMed] [Google Scholar]
- 3.Castellano D, Sepulveda JM, García-Escobar I, et al. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist 2011;16:136–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith MR, Egerdie B, Hernández Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009;361:745–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756–65 [DOI] [PubMed] [Google Scholar]
- 6.Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 2012;48:3082–92 [DOI] [PubMed] [Google Scholar]
- 7.Boikos SA, Hammers H-J. Denosumab for the treatment of bisphosphonate-refractory hypercalcemia. J Clin Oncol 2012;30:e299. [DOI] [PubMed] [Google Scholar]
- 8.Freeman A, El-Amm J, Aragon-Ching JB. Use of denosumab for renal cell carcinoma-associated malignant hypercalcemia: a case report and review of the literature. Clin Genitourin Cancer 2013;11:e24–6 [DOI] [PubMed] [Google Scholar]
- 9.Hu MI, Glezerman I, Leboulleux S, et al. Denosumab for patients with persistent or relapsed hypercalcemia of malignancy despite recent bisphosphonate treatment. J Natl Cancer Inst 2013;105:1417–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence (NICE) Bone metastases from solid tumours—denosumab. Technology appraisals TA265 Issued: October 2012
- 11.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010;28:5132–9 [DOI] [PubMed] [Google Scholar]
- 12.Canon JR, Roudier M, Bryant R, et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis 2008;25:119–29 [DOI] [PubMed] [Google Scholar]
- 13.Datta NS, Abou-Samra AB. PTH and PTHrP signaling in osteoblasts. Cell Signal 2009;21:1245–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011;29:1125–32 [DOI] [PubMed] [Google Scholar]
- 16.Karaca H, Lale A, Dikilitas M, et al. Recovery of paraneoplastic hypercalcemia by sunitinib treatment for renal cell carcinoma: a case report and review of the literature. Med Oncol Northwood Lond Engl 2010;27:1023–6 [DOI] [PubMed] [Google Scholar]
- 17.Bech A, de Boer H. Denosumab for tumor-induced hypercalcemia complicated by renal failure. Ann Intern Med 2012;156:906–7 [DOI] [PubMed] [Google Scholar]