Abstract
Acute genital ulcers, also known as acute vulvar ulcers, ulcus vulvae acutum or Lipschütz ulcers, refer to an ulceration of the vulva or lower vagina of non-venereal origin that usually presents in young women, predominantly virgins. Although its incidence is unknown, it seems a rare entity, with few cases reported in the literature. Their aetiology and pathogenesis are still unknown. The disease is characterised by an acute onset of flu-like symptoms with single or multiple painful ulcers on the vulva. Diagnosis is mainly clinical, after exclusion of other causes of vulvar ulcers. The treatment is mainly symptomatic, with spontaneous resolution in 2 weeks and without recurrences in most cases. We present a case report of a 13-year-old girl with two episodes of acute ulcers that fit the clinical criteria for Lipschütz ulcers.
Background
Acute genital ulcer (AGU) is a condition that the clinicians may sometimes underdiagnose and even misdiagnose with other entities. Hence, it is important to know the workup and the thorough differential diagnosis to establish its diagnosis.
Case presentation
A 13-year-old girl presented with acute ulcers with pain and inflammation in the labia majora that prevented ambulation and was also associated with fever. At 11 years of age, the patient completed the vaccination schedule, menstrual pattern 4/28 and had no sexual intercourse up to that moment. As the only antecedent of interest, 2 years before (when she was 11) she presented with a similar episode, though with more intense local and systemic symptoms, that was diagnosed as a Lipschütz ulcer (LU), after ruling out other conditions (figure 1). During those 2 years, she never had presented oral aphthous ulcers or other significant diseases. The patient denied being sexually active or sexually abused.
Figure 1.
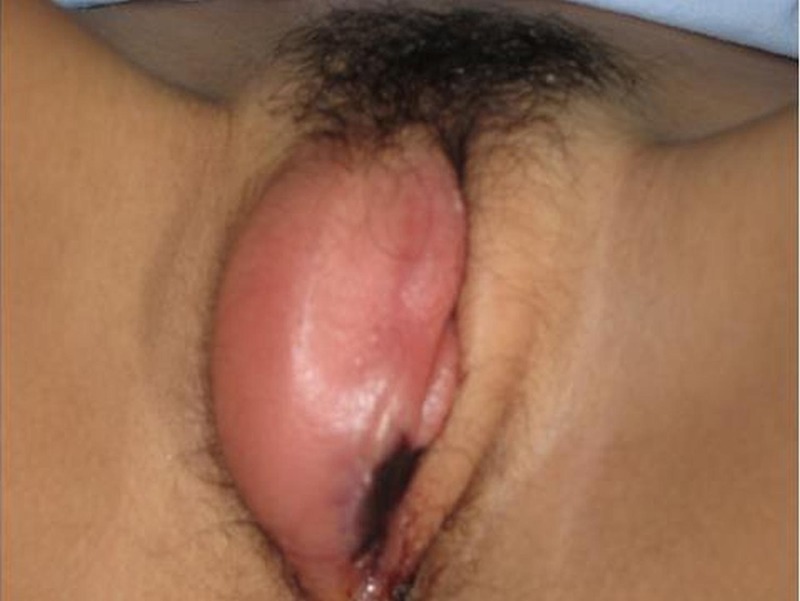
Acute ulcers and inflammation in the labia majora.
Investigations
On physical examination she was afebrile and in good general condition. Cardiopulmonary auscultation was normal with no fatigue, sore throat, cardiac or respiratory signs. She had normal female genital morphology but an ulcerative lesion of about 3 mm diameter at the inner edge of left labia majora and a non-ulcerative smaller lesion at the introitus, both very painful to the touch (figure 2). Blood tests showed a white cell count of 17 280/µL with 61.4% neutrophils, haemoglobin 11.3 g/dL, haematocrit 33.3%, C reactive protein 3.17 mg/L and procalcitonin level 0.06 ng/mL. Owing to the current symptoms and the blood test results, the patient was admitted to the hospital. Serologies for herpes virus, Ebstein-Barr virus (EBV), cytomegalovirus (CMV), HIV and syphilis performed 1 week after admission were negative (as in the first episode). Bacterial culture of the lesion was negative.
Figure 2.
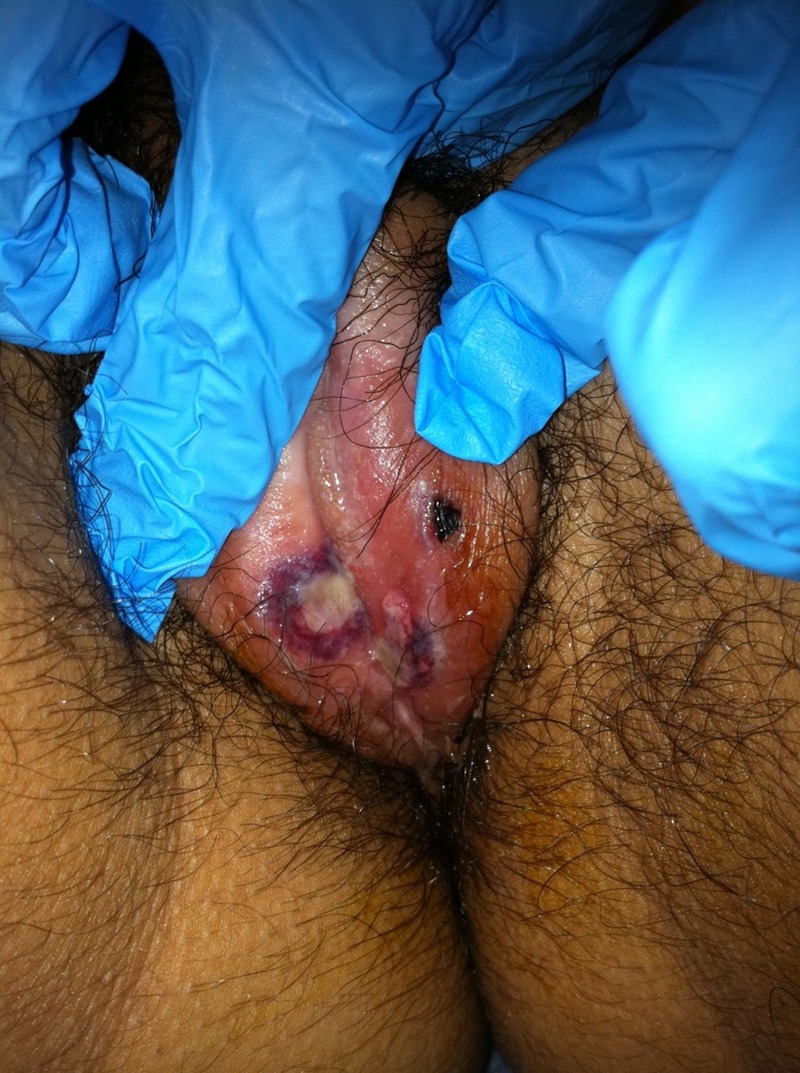
An ulcerative lesion of about 3 mm diameter at the inner edge of left labia majora and a non-ulcerative smaller lesion at the introitus.
Differential diagnosis
The differential diagnosis includes venereal and non-venereal infections, non-infectious diseases, traumatic causes and malignant tumours.
Treatment
Treatment consisted of anti-inflammatory drugs, analgesics and prophylactic broad-spectrum antibiotics (amoxicillin/clavulanic acid and azithromycin). Daily cures under sedation in the operating room were made to remove adherent necrotic material.
Outcome and follow-up
The patient showed a good clinical progress with improvement of the blood test results and was discharged home in good condition and without pain after a total hospital stay of 11 days.
The follow-up showed a total resolution and re-epithelisation of the vulvar lesion at 3 weeks after being discharged from hospital (figure 3). Six months later the patient remained asymptomatic.
Figure 3.
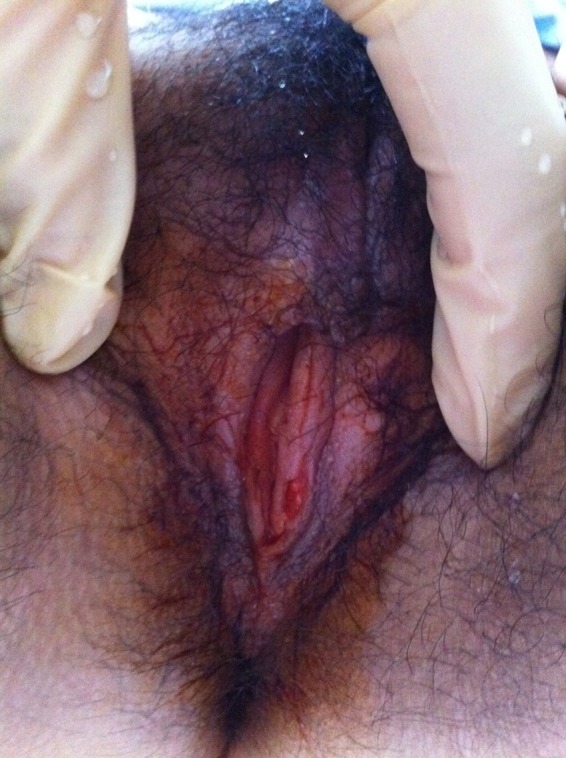
Total resolution and re-epithelisation of the vulvar lesion.
Discussion
In 1913, Lipschütz described an acute clinical entity characterised by the sudden appearance of necrotic painful genital ulcerations, fever and lymphadenopathy that occurred mostly in adolescent and virgin girls.1–3
This characteristic clinical picture is also called acute vulvar ulcers and ulcus vulvae acutum.4
LU seems to be a rare entity, because there are few cases reported in the medical literature but their true incidence is difficult to know as many cases are misdiagnosed with other more frequent causes of genital ulcers.1 5
Since the first description several aetiologies have been discussed. Viral pathogens may cause ulcers. The reported rate of EBV associated with AGU seems to be around 30%.6 Other pathological agents have been found to be associated with episodes of AGU, including CMV, paratyphoid fever, influenza A, Toxoplasma gondii and Mycoplasma.1–4 7 8 Chanal et al5 have also reported a case of AGU associated with mumps. The pathophysiological mechanism of ulcer in these cases is unknown. It has been suggested a direct cytotoxic effect, a role of B lymphocytes EBV-infected or a reactive process typically triggered by a distant infection.1 4
AGU present as single or multiple, shallow ulcers with raised, sharply demarcated borders. Many have an overlying grey exudate or adherent grey-brown eschar. Secondary erythema and oedema may be impressive. Typically, ulcers occur on the medial aspects of the labia minora, but they are also found on the labia majora, perineum and in the lower vagina. ‘Kissing’ ulcers on opposing surfaces are common. The size varies and lesions greater than 1 cm have been described.4 8 Many patients can present as well with malaise, fever, asthenia, myalgia, headache, pharyngitis or tonsillitis and lymphadenopathy.1–4
AGU can be gangrenous, chronic or miliar. Gangrenous vulvar ulcers are the most common form and are painful ulcers with a grey-yellow crust. The chronic form presents as relapsing, undermined ulcers with marked oedema. Miliar vulvar ulcers are purulent, fibrinous, pinhead-sized ulcers with inflammatory edges.3 9
Diagnosis of this entity is made by exclusion, sometimes retrospectively. Women with AGU require a comprehensive evaluation. The workup should start with a sensitive sexual history, which is performed confidentially and includes questions on sexual activity and potential abuse. The review of systems should include queries to uncover other systemic illnesses, with particular attention to ocular, neurological, gastrointestinal and genitourinary symptoms. The clinician should perform a full physical examination looking for oral and skin lesions.4 Laboratory evaluation should always include a complete blood cell count, serological test for syphilis, EBV, HIV and PCR assays for HSV, bacterial culture and viral culture or PCR of the lesion.2–4 Sometimes, skin biopsy from an ulcer edge could be necessary to rule out other conditions but histological examination is not of diagnostic value because findings are non-specific: there are superficial oedema and dilated capillaries with neutrophilic infiltration and ulceration.1 4 9 10
When diagnostic tests are negative, the patient fits the clinical criteria for LU.
Differential diagnosis is broad and includes infections, sexually and non-sexually transmitted, systemic diseases, malignances and drug reactions (see table 1). Behçet disease and vulvar aphthosis (simple and complex) are other conditions that should be included in the differential diagnosis. Behçet disease includes recurrent oral and genital ulceration with multiorgan symptoms (erythema nodosum, uveitis, vasculitis, arthritis, etc). The term ‘complex aphthosis’ describes patients who have recurrent oral and genital ulcers but who do not meet diagnostic criteria for Behçet disease. Simple aphthosis refers recurrent vulvar ulcers only. It has been suggested that oral and genital aphthosis may be on a spectrum of disease ranging from simple aphthosis to complex aphthosis to Behçet disease.4 9 10 Follow-up of these patients is essential to rule out progression to systemic disease.4
Table 1.
Differential diagnosis of acute genital ulcers in an adolescent
| Infection | |
|---|---|
| Sexually transmitted | Herpes simplex virus |
| Syphilis | |
| Lymphogranuloma venereum | |
| Chancroid | |
| HIV | |
| Non-sexually transmitted | Herpes simplex virus |
| Epstein-Barr virus | |
| Cytomegalovirus | |
| Influenza A | |
| Paratyphoid | |
| Systemic disease | Crohn's disease |
| Cyclic neutropenia | |
| PFAPA syndrome (periodic fever, aphthous stomatitis, pharyngitis, adenitis) | |
| MAGIC syndrome (mouth and genital ulcers with inflamed cartilage) | |
| Iron, folate, vitamin B12 deficiency | |
| Behçet's disease | |
| Pemphigus and pemphigoid | |
| Complex and simple aphthosis | |
| Hormone-related | Autoimmune progesterone dermatitis |
| Oestrogen hypersensitivity | |
| Drug reaction | Non-steroidal anti-inflammatory drugs |
| Contact or irritant dermatitis | |
| Malignancy | Lymphoma/leukaemia |
| Trauma | Foreign body |
| Sexual injury | |
| Caustic burns |
The goals of treatment are to provide pain relief, improve healing and prevent scarring. Empiric treatment includes Sitz baths, topical anaesthetic and topical corticosteroids. If the patient has severe pain or malaise, hospitalisation is indicated. Broad-spectrum antibiotics to cover skin flora should be considered. Use of systemic corticoids is indicated if the patient fails to respond to topical agents.1–5 7 9
The condition is self-limited and healing occurs spontaneously, usually in 2 weeks.2 3
Learning points.
Physicians should keep in mind this entity to avoid misdiagnosis, especially with infectious ulcers, and treatments without any benefit.
When the diagnosis of acute genital ulcer (AGU) is made, patients and their families should be reassured because AGU are not sexually transmitted and they heal spontaneously, without sequelae.
Follow-up of these patients would be necessary to exclude early Behçet's disease.
Footnotes
Contributors: SD-G and JCM-E visited the patient and started the diagnosis workup picking up clinical data; AP-M reviewed the case, wrote the case report and got the references and JCM-E provided the figures and critically reviewed the text.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hernández-Núñez A, Córdoba S, Romero-Maté A, et al. Lipchütz ulcers. Four cases. Pediatr Dermatol 2008;25:364–7 [DOI] [PubMed] [Google Scholar]
- 2.Brinca A, Canelas MM, Carvalho MJ, et al. Lipschütz ulcer (ulcus vulvae acutum): a rare cause of genital lesión. An Bras Dermatol 2012;87:622–4 [DOI] [PubMed] [Google Scholar]
- 3.García Reymundo M, Montero Salas A, González Álvarez CM, et al. Úlcera de Lipchütz: causa poco conocida de úlcera genital aguda. An Pediatr 2010;72:443–4 [DOI] [PubMed] [Google Scholar]
- 4.Huppert JS. Lipchütz ulcers: evaluation and management of acute genital ulcers in women. Dermatol Ther 2010;23:533–40 [DOI] [PubMed] [Google Scholar]
- 5.Chanal J, Carlotti A, Laude H, et al. Lipschütz genital ulceration associated whit mumps. Dermatology 2010;221:292–5 [DOI] [PubMed] [Google Scholar]
- 6.Török L, Domján K, Faragó E. Ulcus vulvae acutum. Cutis 2000;65:387–9 [PubMed] [Google Scholar]
- 7.Kluger N, García C, Guillot B. Lipschütz acute genital ulcer. J Gynecol Obstet Biol Reprod 2009;38:528–30 [DOI] [PubMed] [Google Scholar]
- 8.Levy Bencheton A, Agostini A, Mortier I, et al. Acute vulvar ulcer of Lipschütz: a misdiagnosis entity. Gynecol Obstet Fertil 2011;39:e58–60 [DOI] [PubMed] [Google Scholar]
- 9.Lai K, Lambert E, Mercurio MG. Aphtous vulvar ulcers in adolescent girls: case report and review of the literature. J Cutan Med Surg 2010;14:33–7 [DOI] [PubMed] [Google Scholar]
- 10.Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: a manifestation of aphthosis. J Pediatr Adolesc Gynecol 2006;19:195–204 [DOI] [PubMed] [Google Scholar]


